Abstract
Leukotrienes (i.e., products of the 5-lipoxygenase pathway) are thought to be contributors to lung pathologies. Moreover, eosinophils have been linked with pulmonary leukotriene activities both as potential sources of these mediators and as responding effector cells. The objective of the present study was to define the role(s) of leukotrienes in the lung pathologies accompanying eosinophil-associated chronic respiratory inflammation. A transgenic mouse model of chronic T helper (Th) 2–driven inflammation expressing IL-5 from T cells and human eotaxin-2 locally in the lung (I5/hE2) was used to define potential in vivo relationships among eosinophils, leukotrienes, and chronic Th2-polarized pulmonary inflammation. Airway levels of cys-leukotrienes and leukotriene B4 (LTB4) are both significantly elevated in I5/hE2 mice. The eosinophil-mediated airway hyperresponsiveness (AHR) characteristic of these mice was abolished in the absence of leukotrienes (i.e., 5-lipoxygenase–deficient I5/hE2). More importantly, the loss of leukotrienes led to an unexpectedly significant decrease in collagen deposition (i.e., pulmonary fibrosis) that accompanied elevated levels of IL-4/-13 and TGF-β in the lungs of I5/hE2 mice. Further studies using mice deficient for the LTB4 receptor (BLT-1−/−/I5/hE2) and I5/hE2 animals administered a cys-leukotriene receptor antagonist (montelukast) demonstrated that the AHR and the enhanced pulmonary fibrosis characteristic of the I5/hE2 model were uniquely cys-leukotriene–mediated events. These data demonstrate that, similar to allergen challenge models of wild-type mice, cys-leukotrienes underlie AHR in this transgenic model of severe pulmonary Th2 inflammation. These data also suggest that an underappreciated link exists among eosinophils, cys-leukotriene–mediated events, and fibrotic remodeling associated with elevated levels of IL-4/-13 and TGF-β.
Keywords: 5-lipoxygenase, asthma, eosinophils, montelukast, lung
Clinical Relevance
The role(s) of leukotrienes and, more specifically, cys-leukotrienes, in lung remodeling events in chronic eosinophil-associated pulmonary disease is unresolved. Data using a mouse model of chronic respiratory inflammation show that the presence of eosinophils in the lungs may alone elicit sustained expression of pulmonary cys-leukotrienes and, as a result, progressive lung fibrosis. If translatable to human respiratory subjects, it would suggest that the long-term use of antagonists of cys-leukotriene activities (e.g., montelukast) may be a previously underappreciated strategy to impede the fibrotic pulmonary changes that are concomitant with chronic T helper type 2–polarized inflammation.
Leukotrienes represent a biologically active group of low molecular weight lipid mediators (i.e., eicosanoids) formed from arachidonic acid that is derived from membrane phospholipids (1). 5-Lipoxygenase (5-LO) is a primary component to this process, initiating the oxidation of arachidonic acid (1) and the subsequent production of intermediate metabolites (e.g., 5-oxe-eicosatetraenoic acid (2), promoting proinflammatory cell recruitment as well as the eventual formation of the prominent leukotriene subtypes (reviewed in Ref. 1).
5-LO activities and the production of leukotrienes are linked with a variety of leukocytes that are both resident and recruited in response to allergen provocation, including neutrophils, eosinophils, mast cells, macrophages, and basophils (3). As such, leukotriene-mediated pathways are the targets of pharmaceutical intervention, including the targeting of 5-LO (e.g., ziluton [Abbott Laboratories, Abbott Park, IL]), as well as antagonists of the high affinity receptors mediating leukotriene B4 (LTB4) (e.g., RO5101576 [Roche, Basel, Switzerland]; moxilubant maleate [Novartis, Basel, Switzerland]), and leukotriene C4 (LCT4) (e.g., montelukast [Merck Inc., West Point, PA]). The availability of gene knockout mice that completely abolish either leukotriene production (5-LO−/− [4]) or the high-affinity receptors for each leukotriene subtype, LTB4 (BLT-1−/− [5] and LTC4 [CysLT-1−/− (6)]), offer ideal models of drug approaches targeting these pathways in human subjects.
We have previously reported the creation of a transgenic mouse model of chronic T helper (Th) 2–polarized inflammation (I5/hE2) that is characterized by pathological changes that are abolished in the absence of the induced eosinophilia of this model (7). The strain of mice that we created (i.e., constitutive, systemic T cell–driven expression of IL-5 and constitutive airway epithelial cell–driven expression of human eotaxin-2) was a strategic approach to developing a chronic inflammatory model. The importance of this model is that it allows the definition of eosinophil effector functions as causative events leading, and/or contributing to, the pulmonary remodeling events and airways dysfunction associated with sustained Th2-polarized airway inflammation. In addition, unlike any allergen provocation model (acute or chronic) or any previous transgenic or gene knockout/knockin model of pulmonary inflammation, I5/hE2 mice display extensive eosinophil degranulation in the interstitial regions of the lung as well as in the airway lumen. Moreover, the induced pulmonary remodeling/airways dysfunction in the chronic I5/hE2 model are progressive pathologies that increase in magnitude and breadth with time postpartum.
Microarray assessment of gene expression and the development of potential gene regulatory networks showed that the enzymes linked with key leukotriene pathways were up-regulated in the lungs of I5/hE2 mice, particularly pulmonary levels of expression of 5-LO and 5-LO–activating protein. In the studies presented here, metrics of pulmonary pathology were monitored for nearly 2 months (weaning [3/4 wk postpartum] to a postpartum age of 11/12 wk) in the parental I5/hE2 model relative to age-matched negative wild-type controls [C57BL/6J], I5/hE2 mice devoid of leukotriene activities [5-LO−/−/I5/hE2]), and mice targeting the activities linked with each of the two leukotriene subtypes; LTB4 using knockout mice devoid of the high affinity LTB4 receptor (BLT-1−/−/I5/hE2) and LTC4 through the blockade of the cys-leukotriene high-affinity receptor (CysLT-1) with the antagonist drug, montelukast (MK/I5/hE2).
Our studies using the I5/hE2 model confirmed previous reports using allergen provocation models in the mouse (8, 9) showing that airway hyperresponsiveness (AHR) to methacholine challenge in I5/hE2 mice is dependent on cys-leukotriene activities, suggesting that, in rodents, cys-leukotrienes underlie AHR at a fundamental level, with no available concurrent or overlapping pathways. Significantly, the progressive pulmonary fibrosis (i.e., extracellular collagen deposition) occurring in I5/hE2 mice appears to occur as a consequence of leukotriene-mediated activities. In particular, this chronic inflammatory metric was shown to be a function of cys-leukotrienes, and not LTB4. If this result extrapolates to human respiratory subjects, it would suggest that the long-term use of montelukast or other antagonists of cys-leukotriene activities may be a previously underappreciated strategy to slow the fibrotic pulmonary changes that are concomitant with chronic Th2-polarized inflammation.
Materials and Methods
Mice
All studies were performed with mice on the C57BL/6J genetic background, including wild-type controls, I5/hE2 mice ([7], back-crossed [n > 12 generations]), and compound double-transgenic/gene knockout animals (5-LO−/−/I5/hE2 and BLT-1−/−/I5/hE2 mice). Protocols and studies involving animals were performed in accordance with National Institutes of Health and Mayo Foundation institutional guidelines.
Microarray and Molecular Network Analysis
The lungs from eosinophil-sufficient I5/hE2 (n = 4) and eosinophilless PHIL/I5/hE2 (n = 4) mice were taken, RNA was extracted, checked for integrity, and Whole Transcript Expression Array Service (Asuragen, Inc., Austin, TX) was performed and analyzed as single individual assays using Affymetrix GeneChip Mouse Genome 430 2.0 Array (including statistical analysis of the results; Affymetrix, Santa Clara, CA). Gene–protein interaction networks were developed and identified using Ingenuity Pathway Analysis software (Ingenuity Systems, Redwood City, CA; www.ingenuity.com) with differential gene expression cutoffs set at log ratio 1/−1 and P < 0.05 (see Table E1 in the online supplement). The genes significantly up-regulated in I5/hE2 mice (relative to the eosinophil-deficient PHIL/I5/hE2) that are critical to the inflammatory network developed here were validated at the protein level by ELISA, immunohistochemistry, or histochemical staining. These include arachidonate 5-lipoxygenase (ALOX5) (i.e., increased production of leukotrienes [ELISA]), individual eosinophil granule proteins (i.e., major basic protein-1, eosinophil associated ribonucleases, and eosinophil peroxidase [EPX] [ELISA]), IL-4/-5 (ELISA), mucins (histochemical staining), TGF-β1 (immunohistochemistry), and extracellular matrix collagens (histochemical staining).
Administration of Montelukast
Montelukast was administered (as per the manufacturer, Merck & Co., Inc.) to postweaned mice orally (MK/I5/hE2) as part of a powdered formulation of mouse chow that achieved a CMAX of 3.72 μg/ml of maximal concentration of the drug montelukast in the plasma of mice that stably remained at this level throughout the experimental period. Specifically, upon weaning, mice were transferred to cages in which the food source was exclusively montelukast-containing powdered mouse chow (0.25 g montelukast per 1,000 g mouse chow or 50 μg/g body weight/d) contained in light protected feeders. The mice were maintained on this diet for 6 weeks, changing out the montelukast with fresh food twice a week.
Collection of Bronchoalveolar Lavage Fluid and Cell Differentials of Bronchoalveolar Lavage–Derived Leukocytes
Collection of bronchoalveolar lavage (BAL) fluid and cell differential assessments were completed as described previously (7).
Assessment of Pulmonary Cytokine and Leukotriene Levels
Cytokine (IL-4, IL-13, and TGF-β1) levels were measured in cell-free BAL fluid using R&D Quantikine ELISA kits (R&D Systems, Minneapolis, MN) and leukotrienes (LTC4/LTD4/LTE4 and LTB4) (Cayman Chemical Co., Ann Arbor, MI), respectively, according to the manufacturer’s instructions.
Assessments of Pulmonary Histopathology: Epithelial and Airway Smooth Muscle Hyperplasia, Goblet Cell Metaplasia/Epithelial Cell Mucus Accumulation, and Collagen Deposition/Fibrosis
Lungs of mice were inflated and then fixed with 10% formalin before embedding in paraffin. Mid-lung coronal sections (4 μm) were stained with either hematoxylin-eosin (general histological assessments), periodic acid-Schiff (PAS; goblet cell metaplasia/airway epithelial cell mucin accumulation), Masson’s trichrome (MT; collagen deposition/fibrosis), and Picrosirius Red (PSR; collagen deposition/fibrosis).
Quantitative morphometric assessments of PAS-stained (goblet cell metaplasia/airway epithelial cell mucin accumulation) slides were performed as previously described (10). Briefly, mucin index (MI) was calculated from mid-coronal sections of the lungs of each mouse in a given cohort using ImagePro Plus software (Silver Spring, MD). Total counts of bronchioles in a given section were determined, and for each bronchiole the total pixel density of PAS staining (i.e., sum of PAS stained pixels multiplied by average intensity of stained pixels) was normalized by bronchiole epithelial area. MI was calculated for each mouse in a given cohort as mean value resulting from the sum of normalized PAS staining values for each bronchiole divided by the number of bronchioles counted in a given section.
Morphometric assessments quantifying the levels of subepithelial fibrosis occurring in each experimental cohort of mice were determined from PSR-stained lung sections evaluated under polarized light as described previously (11).
Several additional remodeling/inflammatory markers were qualitatively evaluated by immunohistochemistry of sections from cohorts of mice (n = 3–6), including staining with a mouse anti–α smooth muscle antibody (clone 1A4; Dako, Glostrup, Denmark), rabbit polyclonal anti–TGF-β (1, 2) antibody (sc-146; Santa Cruz Biotechnology, Inc., Dallas, TX), and staining for pulmonary Cys-leukotriene receptor (cysLTR1) expression with rabbit polyclonal anti-CysLT1 receptor (no. 120500; Cayman Chemical Co.).
Identification of Lung-Infiltrating Eosinophils and Evidence of Tissue-Associated Degranulation
Immunohistochemical identification of eosinophils and evidence of degranulation was performed using a monoclonal antibody recognizing EPX (12).
Assessments of Airway Luminal Eosinophil Degranulation
Quantitative measures of eosinophil degranulation in the airway of experimental and control mice were determined by EPX-based ELISA of BAL fluid, as recently described (13).
Assessment of Lung Function: Determination of AHR
Pulmonary function phenotypes of the mice were assessed using techniques previously described (7, 14).
Statistical Analysis
GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA) was used for basic analysis, including the generation of graphs and plots. Statistical analysis for comparisons between groups was performed using ANOVA followed by Tukey’s multiple comparisons test or a Student’s t test. Data are expressed as the mean (±SEM). Differences between mean values were considered significant when P was less than 0.05.
Results
Constitutive T Cell–Derived Expression of IL-5 and Ectopic Airway-Specific Expression of Human Eotaxin-2 Elicit Significant and Chronic Respiratory Inflammation Linked with Elevated Airway Levels of Leukotrienes
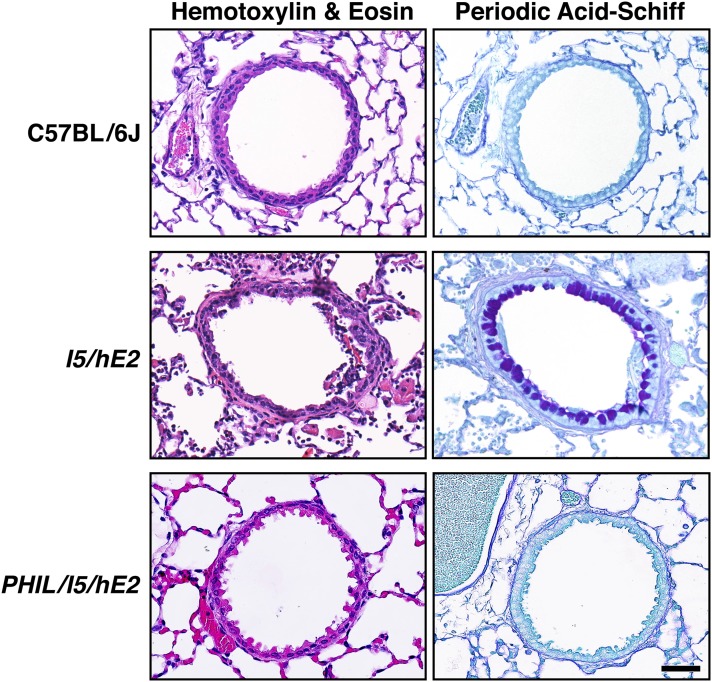
Concomitant expression of the eosinophilic proliferative cytokine, IL-5, systemically from all mature T cells together with ectopic expression of the eosinophil agonist chemokine specifically from lung epithelium creates a unique model (I5/hE2) of life-long chronic pulmonary inflammation that is eosinophil dependent (7). I5/hE2 animals display overt histopathologies, including a prominent proinflammatory cell infiltrate, smooth muscle hyperplasia, as well as fibrotic structural changes to the lung parenchyma, and the appearance of a significant level of goblet cell metaplasia, as well as epithelial cell mucin accumulation. More importantly, all of these characteristic remodeling events are directly linked with the induced pulmonary eosinophilia, as triple-transgenic animals (i.e., PHIL/I5/hE2), created by crossing the I5/hE2 mice with animals devoid of eosinophils (PHIL), have no evidence of pulmonary histopathology, displaying an identical phenotype relative to unmanipulated C57BL/6J control mice (Figure 1).
Figure 1.
The chronic inflammatory pathologies occurring in I5/hE2 mice are completely dependent on one or more eosinophil effector functions. Representative airways from coronal lung sections from wild-type (C57BL/6J) mice are shown in comparison to airways in similar sections from age-matched (12 wk postpartum) double-transgenic (I5/hE2) mice and triple-transgenic (eosinophilless transgenic animals, PHIL/I5/hE2). Sections stained with hematoxylin-eosin (H&E) and periodic acid-Schiff (PAS) provide evidence of the eosinophil-dependent pulmonary changes (inflammatory cell infiltrate and lung structural perturbations [H&E] and goblet cell metaplasia/epithelial cell mucin accumulation [PAS], respectively) that occurred in the parental I5/hE2 model and lost in the absence of eosinophils, but not cytokine/chemokine transgene expression (PHIL/I5/hE2). Scale bar, 50 μm
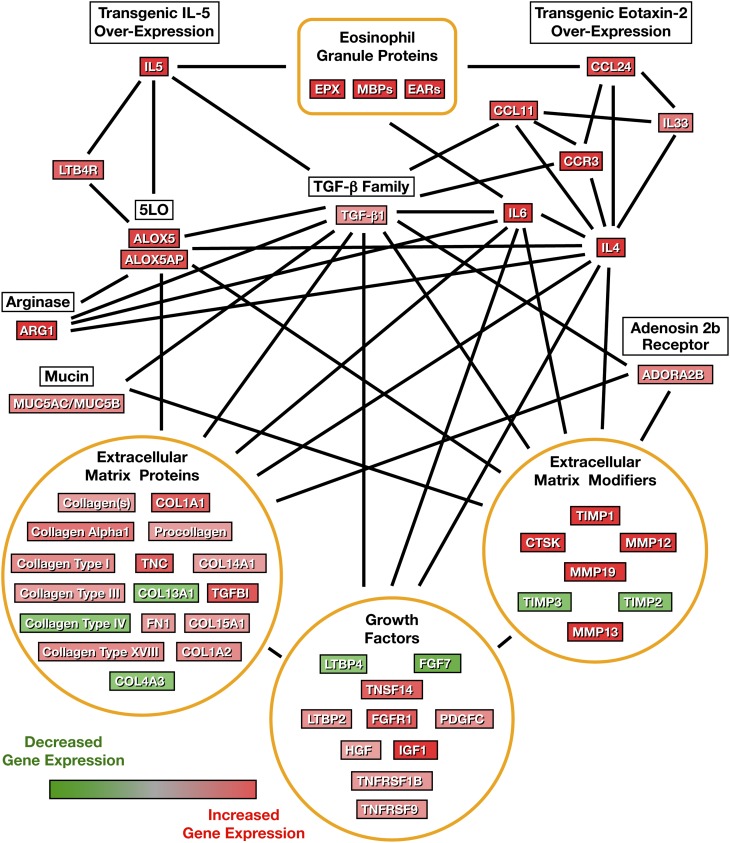
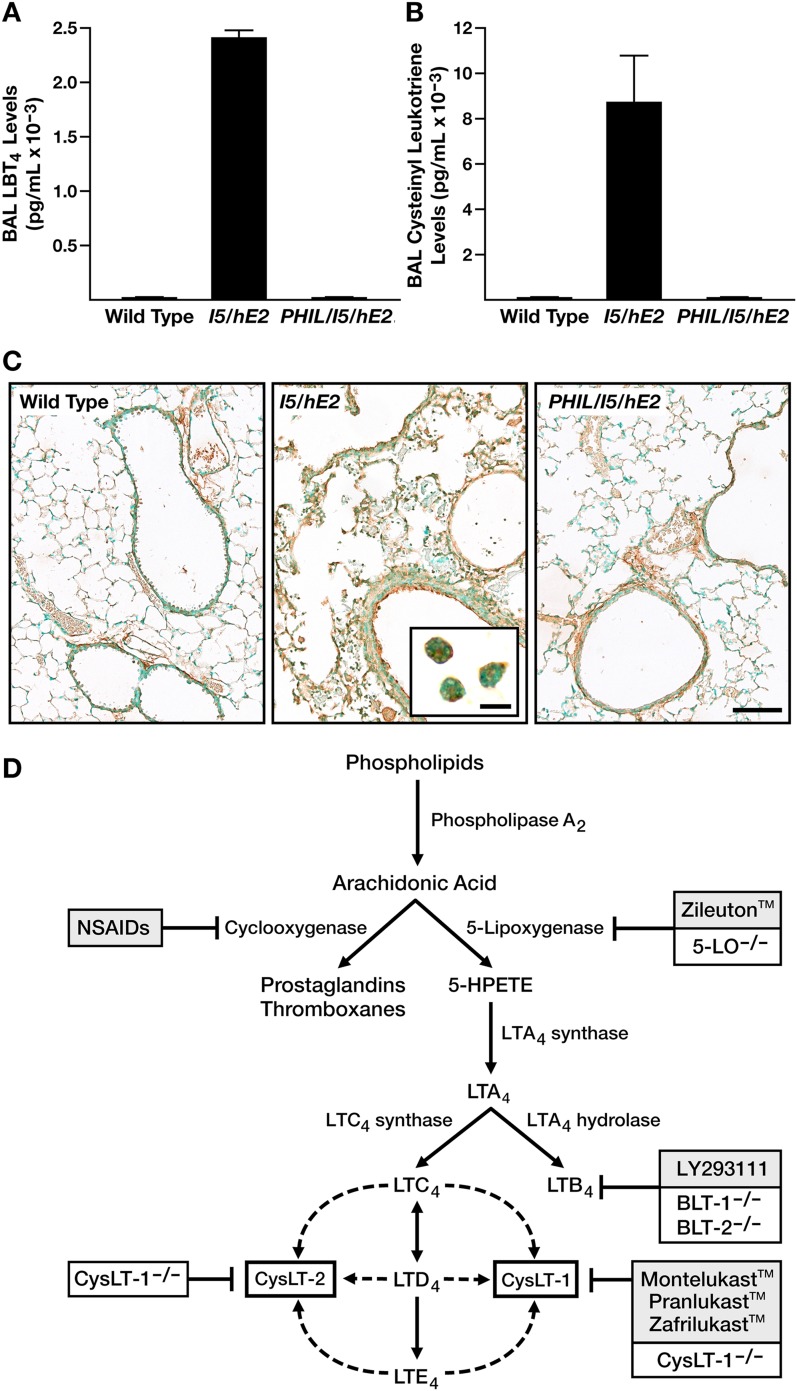
Eosinophil-mediated gene expression in the lungs of the parental eosinophil-sufficient I5/hE2 model was determined by transcript microarray assessment, comparing the relative abundances of mRNAs in the lungs of I5/hE2 mice to those found in eosinophilless animals (PHIL/I5/hE2). These data revealed a large cohort of genes, the differential expression of which was observed in array assessments of log ratio of |1| or greater and P value of less than 0.05 (Table E1). Changes in gene expression were used with Ingenuity Pathway Analysis software to identify multiple molecular networks previously suggested by published studies that are relevant to chronic pulmonary diseases. These pathways include lipid metabolism, airway fibrosis and remodeling, AHR, and asthma (Table E1). Figure 2 represents the overlay of these four molecular networks with the identified changes in associated gene expression. This preliminary molecular pathway analysis of the eosinophil-mediated changes occurring in I5/hE2 mice demonstrated several important nodes of gene expression that appeared to impact both AHR and remodeling events, such as considerably higher transcript levels of genes encoding the proteins in part responsible for leukotriene production (5-LO or ALOX5), as well as its 5-LO–associated protein (ALOX5AP). Indeed, follow-up studies demonstrated that the increase in airway leukotriene levels in I5/hE2 mice were both profound in magnitude and diverse in scope, occurring in both LTB4 (Figure 3A) and cys-leukotrienes (Figure 3B). More importantly, the observed increases in both leukotriene subtypes in I5/hE2 mice was dependent on the presence of eosinophils, as both subtypes returned to unquantifiable levels in eosinophilless transgenic mice (PHIL/I5/hE2). cysLTR1 expression also similarly paralleled eosinophil-mediated changes in the lung among these mice (Figure 3C). Namely, whereas cysLTR1 expression in eosinophil-deficient PHIL/I5/hE2 mice was comparable to C57BL/6J wild-type mice, expression of this receptor was greatly enhanced in several lung cell types in the eosinophil-sufficient parental I5/hE2 model, including airway epithelium, airway smooth muscle, and even the eosinophils infiltrating the lung tissues/airways.
Figure 2.
Compilation of airway molecular pathways derived from assessments of differential gene expression in the lungs of eosinophil-sufficient I5/hE2 versus eosinophilless PHIL/I5/hE2 mice. Ingenuity Pathway Analysis of the transcript array profiles derived from eosinophil-sufficient I5/hE2 versus eosinophilless PHIL/I5/hE2 mice was used in conjunction with previously published studies to identify four molecular networks (lipid metabolism, airway fibrosis and remodeling, airway hyperresponsiveness [AHR], and asthma [Table E1]) relevant to chronic pulmonary diseases. These pathways were then combined to create a larger network scheme of gene expression linked with the eosinophil-mediated inflammation associated with I5/hE2 mice.
Figure 3.
The eosinophil-mediated chronic pulmonary inflammation that occurs in I5/hE2 mice is accompanied by significant increases in both airway cys-leukotriene and leukotriene B4 (LTB4) levels, leading to experimental strategies targeting leukotrienes. The levels of (A) LTB4 and (B) cys-leukotrienes in cell-free bronchoalveolar lavage fluid were assessed by ELISA from wild-type (C57BL/6J), I5/hE2, and eosinophilless PHIL/I5/hE2 transgenic mice (n = 5–8 mice/group; *P < 0.05). Data presented are means (± SEM). (C) Immunohistochemistry (brown staining areas/cells) of lung sections from wild-type (C57BL/6J), I5/hE2, and eosinophilless PHIL/I5/hE2 mice demonstrated that the eosinophil-mediated events in I5/hE2 mice were also accompanied by increases in Cys-leukotriene receptor (cysLTR1) expression that appeared to occur in several lung compartments (scale bar, 100 μm), including the infiltrating eosinophils themselves (see insert; scale bar, 20 μm). (D) Schematic flow diagram reviewing the synthetic pathways leading to the production of arachidonic acid metabolites and the drug strategies in humans and gene knockout approaches in mouse models targeting each of these pathways. BLT-1−/− and BLT-2−/−, knockout mice targeting the LTB4 high- and low-affinity receptors, respectively; CysLT-1−/− and CysLT-2−/−, cys-leukotriene high- and low-affinity receptor knockout mice; 5-LO−/−, 5-lipoxygenase knockout mice; NSAIDs, nonsteroidal anti-inflammatory drugs.
Experimental Strategies Targeting either Total Leukotrienes or the Activities Mediated by Each of the Two Significant Subtypes: LTB4 and Cys-Leukotrienes
Possible strategies to target various leukotriene-mediated pathways in the mouse are facilitated by both the availability of gene knockout mice and the availability of pharmaceutical agents specific for one or all leukotriene subtypes targeting these inflammatory mediators (Figure 3D). The requirement of leukotrienes in the induced pathologies in the I5/hE2 model of chronic respiratory inflammation was accomplished using a multipronged approach exploiting complementary targeting strategies. I5/hE2 mice display life-long chronic inflammation of the lung that progresses from little to no histopathologies/lung dysfunction at weaning to the appearance of extensive structural changes to the pulmonary parenchyma (i.e., lung remodeling) and significant increases in lung dysfunction (i.e., AHR) 4–8 weeks later (7). The experimental strategy investigating leukotrienes in this study targets this 4- to 8-week window of time (Figure E1). Specifically, all leukotriene production in I5/hE2 mice was abolished by crossing these animals with gene knockout mice deficient in the enzyme, 5-LO (5-LO−/−/I5/hE2). Alternatively, targeting the activities mediated by each of the prominent leukotriene subtypes in I5/hE2 mice was achieved either through a cross with knockout mice devoid of the high-affinity receptor for LTB4 (BLT-1−/−/I5/hE2) or the treatment of the parental I5/hE2 model with the cys-leukotriene high-affinity receptor (CysLT1) antagonist (montelukast [MK/I5/hE2]).
Blockade of Leukotriene-Mediated Activities Has No Effect on the Pulmonary Eosinophil Infiltrate or Extent of Eosinophil Degranulation Occurring in I5/hE2 Mice
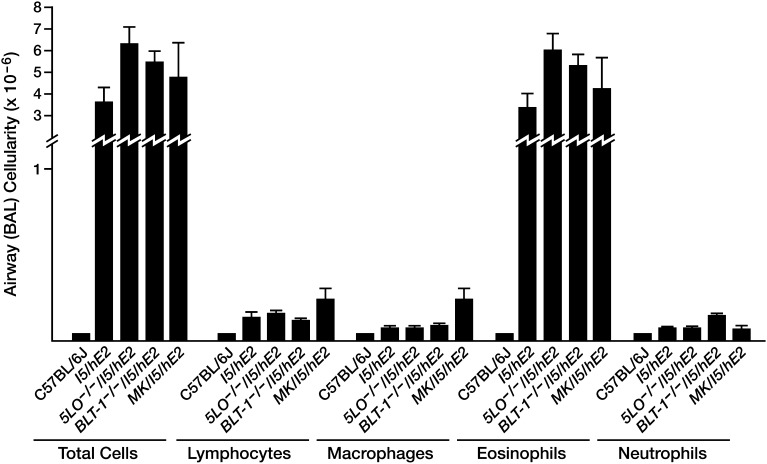
The logistical advantage of using I5/hE2 mice is that the eosinophilia and subsequent events promoting chronic lung inflammation are driven by constitutive ectopic transgene expression. The independence of the induced eosinophilia in I5/hE2 mice from changes in leukotriene activities was suggested by the lack of a decrease in both BAL eosinophil numbers (Figure 4) and tissue eosinophilia (Figure 5) in mice targeting either all leukotrienes (5-LO−/−/I5/hE2) or each of the prominent classes (LTB4 [BLT-1−/−/I5/hE2] and LTC4/LTD4/LTE4 [MK/I5/hE2]).
Figure 4.
The pulmonary eosinophilia displayed by I5/hE2 mice occurred independent of leukotriene-mediated activities. Bronchoalveolar lavage cell counts and differential assessments showed that the increased airway eosinophilia (relative to wild-type [C57BL/6J]) occurring in transgenic mice targeting all leukotrienes (5-LO−/−/I5/hE2), the LTB4 high-affinity receptor (BLT-1−/−/I5/hE2), or I5/hE2 mice administered the cys-leukotriene antagonist drug, montelukast (MK/I5/hE2), remained unchanged relative to the parental I5/hE2 double-transgenic strain of mice.
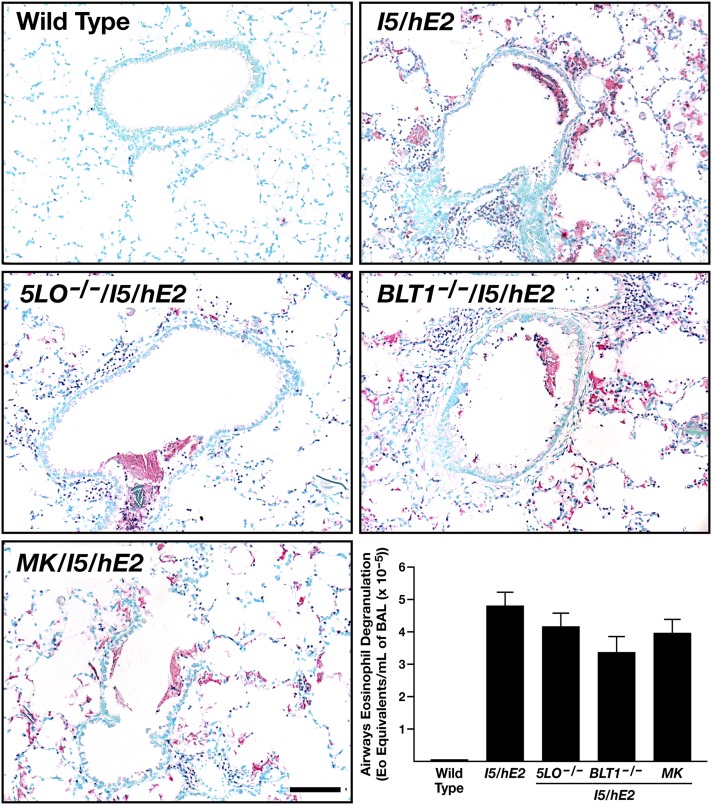
Figure 5.
The extent of eosinophil degranulation associated with the I5/hE2 model is not a function of pulmonary leukotriene-mediated events. Immunohistochemistry of lung sections using a monoclonal antibody that specifically recognizes eosinophil peroxidase (EPX [12]) demonstrated that the induced tissue eosinophilia of the parental I5/hE2 model remained unchanged in I5/hE2 mice devoid of all leukotrienes (5-LO−/−/I5/hE2), LTB4 activities (BLT-1−/−/I5/hE2), or the blockade of cys-leukotriene–mediated events (MK/I5/hE2). Moreover, assessments of extracellular matrix–associated EPX deposition in the lungs of these animals showed that the extensive eosinophil degranulation (i.e., increased EPX) linked with the I5/hE2 model was not a function of leukotriene-mediated activities. Scale bar, 50 μm. ELISA-based assessments of bronchoalveolar lavage (BAL) fluid using paired monoclonal antibodies specific for EPX (13) showed that the level of airway eosinophil degranulation (expressed as the amount of EPX in an eosinophil [Eo]; equivalents per milliliter of BAL) in all groups of mice targeting leukotrienes was no different than the level observed in the parental I5/hE2 model. Scale bar, 100 μm.
The activation state of the eosinophils accumulating in the lungs of I5/hE2 mice was shown to be independent of leukotriene-mediated events (Figure 5), judged by the observed levels of eosinophil degranulation with lung tissue by EPX-specific immunohistochemistry (12) and EPX-based ELISA of cell-free BAL fluid (13).
Leukotriene-Mediated Events Do Not Elicit an Increase in Airway Smooth Muscle Mass, or Contribute to the Central Airways Goblet Cell Metaplasia or the Changes Occurring in the Distal Airway Spaces of the I5/hE2 Mice
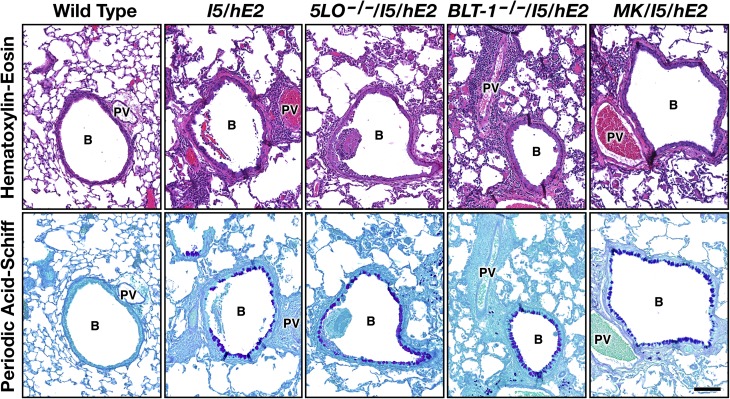
Hematoxylin-eosin–stained lung sections (Figure 6) revealed that gross morphological changes occurring in the lung parenchyma in I5/hE2 mice (i.e., proinflammatory cell infiltrate, airway epithelial hypertrophy, smooth muscle hyperplasia, the development of significant changes in the distal airway spaces, and the appearance of mucus and cellular debris in the central airways) were unaffected by targeting leukotrienes (5-LO−/−/I5/hE2) or either of the prominent leukotriene subtypes (LTB4 [BLT-1−/−/I5/hE2] and LTC4/LTD4/LTE4 [MK/I5/hE2]). For example, immunohistochemistry of lung sections from each cohort of double-transgenic mice using an anti–α–smooth muscle actin antibody showed an increase in airway smooth muscle thickness similar to what occurs in the parental I5/hE2 mice (7). These studies failed to detect significant leukotriene-dependent changes occurred in airway smooth muscle thickness from mice of the various cohorts (Figure E2). In addition, lung sections stained with PAS also showed that neither of the prominent leukotriene subtypes nor total leukotriene-mediated activities had any effect on the observed increase in this histopathologic metric relative to C57BL/6J controls (Figure 6). Quantitative morphometric assessments of this staining (MI) from coronal sections of each mouse (n = 4–10/experimental cohort from three independent studies) demonstrated that the magnitude of the observed goblet cell metaplasia/airway epithelial cell mucin accumulation occurring in these mice (wild-type control animals displayed no evidence of goblet cell metaplasia and/or airway epithelial cell mucin accumulation) was unchanged regardless of the status of leukotriene activities (I5/hE2, MI = 22.9 ± 5.3; 5-LO−/−/I5/hE2, MI = 21.3 ± 4.5; BLT-1−/−/I5/hE2, MI = 18.4 ± 4.9; and MK/I5/hE2, MI = 19.5 ± 2.2). The elevated and unchanging levels of goblet cell metaplasia/airway epithelial cell mucin accumulation observed among the different I5/hE2 animal cohorts reflected a similar and unchanged increase in BAL IL-13 (i.e., relative to its absence in wild-type mice): I5/hE2 (98.9 ± 7.6 pg/ml); 5-LO−/−/I5/hE2 (85.6 ± 6.7 pg/ml); BLT-1−/−/I5/hE2 (89.5 ± 2.2 pg/ml); and MK/I5/hE2 (91.5 ± 7.4 pg/ml). However, the loss of all leukotrienes (5-LO−/−/I5/hE2) did have a nominal, yet statistically significant (P < 0.05), impact on BAL IL-4 levels (I5/hE2, 285.6 ± 29.9 pg/ml versus 5-LO−/−/I5/hE2, 178.9 ± 17.9 pg/ml). The biological significance of this observation is unclear, although a similar, yet not significant (P = 0.06), decrease in IL-4 levels was observed in montelukast-treated mice (MK/I5/hE2, 234.6 ± 52.3 pg/ml), but not animals targeting LTB4 pathways (BLT-1−/−/I5/hE2, 269.6 ± 13.9 pg/ml).
Figure 6.
The chronic pulmonary pathologies displayed by I5/hE2 mice do not occur as a function of leukotriene-mediated events. Representative airways in coronal lung sections from C57BL/6J mice wild-type (negative controls) and age-matched (12 wk postpartum) double-transgenic I5/hE2 mice (positive controls) are shown in comparison to airways in similar sections from transgenic mice targeting all leukotrienes (5-LO−/−/I5/hE2), the LTB4 high-affinity receptor (BLT-1−/−/I5/hE2), or I5/hE2 mice treated with the cys-leukotriene antagonist drug, montelukast (MK/I5/hE2). Sections were stained with H&E and PAS. These data provide evidence of increased inflammatory cell infiltrates, lung structural perturbations (H&E), and goblet cell metaplasia/epithelial cell mucin accumulation (PAS), all of which remained unchanged in the cohorts of double-transgenic mice, despite the targeting of leukotriene pathways activities. Scale bars, 100 μm. B, bronchiole; PV, pulmonary vessel.
Cys-Leukotrienes, but Not LTB4-Mediated Pulmonary Activities, Contribute to the Induced AHR Occurring in I5/hE2 Mice
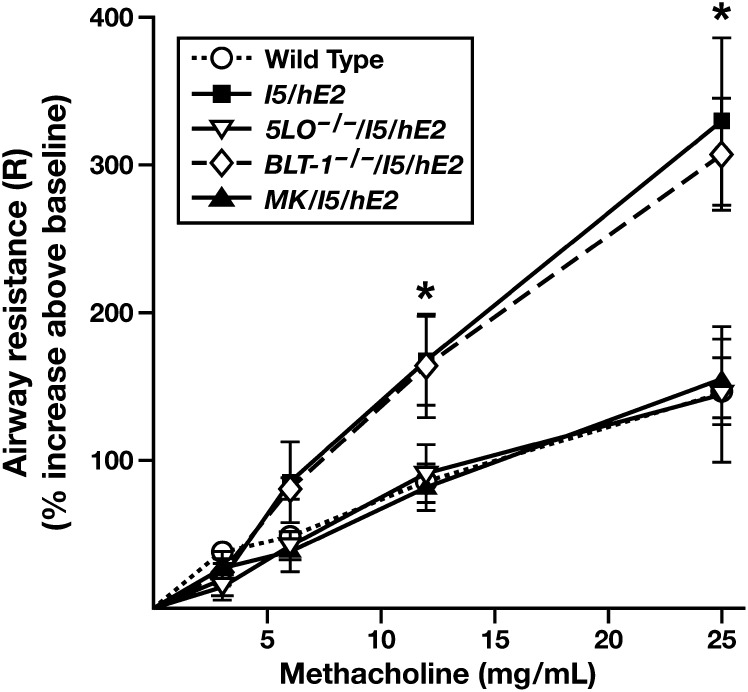
Assessments of the de novo appearance (i.e., without allergen provocation) of AHR in the I5/hE2 model demonstrated that this induced pathophysiological response was dependent on leukotriene-mediated activities (Figure 7). Significantly, this loss of AHR occurred independent of the pulmonary IL-13 levels, which remained elevated in 5-LO−/−/I5/hE2 mice. Figure 7 also shows that this loss of responsiveness to methacholine provocation occurred in mice after a blockade of cys-leukotriene activities (MK/I5/hE2), but not in mice targeting LTB4-mediated events (BLT-1−/−/I5/hE2).
Figure 7.
The dose-dependent airway sensitivity to methacholine provocation occurring in the chronic I5/hE2 inflammatory model is dependent on cys-leukotriene–mediated airway activities. Aerosolized methacholine-induced airway resistance (R) was assessed using an invasive ventilator-based technique (flexiVent; SCIREQ, Montreal, PQ, Canada). Unlike C57BL/6J wild-type controls, I5/hE2 mice displayed enhanced dose-dependent responses to aerosolized methacholine that remained unchanged in transgenic mice deficient in LTB4-mediated activities (BLT-1−/−/I5/hE2). However, elimination of all leukotrienes (5-LO−/−/I5/hE2) and, more specifically, I5/hE2 mice administered the cys-leukotriene antagonist, montelukast (MK/I5/hE2), eliminated all evidence of AHR, returning airway resistance as a function of methacholine dose to levels observed in C57BL/6J wild-type controls. Baseline airway resistance (cm H2O ⋅ s/ml) of each experimental cohort (mean ± SEM): C57BL/6J wild-type, 0.84 ± 0.03; I5/hE2, 1.04 ± 0.08; 5-LO−/−/I5/hE2, 1.10 ± 0.15; BLT-1−/−/I5/hE2, 0.77 ± 0.06; and MK/I5/hE2, 1.12 ± 0.13. *P < 0.05.
Cys-Leukotrienes, but not LTB4-Mediated Pulmonary Activities, Contribute to the Induced Airway Fibrosis that Accompanies Elevated Levels of TGF-β in the Lungs of I5/hE2 Mice
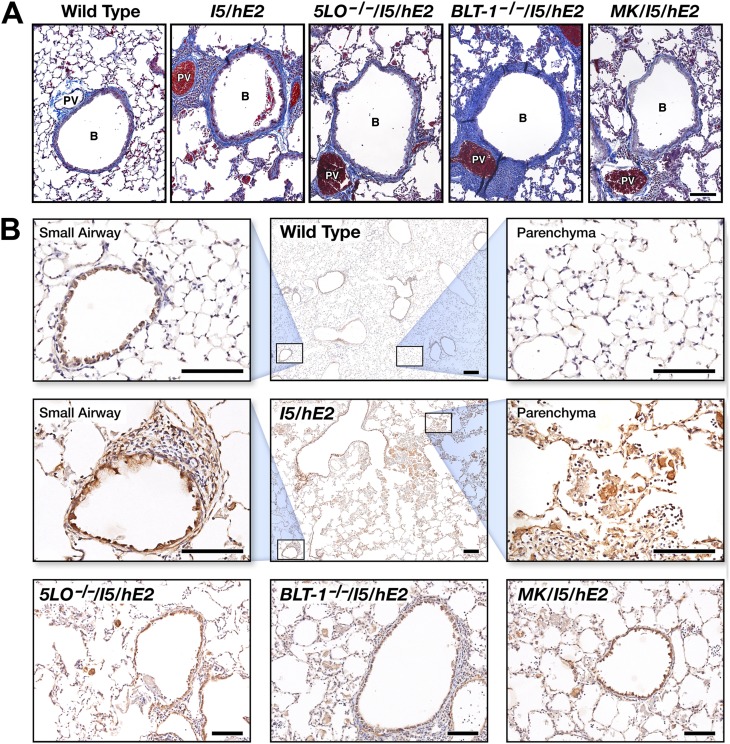
Histopathologic assessments of MT-stained lung sections showed significant increases (relative to wild-type control animals) in collagen deposition around airways in all of the I5/hE2 cohorts examined (i.e., I5/hE2, 5-LO−/−/I5/hE2, BLT-1−/−/I5/hE2, and MK/I5/hE2 mice; Figure 8A). In each case, this increase in fibrosis was accompanied by significant increases in BAL levels of TGF-β relative to wild-type mice (wild-type, 170 ± 39 pg/ml; I5/hE2, 758 ± 50 pg/ml; 5-LO−/−/I5/hE2, 883 ± 97 pg/ml; BLT1−/−/I5/hE2, 1,443 ± 163 pg/ml; and MK/I5/hE2, 1,360 ± 307 pg/ml). In addition, immunohistochemistry using a TGF-β1/-β2 polyclonal antibody provided the spatial distribution of TGF-β expression in the lungs of the various I5/hE2 cohorts and an apparent explanation for the high levels observed in each cohort relative to wild-type mice (Figure 8B). Specifically, elevated TGF-β expression (brown to dark brown-staining areas and cells) was observed in the airway epithelium of these mice. More importantly, the significant number of eosinophils infiltrating the lungs of each I5/hE2 cohort also displayed very high levels of TGF-β expression (Figure 8B).
Figure 8.
Pulmonary fibrosis (i.e., airway collagen deposition) occurring in I5/hE2 mice is accompanied by significantly elevated levels of TGF-β derived from multiple pulmonary cell types. (A) Representative airways from coronal lung sections stained with Masson’s trichrome from C57BL/6J wild-type mice (negative controls) and age-matched (12 wk postpartum) double-transgenic I5/hE2 mice (positive controls) are shown in comparison to airways in similar sections from transgenic mice targeting all leukotrienes (5-LO−/−/I5/hE2), the LTB4 high-affinity receptor (BLT-1−/−/I5/hE2), or I5/hE2 mice administered the cys-leukotriene antagonist, montelukast (MK/I5/hE2). Qualitative assessments of collagen deposition (blue extracellular matrix staining) showed that increased airway fibrosis occurred in all I5/hE2 cohorts examined (relative to wild-type). (B) Immunohistochemical staining (brown to dark brown staining areas and cells) with a TGF-β1/-β2 rabbit polyclonal antisera revealed significant and somewhat invariant expression of TGF-β in the lungs of all the I5/hE2 cohorts. Enhanced expression (relative to wild-type) was observed in multiple lung cell types, with particularly high expression observed in both airway epithelial cells and the robust pulmonary eosinophil infiltrate. Scale bars, 100 μm. B, bronchiole; PV, pulmonary vessel
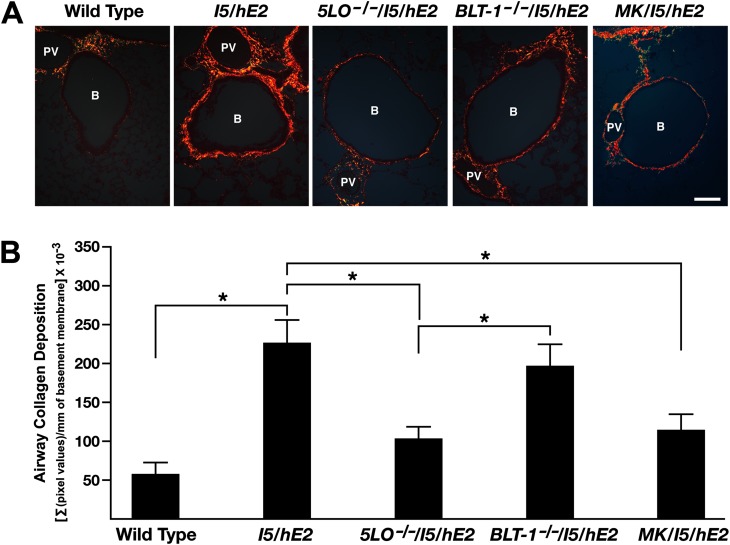
Interestingly, qualitative surveys of the MT-stained lung sections suggested that the extent of this induced pulmonary fibrosis also appeared to be a function of the leukotriene-mediated activities and, in particular, a consequence of cys-leukotriene activities more than LTB4. The significance of these observed qualitative differences were determined by morphometric assessments of PSR-stained coronal sections from each mouse under polarized light (Figure 9A). The interstitial area of all airways in coronal sections from each mouse within an experimental cohort (normalized per millimeter of central airway basement membrane) was evaluated for the extent and magnitude of this PSR staining (Figure 9B). These data were very reproducible and clearly demonstrated that the loss of all leukotrienes (5-LO−/−/I5/hE2) resulted in a significant loss in the airway fibrosis occurring in the I5/hE2 model (Figure 9A). The evaluation of the PSR staining demonstrated that this loss of fibrosis did not occur in the absence of LTB4 activities (BLT1−/−/I5/hE2 mice), and was exclusively linked with the cys-leukotriene subtype (MK/I5/hE2).
Figure 9.
The pulmonary fibrosis in I5/hE2 mice decreases significantly in animals targeting cys-leukotriene–mediated events. (A) Picrosirius red (PSR)-stained slides from each group (visualized under polarized light) provided a quantifiable assessment of the decreased collagen deposition (red extracellular staining) occurring in mice targeting total leukotrienes (5-LO−/−/I5/hE2) and, more specifically, cys-leukotriene events (MK/I5/hE2). Scale bars, 100 μm. B, bronchiole; PV, pulmonary vessel. (B) Quantitative assessments of PSR-stained lung sections demonstrated that the pulmonary fibrosis occurring in I5/hE2 mice was significantly lower in mice targeting either total leukotrienes and, more specifically, cys-leukotriene–mediated activities. Under polarized light, the submucosal interstitial area surrounding all central airways within the entire pulmonary parenchyma represented in coronal lung sections (n = 3–9 mice/group) were evaluated at a magnification of 200× (i.e., 6–21 central airways/section, evaluating all airways ≤0.7 mm in diameter). The extent and intensity of PSR staining was quantified as the sum total (Σ) of pixel values (pixel number × average pixel intensity within the region surrounding the basement membrane of a given central airway) normalized to the total length of central airway basement membrane evaluated. *P < 0.05.
Discussion
The Th2-polarized chronic pulmonary inflammation in I5/hE2 mice induces lung dysfunction as well as structural changes and other remodeling events that are significant in magnitude and progressive with age (our unpublished observations and Ref. 7). Collectively, these characteristics, as well as the eosinophil dependence of I5/hE2 mice, provide a unique mouse model to understand the role(s) of pulmonary leukotrienes, including both cys-leukotrienes and LTB4, in the underlying changes occurring in patients with asthma with chronic and progressive disease. Interestingly, in this model, the observed increase in pulmonary leukotrienes is linked with the induced pulmonary eosinophilia of the model, despite previous studies that showed that, although eosinophils synthesize leukotrienes, their level of production was much smaller than some other resident pulmonary cells (e.g., basophils [15, 16]). These observations suggest that inflammatory conditions that induce a focal accumulation of high numbers of eosinophils in the lung may be sufficient (as in I5/hE2 mice) to increase leukotriene levels and, in turn, contribute to concomitant pulmonary pathologies. The demonstration that local eosinophil-derived cys-leukotrienes induce fibrotic changes in a mouse model of atopic dermatitis after allergen challenge highlights the potential importance of such a mechanism even beyond of the lung (17).
The eosinophil-mediated inflammation in I5/hE2 mice occurs exclusively as a result of ectopic transgene expression and is not a downstream consequence of either the chemotactic activities associated with leukotrienes (18) or potential effects mediated on pulmonary immune responses following allergen provocation (19). The data derived from targeting leukotrienes (5-LO−/−/I5/hE2) or either of the prominent subtypes (LTB4 [BLT-1−/−/I5/hE2] or cys-leukotrienes [MK/I5/hE2]) present clear mechanisms of action that, in some cases, were leukotriene subtype specific. For example, the AHR associated with the chronic eosinophil-mediated pulmonary inflammation occurring in allergen-naive I5/hE2 mice was shown to be dependent on cys-leukotriene activities with no effects linked to LTB4. This pulmonary expression of cys-leukotrienes appears to be an all-or-none phenomenon, reducing responses in I5/hE2 mice to that observed in allergen-naive wild-type C57BL/6J control mice. It is significant that this result replicates observations reported earlier using an ovalbumin sensitization/aerosol challenge model that demonstrated this all-or-nothing effect on AHR mediated by leukotriene activities (9, 20); that is, the association of cys-leukotrienes and AHR was not model specific and, apparently, a generalized mechanism. Moreover, this cys-leukotriene–dependent AHR does not appear to be linked with concomitant leukotriene-dependent changes in airway smooth muscle thickness. However, the increased expression of cysLTR1 levels in the lungs of I5/hE2 mice relative to eosinophil-deficient PHIL/I5/hE2 mice does suggest that cys-leukotrienes may elicit AHR, in part, through eosinophil-mediated increased expression of cysLTR1 receptors.
Cys-leukotriene activities also appear to be downstream of the inflammatory events occurring in the lungs of these mice, as well as any potential effects on AHR mediated by eosinophil degranulation (21). Regardless of the ultimate definition of the causative mechanism(s), this link between cys-leukotrienes and lung dysfunction in the mouse appears to reflect a subgroup of patients with asthma who display demonstrable improved lung function in response to blockade of cys-leukotriene activities or antieosinophil (IL-5) approaches (22).
In contrast to the induced AHR associated with I5/hE2 mice, leukotriene activities did not contribute to the progressive goblet cell metaplasia and the gross morphological changes in the lung (e.g., changes of the distal airspaces) occurring in this model of chronic inflammation. However, leukotriene-mediated events were shown to contribute to the pulmonary fibrosis (i.e., airway collagen deposition) that occurred in the I5/hE2 model. Moreover, this contribution to fibrosis was shown to be a function of cys-leukotriene, but not LTB4-mediated activities. Specifically, the data showed that the pulmonary fibrosis accompanying both elevated levels of IL-13 and TGF-β in I5/hE2 mice is further augmented by elevated levels of cys-leukotrienes in the lungs of these mice. This larger link between IL-13, TGF-β, and cys-leukotrienes was also suggested in allergen provocation studies (reviewed in Ref. 23), and implies that eosinophil-mediated increases in pulmonary cys-leukotrienes may elicit additional and/or alternative fibrotic events extended beyond simply increasing pulmonary IL-13 and TGF-β expression. We suggest that cys-leukotriene–induced eosinophil release of proteases and other extracellular matrix-modulating mediators may represent an additional/alternative fibrotic mechanism. This suggestion is supported both by our assessments of elevated cysLTR1 levels in the lungs of I5/hE2 mice, which were particularly pronounced on the many eosinophils infiltrating the lungs of these mice, and by transcript microarray assessments of gene expression that have implicated multiple candidate pathways connecting eosinophils, cys-leukotrienes, and fibrosis/remodeling events. These include, but are not limited to, the expression of extracellular matrix–activating/–degrading proteases with demonstrable roles in fibrotic events (e.g., matrix metalloproteinase 12, 13, and 19 as well as cathepsin K), and the expression of profibrotic cytokines, such as IL-6 and IL-4. Indeed, the small but reproducible decrease in the BAL IL-4 levels in 5-LO−/−/I5/hE2 and MK/I5/hE2 mice may reflect a link between the cys-leukotriene–induced release of eosinophil-derived IL-4 in specific models and induced pulmonary fibrosis.
Eosinophil release of secondary granule proteins (i.e., degranulation) may also be an additional contributor to pulmonary fibrosis. That is, previous studies in both patients with asthma (24) and mouse models (25) have suggested that eosinophil release of secondary granule proteins is a potential contributor to fibrotic changes occurring in the lung. Moreover, our studies have also shown that the value of the I5/hE2 model, unlike allergen provocation models, is that the significant remodeling events measureable in these transgenic mice correlate with the extensive eosinophil degranulation. This extensive eosinophil degranulation occurs irrespective of the targeting of total leukotrienes (5-LO−/−/I5/hE2) or each of the prominent subtypes (LTB4 [BLT-1−/−/I5/hE2] or cys-leukotrienes [MK/I5/hE2]), suggesting that, in addition to IL-13 and TGF-β expression, the release of eosinophil granule proteins may be a contributing mechanism to the fibrosis observed in I5/hE2 mice. Interestingly, this was also an independently derived conclusion of our differential transcriptome analyses of I5/hE2 mice versus eosinophilless double-transgenic mice (I5/hE2/PHIL).
The unexpected link between cys-leukotriene activities in chronic eosinophilic inflammation and pulmonary parenchymal fibrosis may have potential value to patient care if this mechanism can be extrapolated to human subjects. The data presented here show that eosinophils appear to be a significant local source of cys-leukotrienes that may contribute to fibrotic events mediated by several cell types resident in the lung, including eosinophils themselves. Moreover, the specific blockade of cys-leukotriene–mediated activities (via pharmaceutical intervention) significantly attenuated fibrotic remodeling in the lung, and occurred by a novel unresolved mechanism. This also represents a departure from rationale for the use of agents that block cys-leukotriene activities (e.g., montelukast), which are often prescribed as a treatment for acute/short-term inflammatory symptoms and lung dysfunction (26, 27). We recognize that the use of Th2-polarized chronic inflammatory mice that are specifically engineered to understand the short-term/long-term consequences of pulmonary eosinophil activities is limited as a model of lung dysfunction characteristic of human respiratory disease. Instead, our goal was a reductionist approach to understanding mechanisms potentially mediating eosinophil activities during Th2-driven inflammatory events, which we have suggested are likely shared between humans and mice (28). Thus, if further studies suggest that our observations in mice are translatable to patients with asthma, this result would have significant implications by suggesting that the chronic presence of eosinophils in the lungs of some patients with asthma may elicit sustained expression of pulmonary cys-leukotrienes and, in turn, progressive lung fibrosis. Thus, extended use of a cys-leukotriene–blocking agent, either alone or in combination with other anti-inflammatory agents, may represent a previously underappreciated strategy targeting the lung structural changes accompanying the chronic eosinophilic inflammation in many patients with asthma.
Acknowledgments
Acknowledgments
The authors thank the members of Lee Laboratories who reviewed various drafts of this manuscript and helped in the data collection and organization/infrastructure needed to complete the studies presented. They also acknowledge the invaluable assistance of the Mayo Clinic Arizona medical graphic artist, Marv Ruona, and the excellent administrative support provided to Lee Laboratories by Linda Mardel and Shirley “Charlie” Kern.
Footnotes
This work was supported by Merck research grant IISP 33,689 (J.J.L.), resources from the Mayo Foundation, and National Institutes of Health grants HL058723 (N.A.L.), HL065228 and RR0109709 (J.J.L.), AI0508 (A.D.L.), NIGMS P30 03,115,801 (C.G.I.), as well as American Heart Association grants 05,556,392 (N.A.L.) and 0,855,703 (J.J.L.). These funding sources had no involvement in study design, data collection (including analysis and interpretation), the writing of the manuscript, or the decision to submit for publication.
Author Contributions: The corresponding author (N.A.L.) had full access to all of the data reported in this study and had final responsibility for the decision to submit this report for publication. S.I.O., H.-H.S., A.D.L., C.G.I., J.J.L., and N.A.L. designed the research study; S.I.O., C.A.P., W.L., D.C.C., K.R.Z., and J.J.L. performed the research; S.I.O., C.A.P., W.L., C.G.I., J.J.L., and N.A.L. analyzed the data; S.I.O., J.J.L., and N.A.L. wrote the initial draft of the manuscript, and S.I.O., H.-H.S., C.G.I., J.J.L., and N.A.L. provided critical assessments during the revision process leading to the final, submitted manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0009OC on July 16, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Haeggstrom JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 2.Guilbert M, Ferland C, Bosse M, Flamand N, Lavigne S, Laviolette M. 5-oxo-6,8,11,14-eicosatetraenoic acid induces important eosinophil transmigration through basement membrane components: comparison of normal and asthmatic eosinophils. Am J Respir Cell Mol Biol. 1999;21:97–104. doi: 10.1165/ajrcmb.21.1.3517. [DOI] [PubMed] [Google Scholar]
- 3.Montuschi P, Peters-Golden ML. Leukotriene modifiers for asthma treatment. Clin Exp Allergy. 2010;40:1732–1741. doi: 10.1111/j.1365-2222.2010.03630.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 5.Haribabu B, Verghese MW, Steeber DA, Sellars DD, Bock CB, Snyderman R. Targeted disruption of the leukotriene B(4) receptor in mice reveals its role in inflammation and platelet-activating factor–induced anaphylaxis. J Exp Med. 2000;192:433–438. doi: 10.1084/jem.192.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 7.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O'Neill KR, Colbert DC, Colby TV, et al. Co-expression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–7889. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 8.Hisada T, Salmon M, Nasuhara Y, Chung KF. Cysteinyl-leukotrienes partly mediate eotaxin-induced bronchial hyperresponsiveness and eosinophilia in IL-5 transgenic mice. Am J Respir Crit Care Med. 1999;160:571–575. doi: 10.1164/ajrccm.160.2.9810101. [DOI] [PubMed] [Google Scholar]
- 9.Irvin CG, Tu YP, Sheller JR, Funk CD. 5-lipoxygenase products are necessary for ovalbumin-induced airway responsiveness in mice. Am J Physiol. 1997;272:L1053–L1058. doi: 10.1152/ajplung.1997.272.6.L1053. [DOI] [PubMed] [Google Scholar]
- 10.Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechele TL, O'Neill KR, Ansay TL, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170:3296–3305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- 11.James J, Bosch KS, Zuyderhoudt FM, Houtkooper JM, van Gool J. Histophotometric estimation of volume density of collagen as an indication of fibrosis in rat liver. Histochemistry. 1986;85:129–133. doi: 10.1007/BF00491759. [DOI] [PubMed] [Google Scholar]
- 12.Protheroe CA, Woodruff SA, DePetris G, Mukkada V, Ochkur SI, Janarthanan S, Lewis JC, Pasha S, Lunsford T, Harris L, et al. A novel histological scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis Clin Gastroenterol Hepatol 20097749–755.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochkur SI, Kim JD, Protheroe CA, Colbert D, Moqbel R, Lacy P, Lee JJ, Lee NA. The development of a sensitive and specific ELISA for mouse eosinophil peroxidase: assessment of eosinophil degranulation ex vivo and in models of human disease. J Immunol Methods. 2012;375:138–147. doi: 10.1016/j.jim.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 15.Eglite S, Pluss K, Dahinden CA. Requirements for C5a receptor–mediated IL-4 and IL-13 production and leukotriene C4 generation in human basophils. J Immunol. 2000;165:2183–2189. doi: 10.4049/jimmunol.165.4.2183. [DOI] [PubMed] [Google Scholar]
- 16.Takafuji S, Bischoff SC, De Weck AL, Dahinden CA. IL-3 and IL-5 prime normal human eosinophils to produce leukotriene C4 in response to soluble agonists. J Immunol. 1991;147:3855–3861. [PubMed] [Google Scholar]
- 17.Oyoshi MK, He R, Kanaoka Y, ElKhal A, Kawamoto S, Lewis CN, Austen KF, Geha RS. Eosinophil-derived leukotriene C4 signals via type 2 cysteinyl leukotriene receptor to promote skin fibrosis in a mouse model of atopic dermatitis. Proc Natl Acad Sci USA. 2012;109:4992–4997. doi: 10.1073/pnas.1203127109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargaftig BB, Singer M. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. Am J Respir Cell Mol Biol. 2003;28:410–419. doi: 10.1165/rcmb.2002-0032OC. [DOI] [PubMed] [Google Scholar]
- 19.Vargaftig BB, Singer M. Leukotrienes, IL-13, and chemokines cooperate to induce BHR and mucus in allergic mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L260–L269. doi: 10.1152/ajplung.00226.2002. [DOI] [PubMed] [Google Scholar]
- 20.Henderson WR, Jr, Chiang GK, Tien YT, Chi EY. Reversal of allergen-induced airway remodeling by cysLT1 receptor blockade. Am J Respir Crit Care Med. 2006;173:718–728. doi: 10.1164/rccm.200501-088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CK, Callaway Z, Kim DW, Kita H. Eosinophil degranulation is more important than eosinophilia in identifying asthma in chronic cough. J Asthma. 2011;48:994–1000. doi: 10.3109/02770903.2011.623335. [DOI] [PubMed] [Google Scholar]
- 22.Biernacki WA, Kharitonov SA, Biernacka HM, Barnes PJ. Effect of montelukast on exhaled leukotrienes and quality of life in asthmatic patients. Chest. 2005;128:1958–1963. doi: 10.1378/chest.128.4.1958. [DOI] [PubMed] [Google Scholar]
- 23.Elias JA, Lee CG, Zheng T, Shim Y, Zhu Z. Interleukin-13 and leukotrienes: an intersection of pathogenetic schema. Am J Respir Cell Mol Biol. 2003;28:401–404. doi: 10.1165/rcmb.F264. [DOI] [PubMed] [Google Scholar]
- 24.Benson HL, Wilkes DS. Matrix metalloproteinases in T cell mediated pulmonary diseases. Front Biosci (Elite Ed) 2012;4:2162–2169. doi: 10.2741/533. [DOI] [PubMed] [Google Scholar]
- 25.Pegorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol. 2006;177:4861–4869. doi: 10.4049/jimmunol.177.7.4861. [DOI] [PubMed] [Google Scholar]
- 26.Adachi M, Taniguchi H, Tohda Y, Sano Y, Ishine T, Smugar SS, Hisada S. The efficacy and tolerability of intravenous montelukast in acute asthma exacerbations in Japanese patients. J Asthma. 2012;49:649–656. doi: 10.3109/02770903.2012.690479. [DOI] [PubMed] [Google Scholar]
- 27.Ducharme FM, Noya FJ, Allen-Ramey FC, Maiese EM, Gingras J, Blais L. Clinical effectiveness of inhaled corticosteroids versus montelukast in children with asthma: prescription patterns and patient adherence as key factors. Curr Med Res Opin. 2012;28:111–119. doi: 10.1185/03007995.2011.640668. [DOI] [PubMed] [Google Scholar]
- 28.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, Luo H, Zellner KR, Protheroe CA, Willetts L, et al. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130:572–584. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]