Abstract
Mutations of the tumor suppressor genes tuberous sclerosis complex (TSC)1 and TSC2 cause pulmonary lymphangioleiomyomatosis (LAM) and tuberous sclerosis (TS). Current rapamycin-based therapies for TS and LAM have a predominantly cytostatic effect, and disease progression resumes with therapy cessation. Evidence of RhoA GTPase activation in LAM-derived and human TSC2-null cells suggests that 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor statins can be used as potential adjuvant agents. The goal of this study was to determine which statin (simvastatin or atorvastatin) is more effective in suppressing TSC2-null cell growth and signaling. Simvastatin, but not atorvastatin, showed a concentration-dependent (0.5–10 μM) inhibitory effect on mouse TSC2-null and human LAM–derived cell growth. Treatment with 10 μM simvastatin induced dramatic disruption of TSC2-null cell monolayer and cell rounding; in contrast, few changes were observed in cells treated with the same concentration of atorvastatin. Combined treatment of rapamycin with simvastatin but not with atorvastatin showed a synergistic growth-inhibitory effect on TSC2-null cells. Simvastatin, but not atorvastatin, inhibited the activity of prosurvival serine-threonine kinase Akt and induced marked up-regulation of cleaved caspase-3, a marker of cell apoptosis. Simvastatin, but not atorvastatin, also induced concentration-dependent inhibition of p42/p44 Erk and mTORC1. Thus, our data show growth-inhibitory and proapoptotic effects of simvastatin on TSC2-null cells compared with atorvastatin. These findings have translational significance for combinatorial therapeutic strategies of simvastatin to inhibit TSC2-null cell survival in TS and LAM.
Keywords: TSC, LAM, apoptosis, TSC2, mTOR
Clinical Relevance
This study provides further insights about simvastatin as a potential therapeutic agent and additional preclinical evidence for its use as treatment in tuberous sclerosis and lymphangioleiomyomatosis. Understanding the differences in effectiveness of simvastatin and atorvastatin provides critical information for selection of the better choice as an adjuvant therapy for tuberous sclerosis and lymphangioleiomyomatosis.
Mutational inactivation of the tumor suppressor genes tuberous sclerosis complex (TSC)1 and TSC2 occurs in the hamartoma syndrome tuberous sclerosis (TS) and in pulmonary lymphangioleiomyomatosis (LAM). TS manifests by multiple tumors in the brain, kidney, heart, and skin (1). LAM, which can be sporadic or associated with TS (TS-LAM), is a rare lung disease affecting predominantly women of childbearing age that manifests by neoplastic growth of atypical smooth muscle–like LAM cells, lung cyst formation, obstruction of lymphatics, and spontaneous pneumothoraces (2). A breakthrough in understanding neoplastic pathophysiology in TS and LAM has resulted from the discovery of TSC1/TSC2 tumor suppressor complex as a negative regulator of the mechanistic target of rapamycin (mTOR) (3–6), an integrator of growth factor, nutrient, energy, and stress signaling (7). The TSC2 regulation of mTORC1 (4, 8) and inhibitory effects of rapamycin in preclinical studies (4, 5, 9, 10) have provided a rationale for the clinical use of rapamycin analogs (11–16). Despite promising results of rapamycin analogs in the clinic, after cessation of sirolimus therapy pulmonary function reverts to the diminished levels observed before treatment (11, 14–16), likely because sirolimus does not completely inhibit mTORC1 signaling, without promoting cell death (17). Furthermore, hyperlipidemia occurs as a side effect in patients with LAM and TS on sirolimus (11, 18).
The identification of increased RhoA GTPase activity (19–21) and its requirement for TSC2-null and LAM cell survival (22) has suggested a potential beneficial effect of therapeutic targeting of RhoA in LAM and TS. The development of drugs targeting RhoA GTPase, however, has not been successful (23). Rho GTPases cycle between an inactive GDP-bound form and an active GTP-bound form. Prenylation of small GTPases, which is the posttranslational modification involving the addition of hydrophobic geranylgeranyl functional groups to C-terminal cysteine, facilitates their attachment to the cell membrane. Thus, pharmacological targeting of geranylgeranylation of RhoA GTPase may have therapeutic benefits.
Statins inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, a rate-limiting enzyme in the mevalonate pathway (24). Lipid products of the mevalonate pathway are necessary for cellular functions including cholesterol metabolism, membrane integrity, and cell signaling specifically generating geranylgeranyl pyrophosphate, which is involved in posttranslational modification of membrane-bound small GTPases including Rho, Rac1, and Cdc42 (23). Retrospective study of patients with LAM on statins has cautioned about the potential effects of statins on lung function without taking into account intermolecular differences between statins (25). For example, atorvastatin up-regulates the expression of VEGF-D (26), which is up-regulated in the serum of patients with LAM and is a potential prognostic biomarker for LAM (27). Thus, preclinical comparison of different statins under the same experimental conditions is needed, and their pharmacological characteristics and safety in LAM remain to be determined.
In our published studies, we demonstrated a growth inhibitory effect of simvastatin in vitro and in vivo on TSC2-null cell and tumor growth (22, 28). Finlay and colleagues (20) and Lee and colleagues (29) used atorvastatin. These studies were performed using different cell and animal models, limiting their translational utility and leaving unanswered the question of whether simvastatin or atorvastatin could be used in TS and LAM clinical trials. The goal of this study was to determine whether simvastatin and atorvastatin have differential effects on TSC2-null cell growth. Understanding the differences in effectiveness of these drugs may provide critical information for the selection of the better choice as an adjuvant therapy for TS and LAM.
Results
Effect of Simvastatin and Atorvastatin on Mouse TSC2-Null Cell Growth
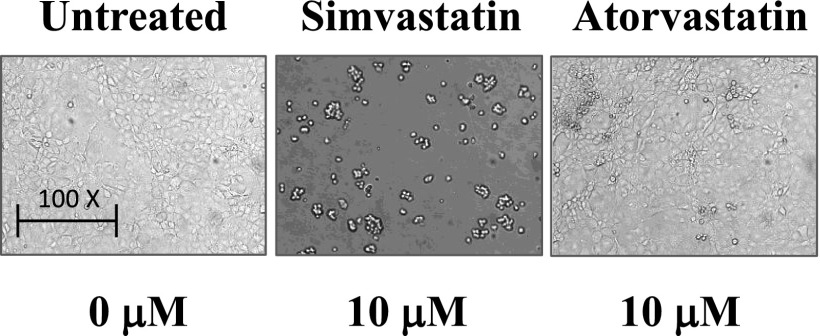
To perform comparative analysis of statins, we used mouse epithelial TSC2-null cells derived from kidney lesions of TSC2+/− mice used in the LAM mouse model (28, 30) and human LAM-derived cells (4). TSC2-null and LAM-derived cells, plated on 6-well plates, were maintained in Dulbecco’s modified Eagle medium supplemented with 10% FBS for 6 days in complete medium that was changed every other day and supplemented with 0.5, 1.0, 5.0, and 10 µm freshly made atorvastatin (Sigma Aldrich, St. Louis, MO) or simvastatin (EMD Millipore, Darmstadt, Germany). Control cells were maintained at the same conditions and treated with diluent. Morphological analysis using a phase-contrast microscope (TE 2000; Nikon, Melville, NY) showed dramatic disruption of TSC2-null cell monolayer and cell rounding at 10 μM simvastatin at Day 6 (Figure 1). In contrast, few morphological changes were observed in TSC2-null cells treated with the same concentration of atorvastatin (Figure 1).
Figure 1.
Simvastatin, but not atorvastatin, induces tuberous sclerosis complex 2 (TSC2)-null cell rounding. A total of 1.5 × 105 TSC2-null cells were plated on 6-well plates and treated with 10 μM of atorvastatin or simvastatin added freshly every other day as described in Results. Untreated cell were used as a control. Representative images were taken using a phase-contrast microscope.
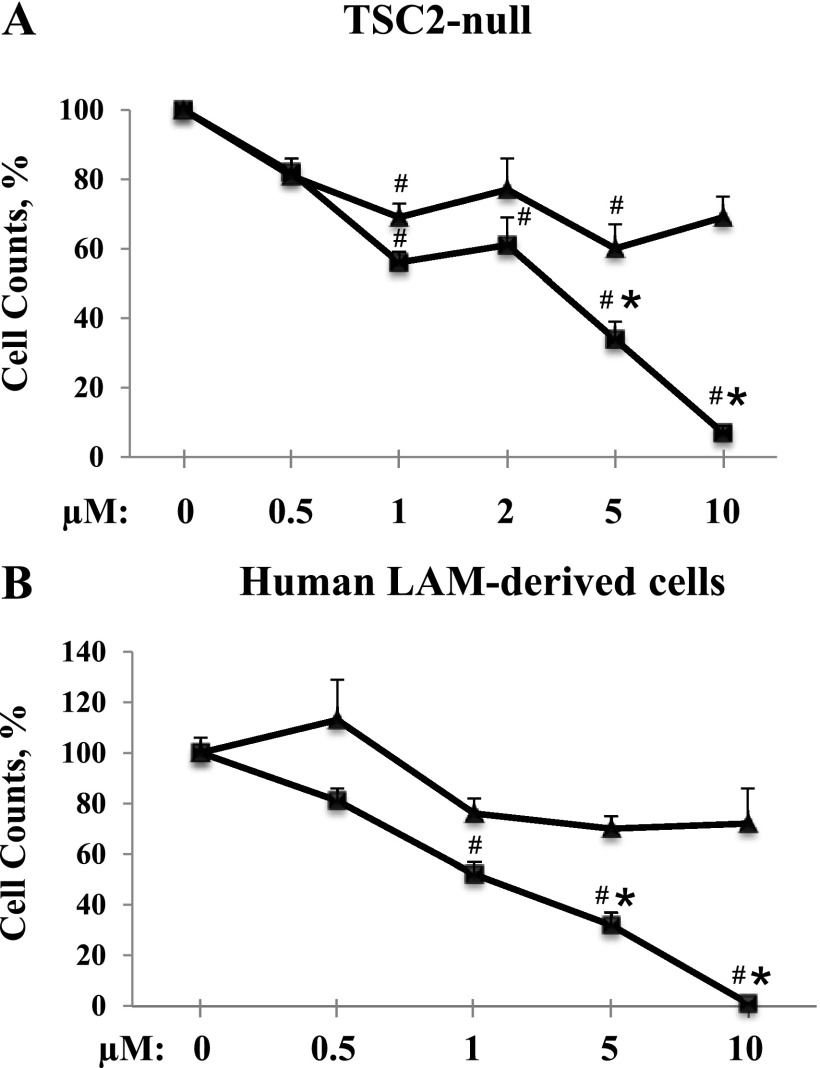
Statins not only inhibit cell proliferation but also promote apoptosis, which decreases cell numbers (22, 31). Thus, to adequately assess the effect of simvastatin and atorvastatin on cell growth, we used cell counts performed on the Countess Automated cell counter (Invitrogen, Grand Island, NY). Treatment with simvastatin exhibits significant dose-dependent inhibition of TSC2-null cell growth (56 ± 3%, 61 ± 8%, 34 ± 5%, and 7 ± 2% of cells were detected after treatment with 1, 2, 5, and 10 μM, respectively; P < 0.001 versus untreated cells). TSC2-null cell counts revealed that, at concentrations of 1 and 2 μM, simvastatin and atorvastatin have comparable inhibitory effects on cell growth. However, at a concentration of 5 μM atorvastatin showed 40% inhibition, and simvastatin showed 66% inhibition (P < 0.005). At 10 μM, only 7 ± 2% of simvastatin-treated cells were detected, in contrast to 69 ± 6% of cells treated with atorvastatin (P < 0.0001) (Figure 2A). Similarly, simvastatin showed marked dose-dependent growth inhibition of human LAM-derived cells with complete loss of cell numbers at 10 μM (52 ± 4%, 32 ± 5%, and 0% of cells were detected after treatment with 1, 5, and 10 μM simvastatin, respectively; P < 0.001 versus untreated cells) (Figure 2B). Unlike simvastatin, atorvastatin does not inhibit cell growth at doses of 1, 5, and 10 μM (76 ± 6%, 70 ± 5%, and 72 ± 14%, respectively). Cell count analysis at 0.5 μM revealed that neither simvastatin nor atorvastatin exhibits inhibitory effects on cell growth in both cell lines.
Figure 2.
Simvastatin, but not atorvastatin, abrogates TSC2-null cell growth. TSC2-null (A) or lymphangioleiomyomatosis (LAM)-derived cells (B) were treated with atorvastatin (triangles) or simvastatin (squares) over the range of concentrations (0.5, 1.0, 5.0, and 10 μM). Untreated cells were used as control. Cell counts were performed and analyzed with three measurements for each condition. Each point represents the mean of three to eight measurements ± SEM. #P < 0.01 for cells treated with simvastatin or atorvastatin versus untreated cells. *P < 0.01 for cells treated with atorvastatin versus simvastatin by analysis of variance (Bonferroni-Dunn).
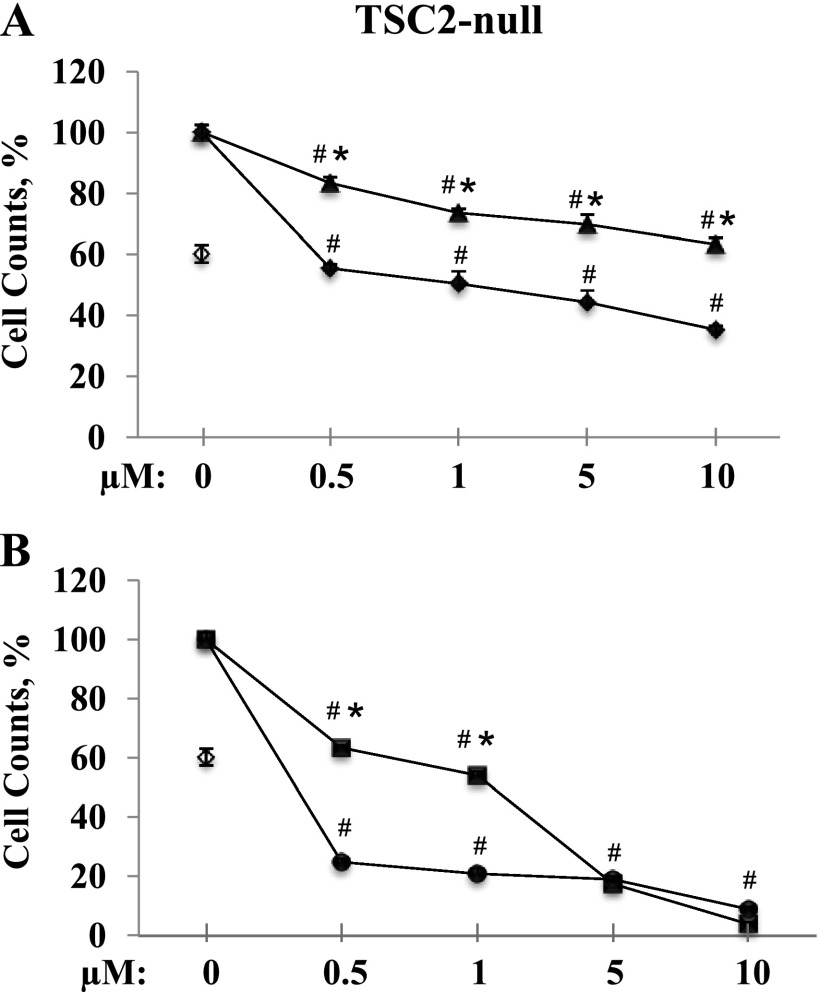
To determine the potential for the combination therapy of statins and rapamycin, TSC2-null cells were treated with 20 nM of rapamycin combined with atorvastatin or simvastatin. The combination of atorvastatin with rapamycin appears to have an additive effect on growth inhibition (Figure 3A). Rapamycin in combination with 0.5, 1, 5, and 10 μM atorvastatin shows 45 ± 1%, 50 ± 4%, 55 ± 4%, and 65 ± 1% growth inhibition, respectively, compared with 40 ± 3% inhibition by rapamycin alone (Figure 3A). In contrast, the combination of simvastatin and rapamycin showed a synergistic inhibitory effect on TSC2-null cell growth at concentrations of 0.5 and 1 μM. Combining rapamycin with 0.5 and 1 μM of simvastatin induced 75 ± 1% and 79 ± 1.6% growth inhibition, respectively, in contrast to 37 ± 1% and 46 ± 1% inhibited by simvastatin alone. Combination with rapamycin had little effect on simvastatin used at the higher doses (19 ± 0.9% and 9 ± 0.6% of cells were detected after treatment with 5 and 10 μM, respectively; P < 0.001 versus untreated cells or rapamycin alone) (Figure 3B) potentially due to a dominant proapoptotic mechanism induced by simvastatin, as demonstrated in a published study (22).
Figure 3.
Rapamycin differentially enhances inhibitory effects of simvastatin or atorvastatin on TSC2-null cell growth. Cells were maintained in Dulbecco’s modified Eagle medium with 10% FBS for 6 days in complete media supplemented with freshly made 20 nM of rapamycin (open diamonds); atorvastatin with the range of concentrations 0.5, 1.0, 5.0, and 10 µM (triangles); or a combination of both (solid diamonds) (A). TSC2-null cells were treated with 20 nM of rapamycin (open diamonds); simvastatin with the range of concentrations 0.5, 1.0, 5.0, and 10 µM (squares); or a combination of both (solid diamonds) (B). Control cells were maintained at the same conditions and treated with diluent. Cell counts were performed and analyzed; two measurements were taken for each condition. Each point represents the mean of two measurements ± SEM. #P < 0.01 for cells with treatment versus untreated cells. *P < 0.01 for cells with single treatment simvastatin or atorvastatin versus combination with rapamycin alone by analysis of variance (Bonferroni-Dunn).
Simvastatin-Induced Down-Regulation of Akt/PKB in TSC2-Null Cells
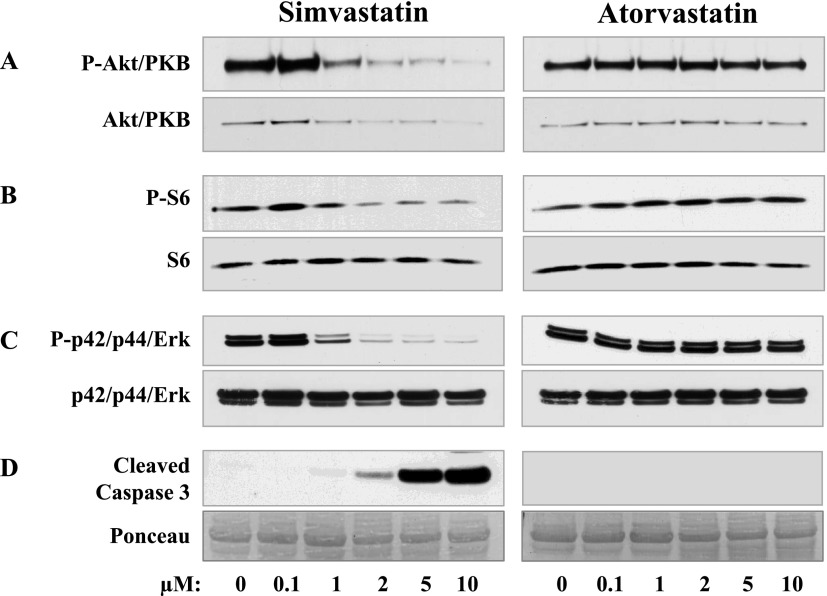
Akt/PKB is a serine-threonine kinase that controls programmed cell death by phosphorylating substrates and regulates apoptosis and cellular metabolism (32). Phosphorylation of Akt/PKB on Serine-473 is regulated by mTORC2 and is required for Akt/PKB activation (33). Whole cell lysates were prepared from cells treated with diluent or with the indicated concentration of simvastatin or atorvastatin. Cells were then washed with ice-cold PBS, and whole cell lysates were prepared. Protein contents were measured using a detergent-compatible protein assay reagent kit (Bio-Rad, Hercules, CA), and equal amounts of protein were subjected to SDS-PAGE. Immunoblot analyses were performed with phospho-Akt and total Akt antibodies purchased from Cell Signaling Technology, Inc. (Boston, MA). Treatment of TSC2-null cells with simvastatin abolished Ser-473 Akt/PKB phosphorylation in a concentration-dependent manner (Figure 4A, upper panel). The Akt/PKB protein level was also markedly decreased in cells treated with 2, 5, and 10 μM simvastatin (Figure 4A, lower panel). In contrast, in cells treated with atorvastatin, phosphorylation levels of Akt/PKB and protein levels were unchanged (Figure 4A).
Figure 4.
Simvastatin-induced down-regulation of Akt/PKB, p42/p44 Erk, and mTORC1 and up-regulation of cleaved caspase-3. TSC2-null cells were treated with 0, 0.5, 1.0, 5.0, and 10 μM of atorvastatin or simvastatin followed by immunoblot analyses with phospho-Ser473-Akt/PKB and Akt/PKB (A), phospho-S6 and ribosomal protein S6 (B), phospho-p42/p44 Erk and total p42/p44 Erk (C), and with cleaved caspase-3 (D) antibodies.
Effect of Simvastatin on mTORC1 and Erk
Cells lacking TSC2 exhibit growth factor–independent activation of mTORC1 that directly phosphorylates the ribosomal protein S6 kinases, inducing phosphorylation of ribosomal protein S6 (7). Antibodies phospho-S6 and total S6 were supplied by Cell Signaling Technology, Inc. Simvastatin at concentrations of 2, 5, and 10 μM markedly inhibited S6 phosphorylation without affecting total S6 protein level in TSC2-null cells (Figure 3B). In contrast, treatment of cells with atorvastatin had little effect on S6 phosphorylation and protein level (Figure 4B).
Extracellular mitogen-regulated protein kinase (Erk) p42/p44 is essential for carcinogenesis and is constitutively activated in a variety of cancers (34). Treatment of TSC2-null cells with 1 μM simvastatin inhibited Erk phosphorylation (Figure 4C), and 2, 5, and 10 μM simvastatin abolished Erk signaling. In contrast, the same concentrations of atorvastatin had little effect on Erk. Antibodies total p42/p44 Erk and phospho p42/44-Erk were also purchased from Cell Signaling Technology, Inc.
Effect of Simvastatin and Atorvastatin on Cleaved Caspase-3 in TSC2-Null Cells
Our published study demonstrated marked induction of TSC2-null cell apoptosis detected by terminal deoxynucleotidyltransferase-mediated dUTPbiotin nick end labeling stating by simvastatin (22). In this study, we examined the level of apoptosis using immunoblot analysis with an antibody for cleaved caspase-3, a marker of cell apoptosis (35). Immunoblot analyses were performed with cleaved caspase-3 antibodies (Cell Signaling Technology, Inc.). At 2 μM, simvastatin induced a slight increase in cleaved caspase-3 levels (Figure 4C). The treatment of cells with 5 and 10 μM simvastatin induced marked up-regulation of cleaved caspase-3 levels (Figure 4C). In contrast, no detectable cleaved caspase-3 was found in TSC2-null cells treated with concentrations of atorvastatin as high as 10 μM (Figure 4C).
Discussion
Published preclinical studies using atorvastatin and simvastatin have provided contradictory evidence, potentially due to the use of different cell types, about the effectiveness of these drugs in TSC2-null cells (20, 22, 28, 29). To address this issue, we tested the therapeutic effect of simvastatin and atorvastatin on the same TSC2-null cells. Our study demonstrates that the proapoptotic effect of simvastatin but not atorvastatin correlates with simvastatin-induced inhibition of TSC2-null cell growth. Combination with rapamycin markedly potentiates the growth-inhibitory effect of simvastatin compared with the apparent additive effect of rapamycin in combination with atorvastatin. Simvastatin also shows effective inhibition of TSC2-null cell signaling, including Akt/PKB, Erk, and mTORC1, in contrast to the lack of atorvastatin effects. This study provides important preclinical data about the differential effects of simvastatin and atorvastatin on TSC2-null cell growth and signaling.
Mutations of tumor suppressor genes TSC1 or TSC2 cause TS, a genetic disease affecting approximately 1 million people worldwide (2). About 30% of those affected by TS, predominantly adult women, develop pulmonary TS-LAM, which manifests as neoplastic lesions that induce destruction of lung parenchyma and progressive loss of pulmonary function. TSC1/TSC2 regulates mTOR, which forms two functionally distinct complexes, rapamycin-sensitive mTORC1 and rapamycin-insensitive mTORC2 (33). Current rapamycin-based therapy for TS and LAM only slows down the disease progression, which is resumed upon the cessation of treatment (14, 15). The limitation of rapamycin as a cytostatic agent indicates the need for novel TS and LAM therapy targeting TSC2-null cell survival.
mTORC1-independent regulation of the actin cytoskeleton occurs through mTORC2-dependent regulation of RhoA and Rac1 GTPases (36), and Rac1 is required for mTOR activation (37). In TSC2-null and human LAM-derived cells, Rho GTPase activity is required for cell adhesion, motility, proliferation, and survival (19, 21, 22). Down-regulation of RhoA in TSC2-deficient, rat-derived TSC2-null ELT3 cells increases apoptosis and up-regulates the proapoptotic proteins Bim, Bok, and Puma (22), suggesting that pharmacological inhibition of RhoA in TSC2-null cells impairs their survival.
Statins are small molecule inhibitors of HMG-CoA reductase, the rate-limiting enzyme in the synthesis of mevalonate, a fatty acid intermediate in de novo cholesterol biosynthesis. Statins including simvastatin, pravastatin, lovastatin, and mevastatin are derived from fungi or made synthetically (e.g., atorvastatin and fluvastatin) (24). All statins are lipophilic except pravastatin (24). These agents are effective in preventing cardiovascular disease largely due to lowering cholesterol levels (38). In noncardiovascular diseases, including cancer (39), rheumatologic (40), and neurological disorders, the beneficial effects of statins are attributed to their “pleiotropic” effects (independent of their lipid-lowering properties). Pleiotropic effects of statins include the inhibition of isoprenoid intermediates involved in geranylgeranylation of Rho GTPases; farnesylation of small GTPases Ras and Rheb; oxidative stress; inhibition of L-type Ca2+ current (41); cell proliferation (22), invasion, and metastasis; and induction of apoptosis in leukemia and in smooth muscle, prostate, and breast cancer (24). Simvastatin has protective effects against oxidative stress, matrix metalloproteinase, and inflammation in preclinical studies (28). Statins also show potential uses in chronic obstructive pulmonary disease, osteoporosis, diabetes, and depression (42). The safety and efficacy of cholesterol-lowering drug statins are well documented as highly effective therapies used by millions of people (24, 38).
Statins differ in their pharmacological properties, such as equipotent doses, bioavailability, protein binding and elimination, pharmacogenetic factors, and cellular effects. An important question remains about the differences in the potency of statins as cholesterol-lowering drugs compared with the cholesterol-independent or “pleiotropic” effects of statins. Evidence from randomized, placebo-controlled trials shows comparable effects of atorvastatin and simvastatin at their standard doses on cardiovascular function (43). The equipotent daily doses needed to reach the therapeutic target of 25 to 30% reduction in low-density lipoprotein cholesterol, a valid surrogate parameter of HMG-CoA reductase inhibition, are 5 mg for atorvastatin and 10 mg for simvastatin (44). Thus, pharmacological and clinical evidence demonstrates higher potency of atorvastatin compared with simvastatin. Less is known about potency of statins in their cholesterol-independent or “pleiotropic” effects (45). Despite strong and consistent associations between the beneficial use of statins, cancer incidence, reduced mortality (46), and improved outcomes of treatment for chronic obstructive pulmonary disease (47), observational studies are inconclusive because they often do not provide information about the specific type of statin on clinical outcome. Further preclinical and clinical research with defined endpoints and defined population is needed to provide evidence for unanswered mechanistic, translational, and clinical questions.
Statins also have different drug interactions (48). Whereas simvastatin and lovastatin are mainly metabolized by cytochrome P450 (CYP) 3A, fluvastatin is metabolized by CYP2C9, and pravastatin is largely unchanged (44). Much less is known about statin differences in their pleiotropic effects, including cell growth and survival. For example, atorvastatin affects Rheb farnesylation (20), whereas simvastatin appears to have no such effect (22). In contrast, in vivo simvastatin induces cell apoptosis, whereas atorvastatin has little effect on cell survival and tumor size (20, 22, 28, 29). Our data present comparative effectiveness of simvastatin and atorvastatin using the same cell type and demonstrate the effectiveness of simvastatin in inducing proapoptotic effects in TSC2-null cells. This study provides further insights about simvastatin as a potential therapeutic agent and additional preclinical evidence for potential treatment in TS and LAM. Understanding the differences in effectiveness of simvastatin and atorvastatin provides critical information for choosing the most effective adjuvant therapy for TS and LAM.
Acknowledgments
Acknowledgments
The authors thank Dr. Reynold A. Panettieri, Jr. and Dr. Elena A. Goncharova for critical reading of the manuscript and helpful discussions; Ms. Sharmin Islam for help in preparing the manuscript and submission; and the National Disease Research Interchange (NDRI) for providing LAM tissue for dissociation of LAM cells.
Footnotes
This work was supported by National Institutes of Health/National Heart, Lung and Blood Institute grants RO1HL090829 and RO1HL114085 (V.P.K.) and by the LAM Foundation (V.P.K.).
Originally Published in Press as DOI: 10.1165/rcmb.2013-0203RC on August 15, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 2.Henske EP, McCormack FX. Lymphangioleiomyomatosis: a wolf in sheep’s clothing. J Clin Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McManus EJ, Alessi DR. TSC1-TSC2: a complex tale of PKB-mediated S6K regulation. Nat Cell Biol. 2002;4:E214–E216. doi: 10.1038/ncb0902-e214. [DOI] [PubMed] [Google Scholar]
- 4.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis. J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 5.Goncharova EA, Goncharov DA, Spaits M, Noonan D, Talovskaya E, Eszterhas A, Krymskaya VP. Abnormal smooth muscle cell growth in LAM: role for tumor suppressor TSC2. Am J Respir Cell Mol Biol. 2006;34:561–572. doi: 10.1165/rcmb.2005-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 7.Yuan H-X, Xiong Y, Guan K-L. Nutrient sensing, metabolism, and cell growth control. Mol Cell. 2013;49:379–387. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002;62:5645–5650. [PubMed] [Google Scholar]
- 9.Lee L, Sudentas P, Donohue B, Asrican K, Worku A, Walker V, Sun Y, Scgmidt K, Albert MS, El-Hashemite N, et al. Efficacy of a rapamycin analog (CCI-779) and IFN-g in tuberous sclerosis mouse models. Genes Chromosomes Cancer. 2005;42:213–227. doi: 10.1002/gcc.20118. [DOI] [PubMed] [Google Scholar]
- 10.Krymskaya VP, Goncharova EA. PI3K/mTORC1 activation in hamartoma syndromes: therapeutic prospects. Cell Cycle. 2009;8:403–413. doi: 10.4161/cc.8.3.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, Witt O, Kohrman MH, Flamini JR, Wu JY, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2012;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 13.Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, Dinopoulos A, Thomas G, Crone KR. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 14.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies DM, de Vries PJ, Johnson SR, McCartney DL, Cox JA, Serra AL, Watson PC, Howe CJ, Doyle T, Pointon K, et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res. 2011;17:4071–4081. doi: 10.1158/1078-0432.CCR-11-0445. [DOI] [PubMed] [Google Scholar]
- 16.Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N, Brakemeier S, de Vries PJ, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–824. doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]
- 17.Krymskaya VP. Treatment option(s) for pulmonary lymphangioleiomyomatosis: progress and current challenges. Am J Respir Cell Mol Biol. 2012;46:563–565. doi: 10.1165/rcmb.2011-0381ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabora SL, Franz DN, Ashwal S, Sagalowsky A, DiMario FJ, Jr, Miles D, Cutler D, Krueger D, Uppot RN, Rabenou R, et al. Multicenter phase 2 trial of sirolimus for TS: kidney angiomyolipomas and other tumors regress and VEGF-D levels decrease. PLoS ONE. 2011;6:e23379. doi: 10.1371/journal.pone.0023379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goncharova E, Goncharov D, Noonan D, Krymskaya VP. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the RAC1 GTPase. J Cell Biol. 2004;167:1171–1182. doi: 10.1083/jcb.200405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finlay GA, Malhowski AJ, Liu Y, Fanburg BL, Kwiatkowski DJ, Toksoz D. Selective inhibition of growth of tuberous sclerosis complex 2 null cells by atorvastatin is associated with impaired Rheb and Rho gtpase function and reduced mTOR/S6 kinase activity. Cancer Res. 2007;67:9878–9886. doi: 10.1158/0008-5472.CAN-07-1394. [DOI] [PubMed] [Google Scholar]
- 21.Goncharova EA, Goncharov DA, Lim PN, Noonan D, Krymskaya VP. Modulation of cell migration and invasiveness by tumor suppressor TSC2 in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2006;34:473–480. doi: 10.1165/rcmb.2005-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncharova EA, Goncharov DA, Li H, Pimtong W, Lu S, Khavin I, Krymskaya VP. mTORC2 is required for proliferation and survival of TSC2-null cells. Mol Cell Biol. 2011;31:2484–2498. doi: 10.1128/MCB.01061-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahai E, Marshal CJ. Rho-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 24.Demierre M-F, Higgins PDR, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 25.El-Chemaly S, Taveira-DaSilva A, Stylianou MP, Moss J. Statins in lymphangioleiomyomatosis: a word of caution. Eur Respir J. 2009;34:513–514. doi: 10.1183/09031936.00012709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loboda A, Jazwa A, Jozkowicz A, Molema G, Dulak J. Angiogenic transcriptome of human microvascular endothelial cells: effect of hypoxia, modulation by atorvastatin. Vascul Pharmacol. 2006;44:206–214. doi: 10.1016/j.vph.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young LR, VanDyke R, Gulleman PM, Inoue Y, Brown KK, Schmidt LS, Linehan WM, Hajjar F, Kinder BW, Trapnell BC, et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138:674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncharova EA, Goncharov DA, Fehrenbach M, Khavin I, Duka B, Hino O, Colby TV, Merrilees MJ, Haczku A, Albelda SM, Krymskaya VP. Prevention of alveolar destruction and airspace enlargement in a mouse model of pulmonary lymphangioleiomyomatosis (LAM) Sci Transl Med. 2012;4:154ra134. doi: 10.1126/scitranslmed.3003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee N, Woodrum C, Nobil A, Rauktys A, Messina M, Dabora S. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol. 2009;9:8. doi: 10.1186/1471-2210-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi T, Minowa O, Kuno J, Mitani H, Hino O, Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line TSC2 mutation in mice. Cancer Res. 1999;59:1206–1211. [PubMed] [Google Scholar]
- 31.Qi XF, Zheng L, Lee KJ, Kim DH, Kim CS, Cai DQ, Wu Z, Qin JW, Yu YH, Kim SK. HMG-CoA reductase inhibitors induce apoptosis of lymphoma cells by promoting ros generation and regulating Akt, Erk and p38 signals via suppression of mevalonate pathway. Cell Death Dis. 2013;4:e518. doi: 10.1038/cddis.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Phys Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 35.Andersen J, Kornbluth S. The tangled circuitry of metabolism and apoptosis. Mol Cell. 2013;49:399–410. doi: 10.1016/j.molcel.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 37.Saci A, Cantley Lewis C, Carpenter Christopher L. Rac1 regulates the activity of mTORC1 and mTORC2 and controls cellular size. Mol Cell. 2011;42:50–61. doi: 10.1016/j.molcel.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heart Protection Study Collaborative Group. Bulbulia R, Bowman L, Wallendszus K, Parish S, Armitage J, Peto R, Collins R. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet. 2011;378:2013–2020. doi: 10.1016/S0140-6736(11)61125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf. 2010;9:603–621. doi: 10.1517/14740331003662620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mihos CG, Artola RT, Santana O. The pleiotropic effects of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in rheumatologic disorders: a comprehensive review. Rheumatol Int. 2012;32:287–294. doi: 10.1007/s00296-011-2008-6. [DOI] [PubMed] [Google Scholar]
- 41.Bergdahl A, Persson E, Hellstrand P, Swärd K. Lovastatin induces relaxation and inhibits l-type Ca2+ current in the rat basilar artery. Pharmacol Toxicol. 2003;93:128. doi: 10.1034/j.1600-0773.2003.930304.x. [DOI] [PubMed] [Google Scholar]
- 42.Tasat DR, Yakisich JS. Expanding the pleiotropic effects of statins: attenuation of air pollution-induced inflammatory response. Am J Physiol Lung Cell Mol Physiol. 2012;303:L640–L641. doi: 10.1152/ajplung.00280.2012. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, Rahme E, Pilote L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention. Am Heart J. 2006;151:273–281. doi: 10.1016/j.ahj.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Neuvonen P, Backman J, Niemi M. Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin. Clin Pharmacokinet. 2008;47:463–474. doi: 10.2165/00003088-200847070-00003. [DOI] [PubMed] [Google Scholar]
- 45.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 47.Janda S, Park K, FitzGerald JM, Etminan M, Swiston J. Statins in COPD: a systematic review. Chest. 2009;136:734–743. doi: 10.1378/chest.09-0194. [DOI] [PubMed] [Google Scholar]
- 48.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]