Abstract
Foxa2 is a member of the Forkhead family of nuclear transcription factors that is highly expressed in respiratory epithelial cells of the developing and mature lung. Foxa2 is required for normal airway epithelial differentiation, and its deletion causes goblet-cell metaplasia and Th2-mediated pulmonary inflammation during postnatal development. Foxa2 expression is inhibited during aeroallergen sensitization and after stimulation with Th2 cytokines, when its loss is associated with goblet-cell metaplasia. Mechanisms by which Foxa2 controls airway epithelial differentiation and Th2 immunity are incompletely known. During the first 2 weeks after birth, the loss of Foxa2 increases the production of leukotrienes (LTs) and Th2 cytokines in the lungs of Foxa2 gene–targeted mice. Foxa2 expression inhibited 15-lipoxygenase (Alox15) and increased Alox5 transcription, each encoding key lipoxygenases associated with asthma. The inhibition of the cysteinyl LT (CysLT) signaling pathway by montelukast inhibited IL-4, IL-5, eotaxin-2, and regulated upon activation normal T cell expressed and presumably secreted expression in the developing lungs of Foxa2 gene–targeted mice. Montelukast inhibited the expression of genes regulating mucus metaplasia, including Spdef, Muc5ac, Foxa3, and Arg2. Foxa2 plays a cell-autonomous role in the respiratory epithelium, and is required for the suppression of Th2 immunity and mucus metaplasia in the developing lung in a process determined in part by its regulation of the CysLT pathway.
Keywords: Foxa2, leukotriene, Th2 inflammation, mucous metaplasia
Goblet-cell metaplasia, mucus hyperproduction, and inflammation are associated with common chronic lung diseases, including asthma (1, 2). Evidence is increasing that the respiratory epithelium plays a role in the regulation of innate and acquired immunity in the setting of aeroallergen exposure (2). Foxa2, a member of the Forkhead family of transcription factors selectively expressed in respiratory epithelial cells, plays a critical role in suppressing Th2-mediated pulmonary inflammation and goblet-cell metaplasia in the developing murine lung (3). The expression of Foxa2 is sufficient to inhibit goblet-cell metaplasia after aeroallergen exposure in adult mice (3). Both Foxa2 and the thyroid transcription factor–1 (TTF-1) inhibit SAM pointed domain containing ETS transcription factor (SPDEF), which is an E–twenty six (Ets)-like transcription factor sufficient and necessary for the activation of genes regulating mucus production and goblet-cell metaplasia after aeroallergen or IL-13 exposure (3, 4).
Lipid-derived mediators play an important role in lung inflammation (5). Proinflammatory lipid mediators derived from arachidonic acid, including leukotrienes (LTs), are the metabolic products of arachidonic acid, and are considered important inflammatory mediators during asthma (6). LTs are synthesized through multiple enzymatic steps from membrane phospholipids. Arachidonic acid is cleaved from membrane phospholipids via phospholipase A2. Arachidonic acid is converted to 5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosate-traenoic acid and then to leukotriene A4 (LTA4) in two oxidative steps by 5-lipoxygenase (Alox5). The Alox5-activating protein is necessary for this conversion. LTA4 is conjugated with the tripeptide glutathione to form the first of the cysteinyl-LTs LTC4. Alternatively, LTA4 can be metabolized to the hydroxyl LTB4 by epoxide hydrolase in neutrophils and other inflammatory cells. LTB4 is a potent neutrophil and eosinophil chemoattractant. LTC4 is exported to the extracellular space and cleaved to LTD4 and LTE4. LTC4, LTD4, and LTE4 all contain a cysteinyl residue and are collectively called the CysLTs (5). LT antagonists, mainly antagonists of the CysLT1 receptor, are used in clinical practice for the treatment of asthma (7). LT responses are mediated through the activation of the cell surface–specific, G-protein–coupled receptors leukotriene B4 receptor 1 (BLT1) and BLT2 for LTB4 signaling, and CysLT1 and CysLT2 for CysLT signaling (8, 9). CysLT1 signaling is blocked by specific antagonists, including montelukast, zafirlukast, and pranlukast, which do not target CysLT2 (7). IL-4, IL-5, and IL-13 augment CysLT production. IL-4 up-regulates LTC4 synthase and CysLT1 receptor gene expression (10). LT synthase is induced by IL-13 in human lung macrophages (11). In Ltc4 snull mice, the production of IL-4, IL-5, and IL-13 by antigen restimulation was reduced (12). IL-13 enhances the expression of the CysLT1 receptor in human lung fibroblasts, increasing eotaxin production in a concentration-dependent fashion (13). How the Th2 cytokine and LT pathways interact during Th2-mediated pulmonary inflammation remains poorly understood.
The role of the LT pathway in Th2-mediated pulmonary inflammation caused by Foxa2 deletion in airway epithelial cells remains unknown. In this study, we hypothesized that Foxa2 regulates the LT pathway to suppress Th2-mediated inflammation in the lung. The present data demonstrate that Foxa2 directly regulated Alox15 and Alox5 gene transcription. Montelukast, a selective antagonist of the CysLT1 receptor, inhibited the Th2-mediated inflammation and mucus metaplasia caused by the deletion of Foxa2 in the developing mouse lung. Activation of the LT pathway in developing Foxa2 gene–targeted mice preceded the increased expression of Th2 cytokines. Taken together, Foxa2 is required for the suppression of Th2-mediated pulmonary inflammation and mucus metaplasia during lung development in a process determined in part by its regulation of the CysLT pathway.
Materials and Methods
More detailed methods are available in the online supplement.
Transgenic Mice and Animal Husbandry
Mice were maintained under pathogen-free conditions according to protocols approved by the Institutional Animal Care and Use Committee of West China Hospital at Sichuan University. Foxa2loxP/loxP mice were kindly provided by Dr. Klaus Kaestner at the University of Pennsylvania (Philadelphia, PA). The conditional deletion of Foxa2 in airway epithelial cells was performed as previously described, using the SPC-rtTA-tet(o)7-CRE system (14). Mice were killed by an injection of anesthetic, and exsanguinated. Foxa2 deletion was confirmed by quantitative PCR.
Montelukast Treatment
Intraperitoneal injections were performed in neonatal Surfactant protein C (SFTPC)/Foxa2Δ/Δ pups and control littermates with either montelukast (10 mg/kg body weight; a gift from Merck Sharp and Dohme Corporation, Rahway, NJ) or the corresponding volume of sterile saline daily from Postnatal Day (PN) 5 to PN15. On PN15, bronchoalveolar lavage fluid (BALF) was collected and differential cell counts were measured, as previously described (3). The left lungs and tracheas were fixed in 4% paraformaldehyde and processed into paraffin blocks for hematoxylin and eosin staining. Lung tissue was collected and stored at −70°C for quantitative PCR, Western blotting, or ELISA.
Measurement of Cytokines and Mediators in the BALF and Lung
Lung tissue from PN5, PN7, and PN15 were prepared. The levels of cytokines (IL-13, IL-4, IL-5, TNF-α, and IL-1β), normal T cells expressed and secreted (RANTES), eotaxin-2, the thymus and activation–regulated chemokine, and LTB4, LTC4, LTD4, LTE4, 12-hydroxy-eicosatetraenoic acid (12-HETE), prostaglandin H2 (PGH2), and prostaglandin E2 (PGE2) in the BALF supernatant and in the supernatant of lung homogenates were measured with ELISA kits, according to the manufacturer’s protocol. Mouse ELISA kits were purchased from Cusabio Biotech Co., Ltd. (Wuhan, Hubei, China).
Western Blot Analysis
Western blot analysis was performed on lung homogenates from SFTPC-Foxa2∆/∆ mice and control littermates at PN5 and PN15. The protein was separated by SDS-PAGE, transferred, and immobilized on nitrocellulose membranes. Membranes were incubated with goat anti-mouse Alox15 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) or the CysLT1 receptor (1:200; Santa Cruz Biotechnology) antibody.
Plasmid Construction and Luciferase Reporter Assay
Alox15 and Alox5 promoter–luciferase constructs were constructed with pGL3 plasmids. The constructs were transiently transfected into H441 cells, and luciferase activity was assessed 48 hours after the transfection.
RNA Extraction and Quantitative Real-Time RT-PCR
Total RNA from lung tissue was extracted with Trizol (Life Technologies, Grand Island, NY). Reverse transcriptase and quantitative real-time PCR analyses were performed.
RNA Microarray Analysis
Lung cDNA was hybridized to murine genome MOE430 chips (Affymetrix, Santa Clara, CA), according to the manufacturer’s protocol, and data processing was performed as previously reported (14). The data were derived from a previous study (3).
Splenocyte Activation and Cytokine Assay
Splenocytes from SPC-Foxa2∆/∆ mice and control littermates were constructed, cultured, and stimulated with anti-CD3 and anti-CD28 (Pharmingen, San Diego, CA), and then IL-13, IL-4, and IFN-γ were measured.
Statistical Analysis
Analyses were performed using SPSS, version 13.0 (SPSS, Inc., Chicago, IL). Data are expressed as means ± SEMs. ANOVA, the Tukey-Kramer test, the Dunnett test, and the unpaired t test were used to compare differences in groups. Differences were considered statistically significant at P < 0.05, according to a two-tailed test.
Results
Conditional Deletion of Foxa2 Induced Th2 Pulmonary Inflammation
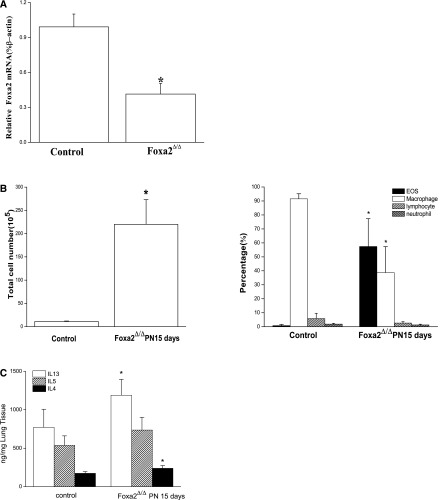
Foxa2 was conditionally deleted from respiratory epithelial cells under the control of SFTPC-rtta, tet(o)7-CRE to mediate the selective deletion of the floxed Foxa2 gene in the epithelia of developing mouse lungs. In whole lungs, Foxa2 mRNA was decreased approximately twofold when assessed on PN 15 (Figure 1A). Extensive pulmonary inflammation was observed in the lungs of Foxa2∆/∆ mice, and this finding was supported by the quantification of inflammatory cells in BALF, wherein eosinophils, macrophages, lymphocytes, and neutrophils were increased (Figure 1B). Consistent with previous findings (3), the deletion of Foxa2 was associated with the increased expression of the Th2 cytokines IL-13 and IL-4 (Figure 1C). However, Th1 cytokines, such as IL-1β and TNF-α, were similar in both groups (Figure E1 in the online supplement).
Figure 1.
Deletion of Foxa2 in respiratory epithelium caused pulmonary eosinophilic inflammation on Postnatal Day (PN) 15. (A) Loss of Foxa2 expression was confirmed by RT-PCR analysis. Foxa2 mRNA was significantly decreased. (B) Numbers of total cells and eosinophils (EOS) in bronchoalveolar lavage fluid (BALF) were increased in Foxa2Δ/Δ mice on PN15. Eosinophils in BALF were increased, as assessed by Diff-Quik (Polysciences, Warrington, PA) staining. (C) Th2 cytokines (IL-4, IL-5, and IL-13) were increased in the lung tissue of Foxa2Δ/Δ mice, as determined by ELISA. The graphs represent means ± SEs (n = 5 for each genotype). *P < 0.05 versus control values, using the Student t test.
Components of the LT Signaling Pathways Are Activated after Foxa2 Deletion in Airway Epithelial Cells
To investigate the role of Foxa2 and its downstream targets known to be associated with Th2 inflammation, an mRNA microarray (3) was used to assess differential gene expression in lung tissue from control and Foxa2-deleted mice. The mRNAs encoding LT signaling pathway–related enzymes (Alox5, 5-lipoxygenase-activating protein [FLAP], and LTC4 s) and Alox15 were significantly increased in lungs from Foxa2Δ/Δ mice, findings confirmed by quantitative RT-PCR. LTB4, LTC4, LTD4, LTE4, and 12-HETE were increased in lung-tissue homogenates from Foxa2Δ/Δ mice, as determined by ELISA assay. These data indicate that the LTs and other components of the lipoxygenase network are activated during the Th2 lung inflammation caused by Foxa2 deletion in airway epithelial cells during postnatal development (Figure 2). We also measured PGH2 and PGE2, the products of the cyclooxygenase-2 (COX-2) pathway, which plays an important role in some inflammation. However, neither PGH2 nor PGE2 changed in lung-tissue homogenates from Foxa2Δ/Δ and control mice (Figure E2), indicating that Foxa2 deletion did not activate the COX-2 pathway.
Figure 2.

Leukotriene (LT) pathways are involved in the Th2 lung inflammation caused by Foxa2 deletion in airway epithelial cells. (A) cDNAs were constructed from Foxa2Δ/Δ mice and control littermates on PN15 (n = 3 of each genotype), and hybridized to murine genome MOE430 chips (Affymetrix). Microarray analysis showed that the loss of Foxa2 in respiratory epithelial cells significantly up-regulated mRNAs encoding arachidonic acid metabolism–related enzymes, including 5-lipoxygenase (Alox5), Alox15, FLAP, and LTC4 s. (B) Increased LT-related enzyme and Alox15 mRNAs in Foxa2Δ/Δ mice on PN15 (n = 5 of each genotype) are shown. Quantitative RT-PCR results were consistent with mRNA microarrays. (C) LTB4, LTC4, LTD4, LTE4 and 12-HETE were increased in Foxa2Δ/Δ mice on PN15, as detected by ELISA. Graphs represent means ± SEs (n = 5 for each genotype for ELISA). *P < 0.05, versus control values determined by the Student t test.
Activation of LT Components Was Independent of Th2 Cytokines in Foxa2Δ/Δ Mice
The expression of mRNAs encoding components of the lipoxygenase signaling pathway and Th2 cytokines was increased in the lungs of Foxa2Δ/Δ mice. To determine the timing of Th2 cytokines and lipid-derived mediators influenced by Foxa2 deletion, levels of LTB4, LTC4, LTD4, LTE4, 12-HETE and the Th2 cytokines IL-4, IL-5, and IL-13 were assessed on PN5 and PN7 by ELISA. The mRNAs of Th2 cytokines and enzymes related to lipid-derived mediator metabolism were also measured by quantitative RT-PCR at the same time. LTB4, LTD4, LTE4, and 12-HETE were significantly increased in lung homogenates from Foxa2Δ/Δ mice as early as PN5, at a time when the expression of IL-4 and IL-13 was similar in control and Foxa2Δ/Δ mice (Figure 3A). Alox5 and LTC4 s mRNAs were increased on PN5 (Figure 3B). On PN7, Alox5, Alox15, FLAP, and LTC4 s mRNAs were increased in Foxa2Δ/Δ mice (Figure 3D), and LTB4 and LTE4 concentrations in lung homogenates were higher in Foxa2Δ/Δ mice than in control mice (Figure 3C). IL-4, IL-5, and IL-13 mRNAs were increased, but not to a statistically significant extent (Figure 3D). In contrast, IL-4, IL-5, and eotaxin-2 protein levels were higher in Foxa2Δ/Δ mice than in control mice (Figure 3C). The CysLT1 receptor was increased in lungs of Foxa2Δ/Δ mice on PN5 (Figure 3E), as assessed by Western blotting.
Figure 3.

Th2 cytokines, LTs, and 12-HETE in Foxa2Δ/Δ mice. (A) ELISA assay results revealed increased LTs and 12-HETE in Foxa2Δ/Δ mice on PN5, but with no difference in protein levels of IL-4, IL-5, and IL-13 in control and Foxa2Δ/Δ mice. (B) Quantitative RT-PCR results indicated that the expression of LT-related enzymes increased in Foxa2Δ/Δ mice, but with no difference in Th2 cytokine mRNA. (C) Th2 cytokines, LTs, and 12-HETE both exhibited a trend to increase significantly, compared with the control group, according to ELISA on PN7. (D) The mRNAs of LT-related enzymes and Th2 cytokines exhibited a trend to increase in Foxa2Δ/Δ mice, measured using quantitative RT-PCR on PN7. (E) Western blots of Alox15 (15-LO), the cysteinyl–leukotriene–1 (CysLT1) receptor, and β-actin in lungs from Foxa2Δ/Δ mice on PN5. The graph data below were derived from three different experiments. *P < 0.05, versus control values determined by two-tailed Student t test (n = 5 for each genotype).
Foxa2 Inhibited the Transcription of Alox15, but Increased the Transcription of the Alox5 Gene In Vitro
Alox15 plays a key role in Th2 inflammation in the lung (15). Alox15 mRNA was significantly increased in the lungs of Foxa2Δ/Δ mice. To determine whether Foxa2 directly regulated Alox15 and Alox5 in respiratory epithelial cells, luciferase reporter constructs containing regulatory regions of the mouse Alox15 and Alox5 genes were cotransfected into H441 cells with Foxa2 expression plasmids. Transfected cells were further stimulated with IL-13, known to induce the expression of Alox15 (15, 16). Foxa2 significantly inhibited the transcription of Alox15 (Figure 4A). IL-13 exerted no effect on the activity of the Alox15 promoter, with or without Foxa2. In contrast, Foxa2 increased Alox5 promoter activity in a dose-dependent manner. This result indicated that Foxa2 differentially regulated the activity of the Alox15 and Alox5 genes (Figures 4B and 4C) in this cell line. The stimulatory effect of Foxa2 on Alox5 promoter activity contrasts with the finding that Alox5 mRNA was increased in the Foxa2-deleted mice.
Figure 4.
Foxa2 regulates Alox15 and Alox5 gene promoter activity. Luciferase reporter constructs containing the regulatory region of the mouse Alox15 and Alox5 gene were cotransfected into H441 cells with Foxa2 expression plasmids. Foxa2 significantly inhibited the activity of Alox15, and increased the transcription of Alox5. Foxa2 increased Alox5 gene activity in a dose-dependent manner. IL-13 exerted no effect on Alox15 and Alox5 promoter activity, with or without Foxa2. *P < 0.05, versus Foxa2 control values determined by two-tailed Student t test. #P < 0.05, versus Foxa2 expression plasmids (0.125 and 0.5 μg), according to the Dunnett test (n = 3 in each group). β-gal, β-galactosidase.
Splenocytes Were Not Activated in Th2-Mediated Pulmonary Inflammation after the Deletion of Foxa2
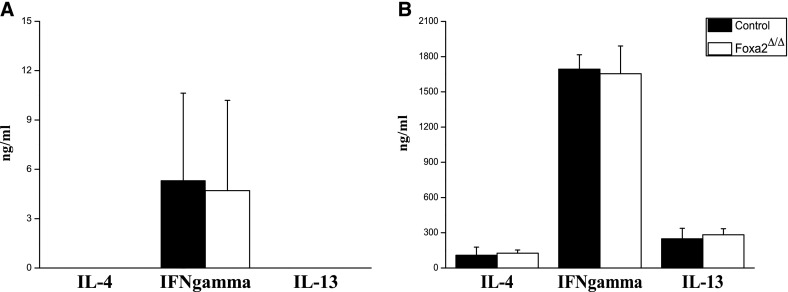
In the present study, Foxa2 was selectively deleted in respiratory epithelial cells. To assess whether the loss of Foxa2 influenced systemic immunity, spleen cells were isolated from Foxa2Δ/Δ and control mice on PN15 and activated by anti-CD3 and anti-CD28. IL-13, IL-4, and IFN-γ were measured by ELISA in supernatants from activated or inactivated splenocytes. IL-4, IL-13, and IFN-γ concentrations were higher in supernatants from activated splenocytes than inactivated splenocytes, but they were not different in Foxa2Δ/Δ and control mice (Figure 5). These findings demonstrate that Th2-mediated inflammation seen after deletion of Foxa2 was likely confined to the lung.
Figure 5.
Cytokines produced by splenocytes from Foxa2Δ/Δ and control mice. (A) Splenocytes from Foxa2Δ/Δ and control mice on PN15, without anti-CD3 and anti-CD28. (B) Splenocytes from Foxa2Δ/Δ and control mice on PN15 were stimulated with anti-CD3 and anti-CD28. The concentrations of IL-13, IL-4, and IFN-γ were measured by ELISA in the supernatants. *P < 0.05, versus control values determined by Student t test (two-tailed; n = 3 for each genotype).
Pulmonary Eosinophilic Inflammation Induced by Foxa2 Deletion Is Inhibited by Montelukast
CysLT signaling is a key pathway regulating Th2 inflammation in asthma. Montelukast inhibits eosinophil adhesion via several CysLT1 receptor–dependent mechanisms (17, 18). To investigate whether pulmonary inflammation caused by the conditional deletion of Foxa2 in the airway epithelium was associated with activation of the CysLT pathway, Foxa2Δ/Δ mice were treated with montelukast daily from PN5–PN15. Inhibition of the CysLT signaling pathway was sufficient to inhibit Th2 inflammation, as indicated by histopathology and the inhibition of expression of Th2 cytokines and chemokines in the lung (Figures 6A and 6E). Montelukast treatment inhibited pulmonary inflammation, as also indicated by the reduction of BALF cells (P < 0.05; Figure 6B). LT production, including LTB4, LTC4, LTD4, LTE4, and the production of Alox15 and 12-HETE were inhibited by montelukast (Figures 6C and 6D), and IL-4, IL-5, RANTES, and eotaxin-2 were significantly decreased. In contrast, levels of IL-13 remained higher, compared with control samples (Figure 6E). Western blot analysis indicated that Alox15 and CysLT1 receptor expression was decreased by montelukast (Figure 6F). These results indicate that Th2 inflammation induced by the deletion of Foxa2 was partly dependent upon the CysLT signaling pathway.
Figure 6.
Montelukast attenuated Th2 lung inflammation in Foxa2Δ/Δ mice. Neonatal SFTPC/Foxa2Δ/Δ pups and control littermates were injected with either montelukast or sterile saline from PN5–PN15. (A and B) Hematoxylin and eosin staining of lung tissues from Foxa2Δ/Δ mice with saline or montelukast. (C) Numbers of cells in BALF were assayed. Decreased numbers of total cells and eosinophils were found in BALF from Foxa2Δ/Δ mice after montelukast treatment. (D) Lung RNAs were extracted from Foxa2Δ/Δ and Foxa2Δ/Δ mice with montelukast on PN15. Alox5, Alox15, FLAP, and LTC4 s mRNAs were decreased in Foxa2Δ/Δ mice after montelukast treatment on PN15, compared with those mRNAs in the control group according to RT-PCR. (E) LTB4, LTC4, LTD4, LTE4, and 12-HETE were decreased in BALF and lung homogenates compared with control samples, according to ELISA. (F) BALF and lung homogenate levels of Th2 cytokines and chemokines, including IL-4, IL-5, RANTES, and eotaxin-2, were decreased in Foxa2Δ/Δ mice after montelukast treatment, compared with control mice. (G) Western blots of Alox15, cysLT1 receptor, and β-actin in lungs from Foxa2Δ/Δ mice after montelukast or saline treatment on PN15. The graph data below were derived from three different experiments. Graph represents means ± SEMs (n = 5 for each genotype) for quantitative RT-PCR and ELISA assays. *P < 0.05, versus control values determined by the Student t test (two-tailed; n = 5 of each genotype).
Goblet-Cell Metaplasia Induced by Foxa2 Deletion Was Inhibited by Montelukast
Because the LT pathway is thought to play a role in goblet-cell metaplasia (19), markers associated with the production of airway mucus were measured after montelukast administration. Spdef, Muc5ac, Arg2, and Foxa3 mRNAs were significantly decreased by montelukast (Figure 7). These results indicate that the goblet-cell metaplasia induced by Foxa2 deletion during neonatal lung development is, at least in part, regulated by the LT pathway.
Figure 7.

Montelukast inhibited mucus metaplasia induced by Foxa2 deletion. Montelukast treatment from PN5–PN15 significantly inhibited Spdef, Foxa3, Muc5ac, and Agr2 mRNAs in lungs of Foxa2-deficient mice. Data represent means ± SEMs (n = 5 for each genotype) according to quantitative RT-PCR. *P < 0.05, versus control values determined by the Student t test (two-tailed).
Discussion
Lung formation and epithelial-cell differentiation are regulated by a complex network of transcription factors, in which TTF-1, GATA-6, and the Forkhead transcription factors FOXA1, FOXA2, FOXF1, and FOXJ1 play important roles (20). Functional analyses of the regulatory regions of several lung-specific genes demonstrated the important role of Foxa2 in regulating the transcription of genes that influence lung morphogenesis and homeostasis (21, 22). The deletion of Foxa2 in respiratory epithelial cells caused spontaneous pulmonary Th2 inflammation after birth, in association with the increased expression of Th2 cell–associated cytokines and chemokines (3). Moreover, the conditional expression of Foxa2 inhibited goblet-cell metaplasia during allergen sensitization in adult mice (3). In the present study, we found that the production of CysLTs and Alox15 were increased during spontaneous pulmonary Th2 inflammation after the deletion of Foxa2. The LT pathway was activated as early as PN5 in the lungs of Foxa2 gene–deleted mice. the activation of LTs and other components of the lipoxygenase pathway preceded the increased expression of the Th2 cytokines, IL-4, IL-5, and IL-13, during development. Foxa2 directly regulated the transcription of Alox15 and Alox5, key enzymes for lipid-derived mediators. Inhibition of the CysLT signaling pathway attenuated the Th2 airway inflammation induced by Foxa2 deletion, without affecting IL-13. Lastly, splenocytes were not activated in the Th2-mediated pulmonary inflammation caused by Foxa2 deletion. Our results indicate that Foxa2 is required for the inhibition of the LT signaling pathway in the developing lung, and that spontaneous activation of the LT pathway is involved in the Th2-mediated pulmonary inflammation resulting from Foxa2 gene deletion in the airway epithelium.
The LT pathway plays an important role in asthma Th2-mediated pulmonary inflammation. Our study demonstrated that the deletion of Foxa2 in airway epithelium activated the LT pathway in the lung, as indicated by the increased expression of Alox5, FLAP, and LTC4 s mRNAs on PN15. The increased expression of Alox15 and the production of 12-HETE indicate that other components of the lipoxygenase pathway were involved in the Th2-mediated pulmonary inflammation caused by Foxa2 deletion. Activation of the lipoxygenase pathway occurred as early as PN5, before the increased expression of Th2 cytokines. Alox15 promoter activity was inhibited, whereas the Alox5 promoter was inhibited by Foxa2 in vitro. Together, these data indicate that Foxa2 is required in the airway epithelium during lung development to regulate the lipoxygenase pathway. Activation of the lipoxygenase pathway may induce Th2-mediated pulmonary inflammation via an increased production of LTs, and other lipoxygenase products that recruit inflammatory cells into the lungs enhance Th2 cytokine expression and mucus production (23, 24). The finding that inhibition of the CysLT1 receptor with montelukast inhibited Th2 inflammation after the deletion of Foxa2 demonstrates the important role of the LT pathway in this Th2-mediated inflammation. Together, these data indicate that both the respiratory epithelium and the lipoxygenase signaling pathway influence Th2-mediated inflammation during postnatal development of the lung.
CysLTs have a clearly defined role in asthma, perpetuating airway inflammation, smooth muscle constriction, and mucus production, and causing airflow obstruction. CysLTs also increase vascular permeability, and contribute to airway remodeling in murine asthma models (23). LTs promote allergic airway inflammation through regulating the expression of Th2 cytokines in animal models (25). Furthermore, LTE4 and LTD4 stimulate the production of Th2 cytokines in human Th2 cells (26). In the Ltc4 snull mice, the production of IL-4, IL-5, and IL-13 after allergen stimulation was reduced (12), indicating that CysLTs play a role in inducing Th2 cytokine expression. Th2 cytokines regulate CysLT1 receptor expression and signaling (26). In human lung macrophages, IL-13 increased CysLT synthesis (11), and IL-13 also enhanced the expression of the principal receptor for CysLTs, CysLT1 (26). In our study, the loss of Foxa2 in lung epithelial cells increased both Th2 cytokine and CysLT production. LTs promote the production of Th2 cytokines, and Th2 cytokines may activate the LT pathway. The finding that increased LTs preceded Th2 cytokine expression supports the concept that the suppression of LTs by Foxa2 is a critical determinant of pulmonary Th2 polarization in this model.
Montelukast decreased the expression of IL-4, IL-5, eotaxin-2, and RANTES, but did not alter IL-13 expression. The decreased expression of Th2 cytokines and chemokines after montelukast treatment is likely to result from inhibition of the LT pathway. Surprisingly, IL-13 in BALF was increased during montelukast treatment. A possible mechanism underlying these findings may be related to the effect of montelukast inhibition of the LT pathway in inflammatory cells expressing IL-5, IL-4, eotaxin-2, and RANTES. The continued expression of IL-13 by subsets of cells lacking the CysLT1 receptor may explain the continued expression of IL-13. IL-13 exerts pleiotropic effects in the lung, including its central role in the development of airway hyperresponsiveness and tissue remodeling (27). IL-13 increased in lungs of patients with asthma (24). IL-13 promotes Th2 inflammatory cell recruitment and airway hypersecretion characteristic of asthma pathology (28, 29). IL-13 mediates its effects by binding to the IL-13 receptor, which is a multimeric complex made up of IL-4Rα and IL-13Rα1 (30). Eosinophilic inflammation in Foxa2Δ/Δ mice was significantly inhibited by the anti–IL-4Rα antibody (3), indicating that the IL-4Rα pathway plays a key role in this Th2-mediated pulmonary inflammation. Our present findings suggest that Th2 cytokine activation occurs after the up-regulation of the LT pathway, supporting the concept that IL-4Rα activation may take place downstream from the LTs in this animal model. The inhibition of LTs inhibits IL-13–mediated bronchopulmonary hyperreactivity, inflammation, and mucosal metaplasia (24, 31), supporting the concept that many effects of IL-13 are mediated through the LT pathway in the setting of allergic asthma.
The loss of Foxa2 in respiratory epithelium enhanced myeloid dendritic-cell (DC) recruitment and activation (3). However, the mechanisms underlying this recruitment remain unknown. Aeroallergen exposure is routinely used to induce Th2 pulmonary inflammation in mice. Splenic cells were activated by antigen stimulation (32). In the present study, splenic lymphocytes were not activated in Foxa2Δ/Δ mice, indicating that the inflammation was limited to the lungs. Because LTs regulate DC activation, migration, and function (33), the activation of DCs in Foxa2∆/∆ mice may result from spontaneous activation of the LT pathway in the absence of Foxa2.
Among the proinflammatory lipid mediators derived from arachidonic acid, LTs are considered to be among the most important inflammatory mediators involved in the pathogenesis of asthma (23). In the developing lung, we demonstrated that the conditional deletion of Foxa2 in respiratory epithelial cells up-regulated the LT pathway and other lipoxygenases, supporting a direct role for Foxa2 in regulating lipid metabolism during postnatal development of the lung. Alox15 promoter activity was directly inhibited by Foxa2. Because Alox15 was found to promote Th2-mediated pulmonary inflammation (15), increased Alox15 expression may promote Th2-mediated pulmonary inflammation after Foxa2 deletion in airway epithelium. Although Alox5 expression increased after Foxa2 deletion in airway epithelial cells, Foxa2 increased the transcription of Alox5 in vitro. This result indicates that the increased expression of Alox5 after Foxa2 deletion may not be regulated directly by Foxa2. Alternatively, the regulation of Alox5 gene expression is dependent on cell type and regulatory elements not present in the Alox5 gene promoter used in the present study.
Antagonists for the CysLT1 receptor have been developed for the treatment of Th2 inflammation diseases, including asthma. Montelukast was recommended for the treatment of mild to severe asthma by the Global Initiative for Asthma (34, 35). In previous studies, Foxa2 expression was decreased in lung tissue from patients with asthma (36). Foxa2 suppressed goblet-cell metaplasia in allergen-sensitized mice (3), and in the present study, the spontaneous activation of the LT pathway was found in Th2-mediated pulmonary inflammation caused by the deletion of Foxa2 in the airway epithelium during lung development. Lung inflammation and goblet-cell metaplasia were inhibited by montelukast. We conclude that the LT pathway plays an important role in Th2-mediated pulmonary inflammation during lung development.
Footnotes
This work was supported by National Natural Science Foundation of China grants 30871118, 30971325, 81270129 (F.L.), and 1107263 (X.J.L.), by Department of Science and Technology of Sichuan Province grants 09ZQ026-020 and 2009SZ0190 (F.L.), and by National Institutes of Health grant HL095580 (J.A.W.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0122OC on July 3, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Roongapinun S, Oh SY, Wu F, Panthong A, Zheng T, Zhu Z. Role of SHIP-1 in the adaptive immune responses to aeroallergen in the airway. PLoS ONE. 2010;5:e14174. doi: 10.1371/journal.pone.0014174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Wan H, Luo F, Zhang L, Xu Y, Lewkowich I, Wills-Karp M, Whitsett JA. Foxa2 programs Th2 cell–mediated innate immunity in the developing lung. J Immunol. 2010;184:6133–6141. doi: 10.4049/jimmunol.1000223. [DOI] [PubMed] [Google Scholar]
- 4.Maeda Y, Chen G, Xu Y, Haitchi HM, Du L, Keiser AR, Howarth PH, Davies DE, Holgate ST, Whitsett JA. Airway epithelial transcription factor NK2 homeobox 1 inhibits mucous cell metaplasia and Th2 inflammation. Am J Respir Crit Care Med. 2011;184:421–429. doi: 10.1164/rccm.201101-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okunishi K, Peters-Golden M. Leukotrienes and airway inflammation. Biochim Biophys Acta. 2011;1810:1096–1102. doi: 10.1016/j.bbagen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa Y, Calhoun WJ. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol. 2006;118:789–800. doi: 10.1016/j.jaci.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Montuschi P, Peters-Golden ML. Leukotriene modifiers for asthma treatment. Clin Exp Allergy. 2010;40:1732–1741. doi: 10.1111/j.1365-2222.2010.03630.x. [DOI] [PubMed] [Google Scholar]
- 8.Dahlén SE. Pharmacological characterization of leukotriene receptors. Am J Respir Crit Care Med. 2000;161:S41–S45. doi: 10.1164/ajrccm.161.supplement_1.ltta-9. [DOI] [PubMed] [Google Scholar]
- 9.Toda A, Yokomizo T, Shimizu T. Leukotriene B4 receptors. Prostaglandins Other Lipid Mediat. 2002;68-69:575–585. doi: 10.1016/s0090-6980(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, Kanaoka Y, Feng C, Nocka K, Rao S, Boyce JA. Cutting edge: interleukin 4–dependent mast cell proliferation requires autocrine/intracrine cysteinyl leukotriene–induced signaling. J Immunol. 2006;177:2755–2759. doi: 10.4049/jimmunol.177.5.2755. [DOI] [PubMed] [Google Scholar]
- 11.Jackson SE, Holloway JW, Warner JA, Sampson AP. Interleukin-13, but not indomethacin, increases cysteinyl–leukotriene synthesis in human lung macrophages. J Allergy (Cairo) 2012;2012:348741. doi: 10.1155/2012/348741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al-Garawi A, Lora JM, Lam BK, Austen KF, et al. Cysteinyl leukotrienes regulate Th2 cell–dependent pulmonary inflammation. J Immunol. 2006;176:4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- 13.Chibana K, Ishii Y, Asakura T, Fukuda T. Up-regulation of cysteinyl leukotriene 1 receptor by IL-13 enables human lung fibroblasts to respond to leukotriene C4 and produce eotaxin. J Immunol. 2003;170:4290–4295. doi: 10.4049/jimmunol.170.8.4290. [DOI] [PubMed] [Google Scholar]
- 14.Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, Whitsett JA. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci USA. 2004;101:14449–14454. doi: 10.1073/pnas.0404424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson CK, Claesson HE, Rydell-Törmänen K, Swedmark S, Hällgren A, Erjefält JS. Mice lacking 12/15-lipoxygenase have attenuated airway allergic inflammation and remodeling. Am J Respir Cell Mol Biol. 2008;39:648–656. doi: 10.1165/rcmb.2007-0443OC. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharjee A, Mulya A, Pal S, Roy B, Feldman GM, Cathcart MK. Monocyte 15–lipoxygenase gene expression requires ERK1/2 MAPK activity. J Immunol. 2010;185:5211–5224. doi: 10.4049/jimmunol.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Profita M, Sala A, Bonanno A, Siena L, Ferraro M, Di Giorgi R, Montalbano AM, Albano GD, Gagliardo R, Gjomarkaj M. Cysteinyl leukotriene–1 receptor activation in a human bronchial epithelial cell line leads to signal transducer and activator of transcription 1–mediated eosinophil adhesion. J Pharmacol Exp Ther. 2008;325:1024–1030. doi: 10.1124/jpet.107.131649. [DOI] [PubMed] [Google Scholar]
- 18.Meliton AY, Munoz NM, Leff AR. Blockade of avidity and focal clustering of beta 2–integrin by cysteinyl leukotriene antagonism attenuates eosinophil adhesion. J Allergy Clin Immunol. 2007;120:1316–1323. doi: 10.1016/j.jaci.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 19.Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso WV. Transcription factors and pattern formation in the developing lung. Am J Physiol. 1995;269:L429–L442. doi: 10.1152/ajplung.1995.269.4.L429. [DOI] [PubMed] [Google Scholar]
- 21.Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004;131:953–964. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- 22.Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem. 2005;280:13809–13816. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- 23.Hallstrand TS, Henderson WR., Jr An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010;10:60–66. doi: 10.1097/ACI.0b013e32833489c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elias JA, Lee CG, Zheng T, Shim Y, Zhu Z. Interleukin-13 and leukotrienes: an intersection of pathogenetic schema. Am J Respir Cell Mol Biol. 2003;28:401–404. doi: 10.1165/rcmb.F264. [DOI] [PubMed] [Google Scholar]
- 25.Faith A, Fernandez MH, Caulfield J, Loke TK, Corrigan C, O’Connor B, Lee TH, Hawrylowicz CM. Role of cysteinyl leukotrienes in human allergen-specific Th2 responses induced by granulocyte macrophage–colony stimulating factor. Allergy. 2008;63:168–175. doi: 10.1111/j.1398-9995.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 26.Thivierge M, Stanková J, Rola-Pleszczynski M. IL-13 and IL-4 up-regulate cysteinyl leukotriene 1 receptor expression in human monocytes and macrophages. J Immunol. 2001;167:2855–2860. doi: 10.4049/jimmunol.167.5.2855. [DOI] [PubMed] [Google Scholar]
- 27.Finkelman FD, Hogan SP, Hershey GKK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 29.Barnes PJ.Current and future therapies for airway mucus hypersecretion Novartis Found Symp 2002248237–253.277–282 [PubMed] [Google Scholar]
- 30.Wills-Karp M, Chiaramonte M. Interleukin-13 in asthma. Curr Opin Pulm Med. 2003;9:21–27. doi: 10.1097/00063198-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Vargaftig BB, Singer M. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. Am J Respir Cell Mol Biol. 2003;28:410–419. doi: 10.1165/rcmb.2002-0032OC. [DOI] [PubMed] [Google Scholar]
- 32.Feng MJ, Shi F, Qiu C, Peng WK. MicroRNA-181a, -146a and -146b in spleen CD4+ T lymphocytes play proinflammatory roles in a murine model of asthma. Int Immunopharmacol. 2012;13:347–353. doi: 10.1016/j.intimp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Harizi H, Gualde N. Eicosanoids: an emerging role in dendritic cell biology. Arch Immunol Ther Exp (Warsz) 2004;52:1–5. [PubMed] [Google Scholar]
- 34.Bousquet J, Demoly P, Humbert M. Montelukast in guidelines and beyond. Adv Ther. 2009;26:575–587. doi: 10.1007/s12325-009-0038-1. [DOI] [PubMed] [Google Scholar]
- 35.Schlick W, Pohl W, Pfeiffer KP, Aigner K, Forche G, Kneussl M, Zwick H. Evaluation of 3–5 months’ add-on therapy with montelukast in patients with non-controlled asthma in Austria: the STAR open-label, real-world, observational study. Curr Med Res Opin. 2010;26:561–570. doi: 10.1185/03007990903523021. [DOI] [PubMed] [Google Scholar]
- 36.Park SW, Verhaeghe C, Nguyenvu LT, Barbeau R, Eisley CJ, Nakagami Y, Huang X, Woodruff PG, Fahy JV, Erle DJ. Distinct roles of Foxa2 and Foxa3 in allergic airway disease and asthma. Am J Respir Crit Care Med. 2009;180:603–610. doi: 10.1164/rccm.200811-1768OC. [DOI] [PMC free article] [PubMed] [Google Scholar]