Abstract
Tissue-specific transgene expression using tetracycline (tet)-regulated promoter/operator elements has been used to revolutionize our understanding of cellular and molecular processes. However, because most tet-regulated mouse strains use promoters of genes expressed in multiple tissues, to achieve exclusive expression in an organ of interest is often impossible. Indeed, in the extreme case, unwanted transgene expression in other organ systems causes lethality and precludes the study of the transgene in the actual organ of interest. Here, we describe a novel approach to activating tet-inducible transgene expression solely in the airway by administering aerosolized doxycycline. By optimizing the dose and duration of aerosolized doxycycline exposure in mice possessing a ubiquitously expressed Rosa26 promoter–driven reverse tet-controlled transcriptional activator (rtTA) element, we induce transgene expression exclusively in the airways. We detect no changes in the cellular composition or proliferative behavior of airway cells. We used this newly developed method to achieve airway basal stem cell–specific transgene expression using a cytokeratin 5 (also known as keratin 5)–driven rtTA driver line to induce Notch pathway activation. We observed a more robust mucous metaplasia phenotype than in mice receiving doxycycline systemically. In addition, unwanted phenotypes outside of the lung that were evident when doxycycline was received systemically were now absent. Thus, our approach allows for rapid and efficient airway-specific transgene expression. After the careful strain by strain titration of the dose and timing of doxycycline inhalation, a suite of preexisting transgenic mice can now be used to study airway biology specifically in cases where transient transgene expression is sufficient to induce a phenotype.
Keywords: airway-specific, aerosolized doxycycline, inhalation, Notch, mucous metaplasia
Clinical Relevance
This work advances the use of existing mouse models for understanding different disease models.
The first tetracycline (tet)-controlled transcriptional activation system was designed nearly a decade ago to control transgene expression temporally. The activation or suppression of a variety of genes in vivo has provided invaluable insights into the function and regulation of these genes in various tissues and cells (1–6). The original tet-controlled transcriptional activator (tTA) is a transcriptional regulator with tight control of target gene expression and a broad range of inducibility (7–13). In tTA-based systems, constitutive gene expression occurs in untreated mice, but is suppressed by the administration of doxycycline. Conversely, the reverse tet-controlled transcriptional activator (rtTA) activates transgene expression in the presence of doxycycline. New variants of tet-based regulators with additional features are still emerging (7–15).
The utility of a tet-inducible transgenic mouse is dependent upon whether the transgenic line demonstrates the faithful activation or suppression of transgene expression in the presence or absence of doxycycline. Doxycycline is generally administered through intraperitoneal injection, drinking water, or animal chow, and is systemically distributed to the entire body through the circulation. Therefore, doxycycline is accessible to almost all of the cell types in the body (3, 16–19). Because most of the murine tTA and rtTA driver lines used to induce transgene expression rely on promoters that drive the expression of genes in multiple tissues, to achieve exclusive expression in an organ of interest with systemic doxycycline administration is often impossible. Furthermore, cell type–specific promoters are often expressed in those particular types of cells in many different organs (20). Thus, these promiscuous driver lines limit our interpretation of the function of a gene in a particular organ when doxycycline is administered systemically. For example, the cytokeratin 5 (CK5)-rtTA driver mouse expresses rtTA in the basal cells of the airway, skin, esophagus, epididymis, and mammary glands (21). Therefore, to use the transgenic mice that bear the CK5-rtTA driver to perform organ-specific gene modulation is difficult. Indeed, in the extreme case, unwanted transgene expression in other organ systems causes lethality that precludes the study of the transgene in the actual organ of interest. Strategies to achieve organ-specific transgene expression involve the use of intersectional transgenic mouse models, in which two separate driver lines are necessary to express a transgene specifically in a particular tissue that can be uniquely identified by two marker genes. However, the generation and maintenance of such models are time-consuming and often expensive (22, 23). In the case of skin, existing transgenic mouse models have been repurposed to create skin-specific transgenic models by using topical skin applications of doxycycline. Thus, a whole suite of experiments has become possible, using mice that were readily available (21, 24). Using a doxycycline-inducible but ubiquitously expressed rtTA driver line, we describe a novel approach to activating transgene expression exclusively in the airway. Moreover, we activate gene expression in mouse basal cells of the proximal airway epithelium, the site that resembles the small airways of the human lung that are affected by major airway diseases such as asthma and chronic obstructive pulmonary disease. We administered aerosolized doxycycline by nebulizer to a mouse bearing the ubiquitously expressed rtTA regulator, and observed airway epithelium–specific transgene expression. Furthermore, this newly developed method can achieve a more rapid and more robust transgene-induced mucous metaplasia phenotype than is achieved using doxycycline administrated in the drinking water.
Materials and Methods
Mouse Models
Rosa26-rtTA, Col1a1-tet(O)H2BGFP, and CK5-rtTA mice were previously described (21, 25). The tet(O)H2BGFP, Tet(O)Cre, and R26-LSL-NICD mice were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were maintained in a pathogen-free environment, and received food and water ad libitum. These animal experiments were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital, in accordance with National Institutes of Health guidelines. In each experiment, at least four mice were used, and each experiment was repeated at least twice.
Doxycycline Administration
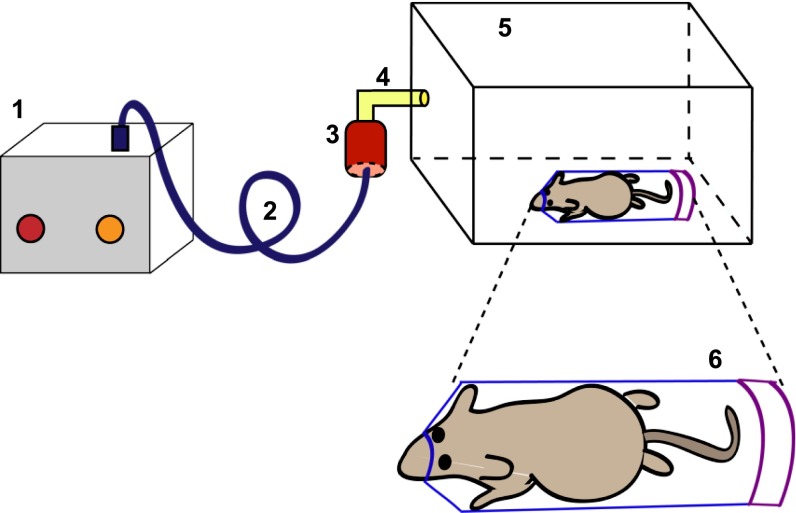
Mice were exposed to isoflurane and oxygen at a ratio of 1:1 for 2 minutes, and placed into a 50-ml conical Falcon tube (REF 352070; BD Falcon, Franklin Lakes, NJ) with a newly created 1-cm opening at the conical end of the tube (designed to fit the snout of the mouse and allow the mouse to breathe normally). Within 2 minutes, we observed the mice moving inside the tube. The mouse in the Falcon tube is then placed into a microisolator cage chamber that is connected to a nebulizer, as shown in Figure 1. Doxycycline (1–50 mg/ml) is aerosolized into the microisolator cage, using a nebulizer (De Villbis Ultraneb 90 nebulizer; DeVillbis Healthcare, Heston, United Kingdom) driven by compressed air. For intermittent doxycycline exposure, doxycycline was aerosolized for 20 minutes, and then shut off for 20 minutes in three cycles. The isolator chamber lid was completely opened to room air during the off cycles. Control mice were treated the same way, except that they received aerosolized PBS alone. Doxycycline is less soluble in PBS at higher concentrations. Therefore, rigorous vortexing (for 1 minute) of the doxycycline solution was necessary just before nebulization. Mice were killed for analyses of transgene expression 24 hours after the final inhalation in all experiments, unless otherwise indicated. For systemic doxycycline administration, doxycycline was dissolved in 2% sucrose water at a concentration of 1 mg/ml, and mice were allowed to drink ad libitum.
Figure 1.
Schematic representation of the experimental setup for administering aerosolized doxycycline. A nebulizer (1) is connected to a doxycycline reservoir (3) by tubing (2). The doxycycline reservoir is connected (4) to a microisolator chamber (5). The mouse is placed inside a 50-ml Falcon tube (REF 352070; BD Falcon, Franklin Lakes, NJ), with its snout snugly positioned through a 1-cm aperture in the tube (6).
Cell Dissociation and Flow Cytometry
Tracheal airway and non–airway cells were dissociated using a two-step sequential enzymatic dissociation method. Airway epithelial cells were liberated using papain dissociation, and non–airway cells were liberated using trypsin-mediated cell dissociation. A detailed description of the protocol is provided in the online supplement. Dissociated cells were stained, and flow cytometric analysis was performed. Airway epithelial cells were first gated for epithelial cell adhesion molecule (EpCAM), and then gated for green fluorescent protein (GFP). This allowed us to quantify the percentage of GFP-expressing epithelial cells in the inhaled doxycycline-treated mice, compared with mice treated with doxycycline in their drinking water.
Immunofluorescence and Cell Quantification
Immunofluorescence was performed as previously described (26), and a complete protocol can be found in the online supplement.
Statistical Analysis
Percentages of each particular cell type were calculated by counting the relative numbers of a specific cell type versus the total number of 4′6-diamidino-2-phenylindole–positive cells. The standard deviation was calculated from the average cell numbers of at least three independent tracheal samples. Data were compared among groups using the Student t test. A P value of less than 0.05 was considered significant.
Results
Aerosolized Doxycycline Activates Tet-Inducible Transgene Expression in the Airways
The conventional method of doxycycline administration for inducible gene expression using rtTA or tTA transcriptional regulators involves intraperitoneal injection, drinking water, or animal chow. Doxycycline administered through these methods is distributed into the circulation, and thereby exerts a systemic effect on any cell expressing both the rtTA and tet-inducible response elements. Because many pharmaceutical agents delivered by aerosolization directly to the respiratory tree escape unwanted systemic side effects, we hypothesized that aerosolized doxycycline could be similarly applied to activate tet-inducible transgene expression solely in the airways. To test this hypothesis, we generated mice bearing both an rtTA transcriptional regulator under the control of the ubiquitous Rosa26 promoter, and a tet-inducible H2BGFP fusion transgene under the control of the ubiquitous Collagen 1a1 (Col1a1) promoter (25). This R26-rtTA/Col1a1-tet(O)H2BGFP mouse (hereafter referred to as R26-H2BGFP) ubiquitously expresses rtTA, which then can bind to and activate a tet-operator in the presence of doxycycline, to activate the expression of the H2BGFP reporter gene.
When free-roaming mice are exposed to aerosolized doxycycline in a chamber, topical application of doxycycline to the skin occurs. We devised a simple procedure to exclusively expose the respiratory tree of a mouse to aerosolized doxycycline. Mice were anesthetized and placed into a 50-ml conical Falcon tube. The conical end of the tube was precut to form a 1-cm opening that allowed the mouse snout to fit snugly through the aperture and breathe normally. The mouse in the Falcon tube is then placed into a microisolator cage chamber that is connected to a nebulizer (Figure 1).
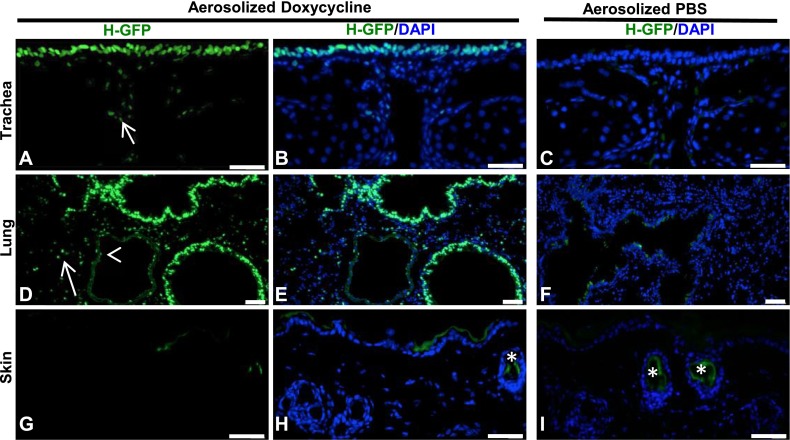
In the first set of experiments, we aerosolized doxycycline (20 mg/ml) or PBS for 1 hour continuously. In this and all subsequent experiments, mice were killed 24 hours after the final inhalation (unless otherwise indicated) for the assessment of transgene activation. Analyses of tissue sections from tracheas and lungs showed H2BGFP expression in the majority of epithelial cells of the airways (Figures 2A, 2B, 2D, and 2E). We also observed H2BGFP expression in some EpCAM+ epithelial cells outside of the airway, some platelet endothelial cell adhesion molecule endothelial cells, and some smooth muscle cells (see Figure E1 in the online supplement). Conversely, we observed little expression in the skin of aerosolized doxycycline–treated mice (Figures 2G and 2H), and no expression in aerosolized PBS–treated mice (Figures 2C, 2F, and 2I). In addition, we detected little expression in the heart, liver, pancreas, or spleen (to be discussed). However, when we exposed mice to aerosolized doxycycline at the same dose for 2 hours (Figure E2) and 6 hours (data not shown) continuously, we detected H2BGFP expression in the heart, liver, skin, and spleen. This suggests that prolonged inhalation causes the widespread distribution of doxycycline, compared with a 1-hour exposure. In addition, we note that mice treated with aerosolized doxycycline for a continuous 6-hour exposure demonstrated no aberrant phenotype after the inhalation, or at 1 month after the inhalation.
Figure 2.
Aerosolized doxycycline activates transgene expression in the airway. (A and B) The expression of nuclear H2BGFP (green) in the tracheal epithelium of R26-H2BGFP mice after aerosolized doxycycline administration. Nuclei are stained with 4′6-diamidino-2-phenylindole (DAPI), and DAPI/H2BGFP merges are shown in the middle. (D and E) H2BGFP expression in the small airway epithelia and alveoli. Of note, occasional H2BGFP+ cells are evident in the tracheal mesenchyme (A, arrow) and lung parenchyma (D, arrow) and in the endothelial lining of blood vessels (D, arrowhead). (G and H) H2BGFP is not expressed in the skin epidermis after the administration of aerosolized doxycycline. (C, F, and I) No H2BGFP expression is evident in the absence of doxycycline. Asterisks in H and I represent the green background signal seen in cross sections of the hair shaft. Scale bars, 20 μm. H-GFP, H2BGFP.
Titration of Aerosolized Doxycycline to Activate Tet-Inducible Transgene Expression Exclusively in the Airway Epithelium
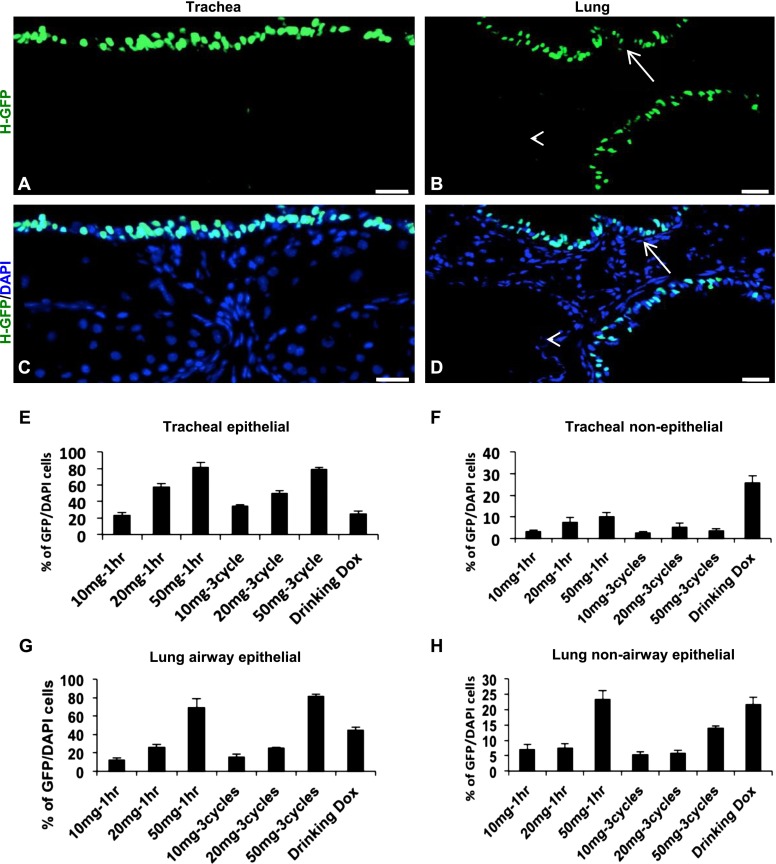
Although aerosolized doxycycline activates tet-inducible transgene expression in the airway epithelium, we also observed transgene expression in airway nonepithelial cells, such as subepithelial mesenchymal cells and the lung parenchyma, at a dose of 20 mg/ml for 1 hour (Figures 2 and E1). To activate the expression of a transgene exclusively in the airway epithelium, we tested different doses of doxycycline at different durations of exposure in R26-H2BGFP mice. Mice were exposed to various concentrations (10 mg/ml, 20 mg/ml, and 50 mg/ml) of doxycycline either continuously or intermittently for 1 hour, and were killed 24 hours after the final inhalation. In parallel, we also compared the efficiency of transgene expression in mice treated with doxycycline in their drinking water. The quantification of GFP+ cells in the airway epithelium indicated that the 50 mg/ml concentration produced the most efficient transgene expression, compared with the other concentrations tested (Figures 3A–3H). The quantification of GFP+ cells in the airways after a regimen of three intermittent 20-minute on–off exposure cycles of doxycycline revealed that transgene expression is largely restricted to the airway epithelium of the trachea and small airways (Figures 3A–3H). Conversely, doxycycline administration through drinking water resulted in widespread transgene expression when compared with inhaled doxycycline in all of the various tissues tested (Figures E3A–E3J). Of note, we demonstrated that 1-hour continuous inhalation led to more GFP activation in nonepithelial cells of the lung and trachea, compared with three intermittent 20-minute on–off cycles. With our newly developed method of doxycycline administration by inhalation (three 20-minute inhalations of 50 mg/ml doxycycline, separated by 20-minute intervals), we observed transgene expression as early as 30 minutes after inhalation (data not shown). To ascertain how long GFP expression persists after inhalation, we performed RT-PCR analysis from RNA samples isolated from doxycycline-treated mice at 24, 72, and 120 hours after inhalation. We detected GFP transcripts up to 72 hours after exposure, but the expression ceased by 120 hours after inhalation (Figure E4).
Figure 3.
Optimization of doxycycline (Dox) exposure to activate transgene expression exclusively in the airway epithelium of R26-H2BGFP mice. (A and C) Expression of H2BGFP (green) occurred mainly in the tracheal airway epithelium. (B and D) Expression of H2BGFP occurred mainly in the small airway epithelium of the lung. Tracheal and lung mesenchymal, lung parenchymal, and endothelial cells do not show H2BGFP expression. (E–H) Quantification of H2BGFP-expressing cells in the airway epithelial and nonepithelial cells of the trachea and lung after different doses, regimens, and routes of doxycycline administration. In B and D, the arrow and arrowhead indicate labeled airway epithelium and unlabeled endothelial cells, respectively. Scale bars, 20 μm.
Aerosolized Doxycycline Induced Transgene Expression in the Airway More Efficiently and More Rapidly than Doxycycline in the Drinking Water
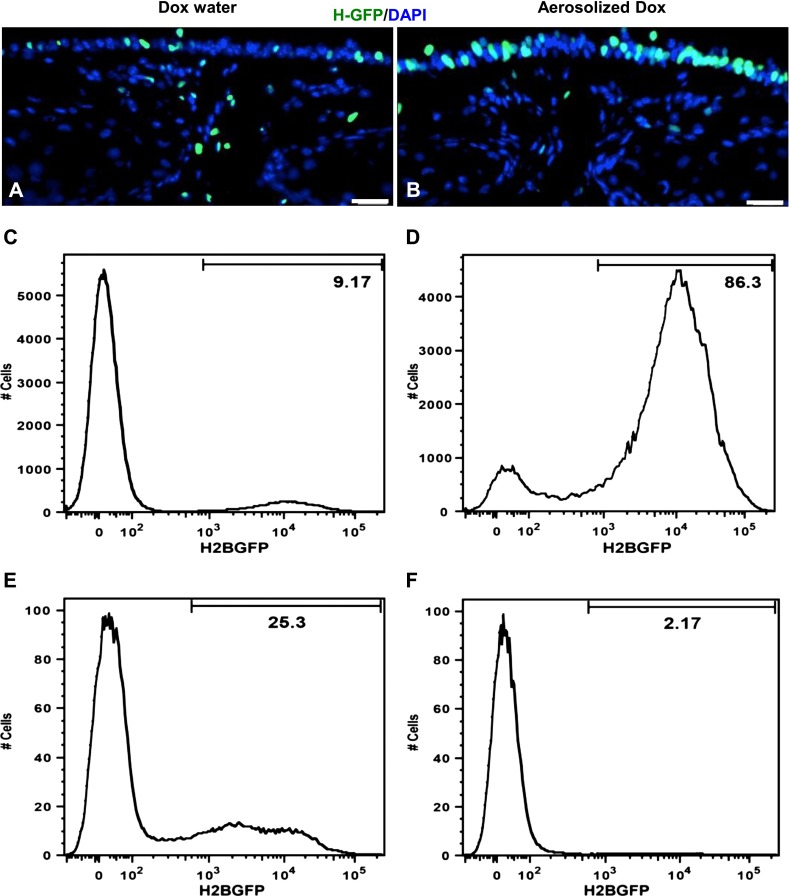
To compare the two methods of doxycycline administration quantitatively (inhalation versus drinking water), we again used R26-H2BGFP transgenic mice. These transgenic mice were exposed to aerosolized doxycycline, as already described, for 2 consecutive days. In parallel, we administered doxycycline by drinking water continuously for 2 days. These mice were killed 24 hours after their final inhalation of doxycycline or 24 hours after their final exposure to doxycycline in drinking water. We then analyzed the expression of H2BGFP on frozen sections, and found that mice treated with aerosolized doxycycline exhibited a greater percentage of airway epithelial cells labeled with GFP than did control mice (Figures 3, 4A, and 4B). To quantify the number of GFP-expressing epithelial cells versus GFP-expressing nonepithelial cells, we used a two-step sequential enzymatic cell dissociation protocol. We used papain digestion (27) to liberate epithelial cells, followed by collagenase/trypsin–EDTA dissociation to isolate the remaining nonepithelial cells. We then performed flow-cytometric analysis. We found that 70–80% of EpCAM-gated airway epithelial cells expressed GFP in the aerosolized doxycycline–treated mice, whereas only approximately 10–20% of the total epithelial cells expressed GFP in mice that received doxycycline through their drinking water (Figures 3, 4C, and 4D). In contrast, we observed more transgene expression in nonepithelial cells in mice that received doxycycline through their drinking water, compared with those receiving aerosolized doxycycline (Figures 3, 4E, and 4F). This is consistent with the nonspecific activation associated with systemic doxycycline exposure.
Figure 4.
Efficient and airway-specific transgene expression via aerosolized doxycycline. H2BGFP expression (green) was determined in cross sections of tracheas from R26-H2BGFP mice that were exposed to doxycycline, either by drinking water (A) or by inhalation in the aerosolized form (B). Airway-specific and efficient expression of H2BGFP was observed in mice that received doxycycline in aerosolized form, compared with doxycycline received through drinking water. Flow cytometric analysis of GFP expression was performed in papain-dissociated epithelial cell adhesion molecule airway epithelial cells from R26-H2BGFP mice that received doxycycline either by drinking water (C) or inhalation (D). Collagenase/trypsin/EDTA-dissociated nonepithelial tracheal cells from R26-H2BGFP mice that received doxycycline via either drinking water (E) or inhalation (F) were analyzed by flow cytometry. Scale bars, 20 μm.
Aerosolized Doxycycline Inhalation Does Not Alter Airway Epithelial Cell Composition
Although we did not observe any obvious phenotype in mice after exposure to aerosolized doxycycline, we examined whether any change was evident in airway epithelial cell composition. We performed immunofluorescence staining for airway epithelial cell markers on frozen sections from CK5-rtTA/tet(O)H2BGFP (hereafter referred to as CK5-H2BGFP) mice treated with aerosolized doxycycline, using a regimen of three 20-minute exposures to 50 mg/ml of doxycycline, separated by 20-minute off cycles. In this mouse model, rtTA is expressed specifically in basal cells under the control of the CK5 promoter. Therefore, aerosolized doxycycline administration causes H2BGFP transgene expression only in airway basal cells, but not in superficial club cells (Clara cells) or ciliated cells (Figure 5). In parallel, we administered doxycycline through drinking water continuously over 2 days to another set of mice. All mice were killed 24 hours after their final exposure to doxycycline. GFP expression in the large airway epithelium colocalized with cytokeratin-5–expressing basal cells, demonstrating that inhaled doxycycline induces transgene expression faithfully in the appropriate cell type. In addition, we observed a greater number of GFP-expressing basal cells, as marked by the coexpression of CK5 and GFP, in the mice that were treated with inhaled doxycycline, compared with those treated with doxycycline water (Figures 5A and 5B).
Figure 5.
Aerosolized doxycycline does not alter airway epithelial cell composition. (A–D and E–H) Representative images from sections obtained from cytokeratin 5 (CK5)-H2BGFP mice treated with aerosolized doxycycline or doxycycline through drinking water, respectively. H2BGFP is shown in green, and coimmunofluorescence staining for CK5 (A and E), Clara cell (club cell)–specific protein (CC10) (B and F), FoxJ1 (C and G), and bromodeoxyuridine (BrdU) (D and H) are shown in red. Of the total CK5+ basal-cell population, a larger fraction is labeled with green fluorescent protein (GFP) in mice treated with doxycycline inhalation (A), compared with mice treated with doxycycline in their drinking water (E). Graphical representation of the quantification of CK5+ basal cells (I), FoxJ1+ ciliated cells (J), and CC10+ Clara cells (club cells) (K) from tracheas of the CK5-H2BGFP transgenic mice treated with either inhaled doxycycline or doxycycline in drinking water. The Y-axis represents percentages of the respective cell types, compared with the total number of airway epithelial cells. The quantification of GFP-expressing cells in the tracheas (L) and esophagi (M) of CK5-H2BGFP transgenic mice that received doxycycline by either inhalation or drinking water was determined. Scale bar, 20 μm. neb, nebulized.
We found no difference in the actual numbers of basal stem cells of the airway epithelia in mice treated with inhaled doxycycline or inhaled PBS, compared with mice that received doxycycline by drinking water (Figure 5). Similarly, we did not observe any significant difference in the numbers of ciliated cells as marked by FoxJ1, a transcription factor that is specifically expressed by ciliated cells (Figure 5), or in the number of Clara cells (club cells), as marked by the Clara cell (club cell)–specific protein (CC10) (Figure 5). To further assess whether any changes in the proliferation of airway epithelial cells occurred, we administered bromodeoxyuridine (BrdU) in drinking water, and performed immunostaining for BrdU. We found no changes in the numbers of proliferating cells of the airway epithelium, compared with control mice (Figure 5). We also compared the efficiency of transgene expression in the trachea and esophagus between CK5-H2BGFP mice that received doxycycline in aerosolized form (three cycles of 20-minute intermittent on–off exposure cycles) and/or in their drinking water. We observed a much greater percentage of GFP expression in the CK5+ cells of the trachea of mice treated with inhaled doxycycline, compared with mice treated with doxycycline water. Notably, little transgene expression was evident in the esophagi of mice treated with inhaled doxycycline, as opposed to mice treated with doxycycline water (Figures 5L and 5M).
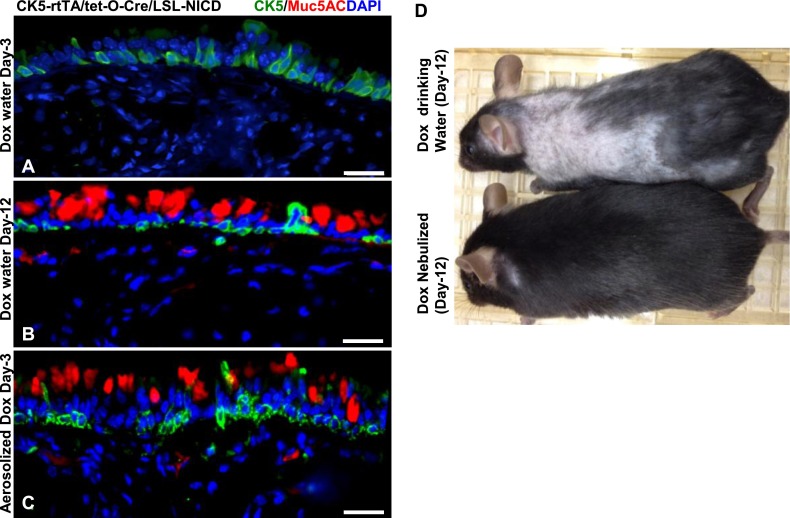
Aerosolized Doxycycline Administration Causes Notch-Induced Airway-Specific Mucous Metaplasia More Rapidly and Efficiently than Systemically Administered Doxycycline, and Avoids Off-Target Transgenic Phenotypes
Because we observed efficient and rapid transgene expression using doxycycline inhalation, we wondered how well this system would compare with the conventional systemic administration of doxycycline in eliciting a specific cellular phenotype. To address this, we used a triple transgenic mouse model that consists of rtTA under the promoter of the CK5 basal-cell promoter (CK5-rtTA), tet-operator driven Cre-recombinase (tet(O)Cre), and the active Notch intracellular domain, preceded by the LoxP-flanked transcriptional STOP cassette (LSL-NICD). This transgenic mouse model expresses rtTA under the promoter of CK5, which upon binding to doxycycline activates the tet-operator and mediates the transcription of Cre recombinase. The expressed Cre recombinase then removes the STOP cassette in front of the notch intracellular domain (NICD) transgene. NICD is transcribed and translated in the CK5-expressing basal cells in a doxycycline-dependent manner, thereby activating Notch signaling in basal cells. Ectopic Notch signaling has previously been shown to induce mucus-producing cells in the airway epithelium at 2 weeks after the systemic administration of tamoxifen in a CK5-CreER mouse model (28, 29). To test whether we could activate this triple transgenic system using our newly formulated doxycycline administration method, we exposed mice to aerosolized doxycycline, using our optimized protocol for 2 consecutive days, and killed the mice on Day 3 after the final inhalation. In parallel, we administered doxycycline in drinking water continuously for 2 and 12 consecutive days, and killed the mice on Day 3 (after the final doxycycline treatment) and Day 12, respectively. We then analyzed frozen sections from both groups of mice and performed immunohistochemistry for Muc5AC, a marker of secretory mucous cells. In accordance with previous reports, we did not observe any mucous cells on Day 3 (Figure 6A), but observed many Muc5AC-expressing cells on Day 12 in transgenic mice treated with doxycycline in their drinking water (Figure 6B). On the other hand, we observed Muc5AC-expressing cells on Day 3 in transgenic mice treated with aerosolized doxycycline (Figure 6C). Moreover, mice that received doxycycline in their drinking water showed hair loss by Day 12, consistent with a Notch effect on epidermal tissue (30), a phenotype not observed in mice treated by doxycycline inhalation (Figure 6D). We then wondered whether inflammatory responses were produced by the inhalation of doxycycline in the airways. To check this, we exposed C57Bl/6 and CD1 mice to aerosolized doxycycline or PBS (three 20-min on and off cycles for 4 consecutive days) and collected the bronchoalveolar lavage (BAL) on Day 1 and Day 3 (72 h after inhalation). We then measured the numbers of total leukocytes, mononuclear cells, and granulocytes, as well as the total protein concentration, in the BAL. We found no significant differences in the numbers of different leukocytes or the total protein concentration between the mice inhaling doxycycline and those inhaling PBS (Figure E5). Therefore, we conclude that inhaled doxycycline by itself does not cause significant inflammatory responses in the airways. Inhaled doxycycline is therefore a very rapid and efficient method to activate transgenes specifically in the airway epithelium, and to avoid off-target effects.
Figure 6.
Rapid and airway epithelial–specific transgene expression by aerosolized doxycycline. (A and B) Representative images from sections obtained from Ck5rtTA/tet(O)Cre/LSL-NICD mice after receiving doxycycline through their drinking water on Day 3 (A) or Day 12 (B), or aerosolized doxycycline on Day 3 (C). Mice receiving aerosolized doxycycline developed Muc5AC-positive mucous cells, whereas no Muc5AC-positive cells were detected in mice that received doxycycline through drinking water at this early time point. CK5-positive cells represent the basal cells of the large airway epithelium, where notch intracellular domain (NICD) is ectopically expressed in transgenic mice upon doxycycline treatment. (D) Aerosolized doxycycline administration (bottom) eliminated a skin phenotype associated with NICD expression in transgenic mice treated with systemic doxycycline. Mice treated with doxycycline through drinking water (top mouse) showed hair loss, whereas mice treated with aerosolized doxycycline (bottom mouse) did not show a hair-loss phenotype on Day 12. Scale bars, 20 μm. Cre, Cre recombinase; LSL, LoxP-STOP-LoxP; rtTA, reverse tetracycline-controlled transcriptional activator (rtTA); tet, tetracycline.
Discussion
Here we describe a novel method to activate tet-inducible transgene expression exclusively in the airways. This method not only allows for organ and cell-type specificity, but it also provides more efficient and robust transgene expression compared with conventional methods. Our approach eliminates the necessity to generate intersectional transgenic mouse models for airway gene modulation (20, 22, 23). For example, one could generate Nkx2-1-rtTA transgenic mice in combination with Rosa26-tet(O)Cre–driven CK5-LSL-NICD to drive NICD in the cells that express both Nkx2-1 and CK5. However, approaches such as this are often expensive, time-consuming, and impossible with current driver lines.
Importantly, in addition to airway epithelial cell specificity, we demonstrate that by using aerosolized doxycycline, one can activate transgene expression as early as 30 minutes after inhalation. This onset of transgenic expression is very rapid when compared with that in mice treated with doxycycline water, which requires at least 2 days for appreciable transgene expression. However, doxycycline administered via drinking water or food has been reported to occur within 4 to 16 hours (14), highlighting the need to study transgene kinetics in any set of experiments independently. We note, however, that doxycycline administered systemically by intraperitoneal injection can activate transgene expression in 2 hours (14). We speculate that the higher efficiency and rapid transgene expression by aerosolized doxycycline may be attributable to the ability to generate a higher local doxycycline concentration in the airway, as well as its more rapid distribution to its target cells when compared with systemic administration. In addition, we also found that some airway specificity was lost with a 1-hour continuous inhalation, compared with three intermittent 20-minute on–off cycles. We speculate that less airway specificity is evident after a 1-hour continuous exposure than after three intermittent 20-minute on–off cycles, because there is less time for the redistribution of deposited doxycycline relative to the time of deposition itself. Clearly, different kinetics in different strains may exert a significant effect on the necessary doses and periods of doxycycline administration by aerosolization.
The method of gene modulation by inhaled doxycycline allows us the flexibility to activate transgene expression in a dose-dependent manner. By fine-tuning the dose and duration of the exposure, one could ectopically express transgenes at desired levels. This has clear implications for studying the importance of transgene levels in affecting in vivo biology. In addition, for many precisely timed biological effects, the rapid onset of action in our protocol allows for the quantification of precisely timed physiologic outcomes. Because knowing how much doxycycline is actually consumed by a mouse in any given period is difficult, oral doxycycline dose titration is much more difficult, and the slow onset of action makes timing very difficult.
Accounting for possible phenotypes in other parts of the respiratory or upper digestive tract is important when using aerosolized doxycycline. Indeed, by using particulate forms of doxycycline, delivering the drug to specific regions of the airway or alveoli based upon particle size may be possible. We also note that a previous report showed how the inhalation of doxycycline reduced allergen-induced mucous metaplasia (31). Therefore, antimicrobial, anti-inflammatory, and other effects of inhaled doxycycline must be carefully controlled in both the aerosolized and systemic administration of doxycycline. We also advise avoiding continuous prolonged inhalation or more than four pulsed intermittent inhalations, because these may lead to leakage into other tissues. Care must be taken in each new transgenic model to validate the necessary doses and intervals necessary to ensure airway specificity.
As a final caveat, we note that we tested the applicability of aerosolized doxycycline in a limited number of tet-inducible transgenic mice. Phenotypic outcomes may vary between different strains and transgenes, and likely require optimization for each experiment. For example, only one dose of aerosolized doxycycline is required to detect H2BGFP expression using the CK5-rtTA driver, whereas ectopic NICD expression was detected after three doses of aerosolized doxycycline. We note that doxycycline distribution and metabolism may vary with strain, and that various transgenes respond in differing degrees to doxycycline. In our experiments, we switched between using R26-H2BGFP mice and CK5-H2BGFP mice to check the efficiency of doxycycline inhalation in a different strain, because the R26-rtTA mouse line is of a C57/bl6 background, and the CK5-rtTA mouse line is of a FVB/N background. Using these two different lines, we found no difference in transgene activation efficiency after doxycycline inhalation. It is also important to note that different transgenes may be activated more rapidly than others, or may persist for longer than others. In this case, repeated doxycycline inhalations may need to be used for some strains but this may lead to an increase in unspecific systemic gene activation. As a result, airway specificity may not be achievable in strains that require higher exposures of doxycycline to initiate transgene expression. In each new experimental cross, investigators will need to ensure that repeated administrations of doxycycline cause transgene effects that are limited to the lung, and they will likely need to modify the doxycycline dosing.
Overall, we believe that our novel method of airway epithelial transgene activation will serve as a useful tool for the lung research community. Although the methodology will require controls and optimization for each individual set of transgenic experiments, we hope that investigators studying lung biology will save both the time and costs associated with the need for the multiplicity of transgenic lines that would otherwise be necessary to achieve airway-specific gene expression.
Acknowledgments
Acknowledgments
The authors thank Hanno Hock for providing the R26-M2rtTA and tet(O)H2BGFP knock-in mice, and Adam Glick for providing the CK5-rtTA mouse. The authors also thank all members of Jayaraj Rajagopal’s laboratory for their constructive criticisms and comments, and for valuable discussions and support.
Footnotes
This work was supported by National Institutes of Health–National Heart, Lung, and Blood Institute Early Career Research New Faculty (P30) Award 5P30HL101287-02 (J.R.), by a Harvard Stem Cell Institute Junior Investigator Grant (J.R.), and by National Institutes of Health–National Heart, Lung, and Blood Institute grant R01HL108975 (A.M.T.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0412OC on July 12, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- 2.Baron U, Gossen M, Bujard H. Tetracycline-controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res. 1997;25:2723–2729. doi: 10.1093/nar/25.14.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lübbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z-H, Miao X-Y, Zhu R-L. New advances in animal transgenic technology. Yi Chuan Hereditas Zhongguo Yi Chuan Xue Hui Bian Ji. 2010;32:539–547. doi: 10.3724/sp.j.1005.2010.00539. [DOI] [PubMed] [Google Scholar]
- 6.Ting DT, Kyba M, Daley GQ. Inducible transgene expression in mouse stem cells. Methods Mol Med. 2005;105:23–46. doi: 10.1385/1-59259-826-9:023. [DOI] [PubMed] [Google Scholar]
- 7.Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bockamp E, Christel C, Hameyer D, Khobta A, Maringer M, Reis M, Heck R, Cabezas-Wallscheid N, Epe B, Oesch-Bartlomowicz B, et al. Generation and characterization of tTS-H4: a novel transcriptional repressor that is compatible with the reverse tetracycline-controlled TET-ON system. J Gene Med. 2007;9:308–318. doi: 10.1002/jgm.1012. [DOI] [PubMed] [Google Scholar]
- 9.Qu Z, Thottassery JV, Van Ginkel S, Manuvakhova M, Westbrook L, Roland-Lazenby C, Hays S, Kern FG. Homogeneity and long-term stability of tetracycline-regulated gene expression with low basal activity by using the rtTA2S-M2 transactivator and insulator-flanked reporter vectors. Gene. 2004;327:61–73. doi: 10.1016/j.gene.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Z, Ma B, Homer RJ, Zheng T, Elias JA. Use of the tetracycline-controlled transcriptional silencer (tTS) to eliminate transgene leak in inducible overexpression transgenic mice. J Biol Chem. 2001;276:25222–25229. doi: 10.1074/jbc.M101512200. [DOI] [PubMed] [Google Scholar]
- 11.Kämper MR, Gohla G, Schlüter G. A novel positive tetracycline-dependent transactivator (rtTA) variant with reduced background activity and enhanced activation potential. FEBS Lett. 2002;517:115–120. doi: 10.1016/s0014-5793(02)02587-5. [DOI] [PubMed] [Google Scholar]
- 12.Lamartina S, Roscilli G, Rinaudo CD, Sporeno E, Silvi L, Hillen W, Bujard H, Cortese R, Ciliberto G, Toniatti C. Stringent control of gene expression in vivo by using novel doxycycline-dependent trans-activators. Hum Gene Ther. 2002;13:199–210. doi: 10.1089/10430340252769734. [DOI] [PubMed] [Google Scholar]
- 13.Go WY, Ho SN. Optimization and direct comparison of the dimerizer and reverse tet transcriptional control systems. J Gene Med. 2002;4:258–270. doi: 10.1002/jgm.271. [DOI] [PubMed] [Google Scholar]
- 14.Perl A-KT, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–29. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- 15.Duerr J, Gruner M, Schubert SC, Haberkorn U, Bujard H, Mall MA. Use of a new-generation reverse tetracycline transactivator system for quantitative control of conditional gene expression in the murine lung. Am J Respir Cell Mol Biol. 2011;44:244–254. doi: 10.1165/rcmb.2009-0115OC. [DOI] [PubMed] [Google Scholar]
- 16.Fedorov LM, Tyrsin OY, Krenn V, Chernigovskaya EV, Rapp UR. Tet-system for the regulation of gene expression during embryonic development. Transgenic Res. 2001;10:247–258. doi: 10.1023/a:1016632110931. [DOI] [PubMed] [Google Scholar]
- 17.Chikama T-I, Hayashi Y, Liu C-Y, Terai N, Terai K, Kao CW-C, Wang L, Hayashi M, Nishida T, Sanford P, et al. Characterization of tetracycline-inducible bitransgenic Krt12rtTA/+/tet-O-LacZ mice. Invest Ophthalmol Vis Sci. 2005;46:1966–1972. doi: 10.1167/iovs.04-1464. [DOI] [PubMed] [Google Scholar]
- 18.Sheng Y, Lin C-C, Yue J, Sukhwani M, Shuttleworth JJ, Chu T, Orwig KE. Generation and characterization of a Tet-On (rtTA-M2) transgenic rat. BMC Dev Biol. 2010;10:17. doi: 10.1186/1471-213X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawlins EL, Perl A-K. The a“MAZE”ing world of lung-specific transgenic mice. Am J Respir Cell Mol Biol. 2012;46:269–282. doi: 10.1165/rcmb.2011-0372PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 22.Dymecki SM, Ray RS, Kim JC. Mapping cell fate and function using recombinase-based intersectional strategies. Methods Enzymol. 2010;477:183–213. doi: 10.1016/S0076-6879(10)77011-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim JC, Dymecki SM. Genetic fate-mapping approaches: new means to explore the embryonic origins of the cochlear nucleus. Methods Mol Biol. 2009;493:65–85. doi: 10.1007/978-1-59745-523-7_5. [DOI] [PubMed] [Google Scholar]
- 24.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, Hock H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JK, Vinarsky V, Wain JC, Zhao R, Jung K, Choi J, Lam A, Pardo-Saganta A, Breton S, Rajagopal J, et al. In vivo imaging of tracheal epithelial cells in mice during airway regeneration. Am J Respir Cell Mol Biol. 2012;47:864–868. doi: 10.1165/rcmb.2012-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardo-Saganta A, Law BM, Gonzalez-Celeiro M, Vinarsky V, Rajagopal J. Ciliated cells of pseudostratified airway epithelium do not become mucous cells after OVA challenge. Am J Respir Cell Mol Biol. 2012;48:1–41. doi: 10.1165/rcmb.2012-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock JR, Gao X, Xue Y, Randell SH, Kong Y-Y, Hogan BLM. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uyttendaele H, Panteleyev AA, De Berker D, Tobin DT, Christiano AM. Activation of Notch1 in the hair follicle leads to cell-fate switch and Mohawk alopecia. Differentiation. 2004;72:396–409. doi: 10.1111/j.1432-0436.2004.07208006.x. [DOI] [PubMed] [Google Scholar]
- 31.Gueders MM, Bertholet P, Perin F, Rocks N, Maree R, Botta V, Louis R, Foidart J-M, Noel A, Evrard B, et al. A novel formulation of inhaled doxycycline reduces allergen-induced inflammation, hyperresponsiveness and remodeling by matrix metalloproteinases and cytokines modulation in a mouse model of asthma. Biochem Pharmacol. 2008;75:514–526. doi: 10.1016/j.bcp.2007.09.012. [DOI] [PubMed] [Google Scholar]