Abstract
Systemic sclerosis (SSc) is a systemic autoimmune disease that causes inflammation, vasculopathy, and fibrosis of the skin and internal organs. One of the most severe complications of SSc involves the development of pulmonary fibrosis. Endothelial cell injury precedes the development of fibrosis, and is believed to be an initiating event. Therefore, we aimed to characterize the role of endothelial cells in the progression of pulmonary fibrosis, using a well-established bleomycin (BLM) model of pulmonary fibrosis. Endothelial cells were isolated by cell sorting, and the analysis of gene expression was performed with quantitative RT-PCR. Endothelial injury was induced between the first and second week, as shown by the elevated expression of the vascular injury markers matrix metallopeptidase–12 and von Willebrand factor. After injury, endothelial activation was indicated by the up-regulation of selectins, CCL chemokines, and inflammatory mediators, including complement anaphylatoxin receptors (C3aR and C5aR), oncostatin M, and leukemia inhibitory factor. The endothelial cell overexpression of fibrotic mediators, including connective tissue growth factor, plasminogen activator inhibitor–1, osteopontin, fibronectin, and fibroblast specific protein–1, was observed in the second and fourth weeks. This study suggests that endothelial cells actively contribute to the disease process via multiple mechanisms, including the recruitment of inflammatory cells and the establishment of a profibrotic environment during the development of BLM-induced pulmonary fibrosis.
Keywords: bleomycin, endothelial cells, pulmonary fibrosis, systemic sclerosis, idiopathic pulmonary fibrosis
Clinical Relevance
Endothelial cell injury is considered an initiating event in the pathogenesis of scleroderma (SSc), but the role of injured endothelium in the development of SSc interstitial lung disease remains poorly understood. In this work, we demonstrate the up-regulation of numerous inflammatory and profibrotic genes in pulmonary endothelial cells in response to subcutaneously administered bleomycin. This study suggests that endothelial cells actively contribute to pulmonary fibrosis via multiple mechanisms, including the recruitment of inflammatory cells and the establishment of a profibrotic environment.
Systemic sclerosis (SSc) is a progressive autoimmune disease characterized by vascular abnormalities, immune dysfunction, and fibrosis of the skin and internal organs (1). The pathogenesis of SSc is not completely understood, but is believed to be initiated by an early and persistent damage to the endothelium (2). Patients with SSc manifest symptoms of endothelial dysfunction, including irreversible structural changes because of remodeling, functional abnormalities controlling vascular tone, and altered permeability (2). In addition, the apoptosis of endothelial cells seems to play an early and important role in the initiation of inflammation and the eventual activation of fibroblasts, leading to an excess deposition of interstitial collagens (3).
Bleomycin (BLM)–induced pulmonary fibrosis is the most commonly used animal model in rodents to study interstitial lung disease (ILD), including SSc-mediated ILD and idiopathic pulmonary fibrosis (IPF). The subcutaneous administration of BLM represents a clinically relevant model of chronic lung injury, with many histological features similar to those of pulmonary fibrosis in SSc patients, including mononuclear cell infiltration and patchy interstitial fibrosis (4). Similar to human ILD, fibrotic areas in animals treated with subcutaneous BLM develop subpleural and perivascular lesions, where fibrotic areas in the intratracheal instillation are localized predominantly to peribronchial and peribronchiolar areas (4). Similar to SSc, endothelial cells are believed to be a major target of BLM-induced injury when administered via intravenous or subcutaneous routes. Bleomycin can induce apoptosis in cultured endothelial cells (5), but whether endothelial cells undergo apoptosis after BLM treatment in vivo has not been clearly shown, except for one report where subendothelial blebbing was described (6). Although endothelial cells are widely accepted as a primary target of the drug, the extent to which endothelial cells contribute to BLM-induced pulmonary inflammation and fibrosis, and the extent of vasculopathy in this model, are debated.
Endothelial cells may contribute in several ways to the development of tissue fibrosis, involving inflammatory roles and interactions with fibroblasts. Activated endothelial cells are known to secrete cytokines and profibrotic mediators such as transforming growth factor–β (TGF-β), connective tissue growth factor/CCN family member 2 (CTGF/CCN2), and plasminogen activator inhibitor–1 (PAI-1), which directly recruit and activate fibroblasts to produce collagen. The direct treatment of endothelial cells with BLM in vitro has been shown to induce the secretion of certain profibrotic mediators (7, 8), but little is known about the effects of BLM on endothelial cells in vivo. Moreover, recent studies have suggested that a process known as the endothelial-to-mesenchymal transition (EndMT) may be activated during fibrotic processes, creating fibroblast-like cells that contribute to excess collagen production (8, 9). Finally, the activation of endothelial cells may contribute to prolonged tissue injury by promoting a proinflammatory environment. The expression of adhesion molecules and chemokines at the vascular wall contribute to leukocyte homing and the extravasation of cells at sites of inflammation. Bleomycin has been shown to increase the expression of chemokines (10, 11) and adhesion molecules (10, 11) in endothelial cells in vitro. With the exception of a few studies (12–14), very few efforts have been undertaken to confirm the proinflammatory effects of BLM on endothelial cells in vivo.
The activation of the immune system contributes to persistent tissue damage, causing the eventual activation of fibroblasts and the overproduction of collagen through a poorly understood mechanism (15). Macrophages are central to the pathogenesis of ILD, infiltrating the lung in response to tissue injury, or damaging agents such as bleomycin. In the lung, they contribute further to tissue damage by secreting cytokines, lipid mediators, and reactive oxygen species. This creates a vicious cycle of tissue damage and repair, eventually resulting in fibrosis (16). Understanding the crosstalk between endothelial cells and other cell types implicated in the pathogenesis of fibrosis, including immune cells and fibroblasts, is vital to understanding and treating SSc-mediated ILD.
The purpose of this study was to determine the role of endothelial cells in BLM-induced pulmonary fibrosis. We isolated endothelial cells from lung tissue after BLM injury by cell sorting, and used gene expression analysis to determine the role these cells play in the pathogenesis of pulmonary fibrosis. We observed an increased expression of numerous genes, including genes related to endothelial cell injury, inflammation, and fibrosis, suggesting the active participation of endothelial cells during the progression of pulmonary fibrosis.
Materials and Methods
Reagents
Several antibodies were obtained from BD Pharmingen (San Jose, CA), including Fc block rat anti-mouse CD16/CD32 (1:100), Allophycocyanin (APC) rat anti-mouse CD45 (1:100), R-phycoerythrin (PE) rat anti-mouse CD31 (1:100), and rat anti-mouse macrophage antigen-3 (MAC3) (1:50). Rabbit anti-human von Willebrand factor (vWF; 1:1,200) was purchased from Dako. Several reagents were purchased from Vector (Burlingame, CA), including antigen unmasking solution (catalogue number H-3300), rabbit serum (catalogue number S-5000), biotinylated rabbit anti-rat, an Avidin/Biotinylated Enzyme Complex (ABC) kit, and a diaminobenzidine (DAB) Kit.
BLM Subcutaneous Injection Model
Fibrosis was induced in 8- to 12-week-old male C57BL/6 mice (Charles River Laboratories, Wilmington, MA) by a daily subcutaneous injection with 100 μl of bleomycin (1 mg/ml; Enzo, Farmingdale, NY) or saline for 7, 14, or 28 days. Animals were killed 24 hours after the final treatment. All experiments were performed according to guidelines of the Institutional Animal Care and Use Committee at Boston University.
Tissue Collection and Histology
For histology, the right lung was tied off and saved in RNA Later (Life Technologies, Grand Island, NY). The left lung was fixed by tracheal perfusion with 4% paraformaldehyde, and transferred into 70% ethanol. Samples were dehydrated in a series of ethanol and xylene, and embedded in paraffin. For histology, sections were stained with hematoxylin and eosin or Gomori trichrome, or used for immunohistochemistry.
Immunohistochemistry
Tissue sections were deparaffinized and rehydrated. Antigen retrieval was performed in citrate buffer. The Fc receptor was blocked for 30 minutes, and endogenous peroxidase activity was blocked by incubating slides for 30 minutes in 0.3% H2O2. Slides were incubated in 0.1 M glycine for 45 minutes at 4°C (Mac3 only). Blocking was performed in 3% rabbit serum (Mac3) or 3% goat serum (vWF) for 1 hour, and incubated overnight with primary antibody. Slides were incubated in the secondary antibody for 30 minutes. For visualization, the ABC and DAB substrate kits were used according to the manufacturer’s protocol (Vector), and counterstained in hematoxylin.
Flow Cytometry and Cell Sorting
Lung tissue was digested by mechanical disruption, followed by enzymatic digestion for 1 hour at 37°C in a 0.01% collagenase I (Worthington Biochemical Corp., Lakewood, NJ)/Dispase (STEMCELL Technologies, Vancouver, BC, Canada) solution with CaCl2, passed through a 70-μm filter, centrifuged, and resuspended in buffer. Cells were incubated in the Fc block, followed by immunostaining for 30 minutes with APC-labeled CD45 and PE-labeled CD31. Cells were washed and sorted by flow cytometry to separate the endothelial (CD45− CD31+) and immune (CD45+ CD31−) cell populations. In a separate experiment, cells were sorted by the methods described, using APC-labeled CD11c and PE-labeled CD11b for the staining of macrophage and dendritic subpopulations (17).
For the isolation of RNA, cells were lysed in RLT buffer (Qiagen, Valencia, CA) with the QIAshredder column (Qiagen). RNA was isolated with an RNeasy Mini Kit (Qiagen), and real-time quantitative RT-PCR was performed as will be described.
Real-Time Quantitative PCR
Each total lung was homogenized and RNA was extracted by column purification, using the RNeasy Mini Kit (Qiagen). One microgram of RNA was reverse-transcribed, using random hexamers. Endothelial cells were sorted, followed by a cDNA amplification step using the Ovation PicoSL WTA V2 kit (NuGEN Technologies, San Carlos, CA). Quantitative PCR was performed using SYBR Green (Applied Biosystems, Grand Island, NY) with cDNA in triplicate, and with β-actin as the internal control. The primers are listed in Table 1. Results were normalized to a saline-injected lung, and fold change was determined using the ΔΔCt method. Statistics are reported as the results of a two-tailed, unpaired Student t test.
TABLE 1.
PRIMERS FOR REAL-TIME QUANTITATIVE PCR (5′–3′)
| Forward | Reverse | |
|---|---|---|
| β-actin | AAGGCCAACCGTGAAAAGAT | GTGGTACGACCAGAGGCATAC |
| Col1a1 | GCCAAGAAGACATCCCTGAAG | TGTGGCAGATACAGATCAAGC |
| Col1a2 | GCCACCATTGATAGTCTCTCC | CACCCCAGCGAAGAACTCATA |
| Col3a1 | TTTGTGCAAGTGGAACCTG | TGGACTGCTGTGCCAAAATA |
| Col5a1 | GGACTAGTCCGCTTTCCCTGTCAACTTGTCCGATGG | GTGGTCACTGCGGCTGAGGAACTTC |
| Col5a2 | CAGAAGCCCAGACGTATCG | GGTGGTCAGGCACTTCAGAT |
| PAI-1 | AGGATCGAGGTAAACGAGAGC | GCGGGCTGAGATGACAAA |
| CTGF | TGACCTGGAGGAAAACATTAAGA | AGCCCTGTATGTCTTCACACTG |
| TGF-β1 | TGGAGCAACATGTGGAACTC | CAGCAGCCGGTTACCAAG |
| MCP-1/CCL2 | CATCCACGTGTTGGCTCA | GATCATCTTGCTGGTGAATGAGT |
| EMR1 | CCTGGACGAATCCTGTGAAG | GGTGGGACCACAGAGAGTTG |
| vWF | CCAAGGAGGGTCTGCAACT | AAAGGAAGACTCTGGCAAGCTA |
| MMP12 | TGATGCTGTCACAACAGTGG | GTAATGTTGGTGGCTGGACTC |
| ICAM-1 | CCCACGCTACCTCTGCTC | GATGGATACCTGAGCATCACC |
| VCAM | TGGTGAAATGGAATCTGAACC | CCCAGATGGTGGTTTCCTT |
| E-selectin | TCCTCTGGAGAGTGGAGTGC | GGTGGGTCAAAGCTTCACAT |
| P-selectin | ATGCCTGGCTACTGGACACT | GACTGAGCATAGGGGCACA |
| CD34 | GGGTAGCTCTCTGCCTGATG | TCCGTGGTAGCAGAAGTCAA |
| CXCL1 | AGACTCCAGCCACACTCCAA | TGACAGCGCAGCTCATTG |
| CXCL2 | AAAATCATCCAAAAGATACTGAACAA | CTTTGGTTCTTCCGTTGAGG |
| CCL3 | TGCCCTTGCTGTTCTTCTCT | GTGGAATCTTCCGGCTGTAG |
| CCL6 | TCTTTATCCTTGTGGCTGTCC | TGGAGGGTTATAGCGACGAT |
| CCL7 | TTCTGTGCCTGCTGCTCATA | TTGACATAGCAGCATGTGGAT |
| CCL9 | TGGGCCCAGATCACACAT | CCCATGTGAAACATTTCAATTTC |
| C3aR | GTGGCTCGCAGATCATCA | AAGACTCCATGGCTCAGTCAA |
| C5aR | GCATCCGTCGCTGGTTAC | TGCTGTTATCTATGGGGTCCA |
| IL-6 | GCTACCAAACTGGATATAATCAGGA | CCAGGTAGCTATGGTACTCCAGAA |
| OSM | TGCTCCAACTCTTCCTCTCAG | CAGGTTTTGGAGGCGGATA |
| LIF | AAACGGCCTGCATCTAAGG | AGCAGCAGTAAGGGCACAAT |
| Fibronectin | TGGGTCTGAGTACACCGTGA | GTGGAATGGAGCGCAGAG |
| FSP-1 | GGAGCTGCCTAGCTTCCTG | TCCTGGAAGTCAACTTCATTGTC |
| OPN | CCCGGTGAAAGTGACTGATT | TTCTTCAGAGGACACAGCATTC |
Definition of abbreviations: C3aR, complement 3a receptor; C5aR, complement 5a receptor; Col, collagen; FSP-1, fibroblast specific protein–1; CTGF, connective tissue growth factor; LIF, leukemia inhibitory factor; MCP-1, monocyte chemotactic protein–1; CCL2, chemokine (C-C motif) ligand 2; EMR1, EGF-like module containing, mucin-like, hormone receptor-like sequence 1; MMP12, matrix metallopeptidase–12; ICAM-1, intercellular adhesion molecule 1; OPN, osteopontin; OSM, oncostatin-M; PAI-1, plasminogen activator inhibitor–1; TGF-β, transforming growth factor–β; VCAM, vascular cell adhesion molecule 1; vWF, von Willebrand factor.
Results
Subcutaneous Injection of Bleomycin Induces Inflammation and Extensive Pulmonary Fibrosis
Mice were subjected to a daily subcutaneous administration of BLM, and tissue was first analyzed for evidence of inflammation and fibrosis. A decline in health of the BLM-treated animals was evidenced by progressive weight loss, showing a significant difference from the saline control group by the first week, with this significant difference sustained into the fourth week of the experiment. Further evidence of pulmonary inflammation was indicated by histology of the lungs, using a standard hematoxylin and eosin stain. Lungs from BLM mice showed thickened alveolar walls and increased cellularity by Day 14 because of the infiltration of immune cells (18) (see Figures E1A and E1B in the online supplement).
Fibrosis of lung tissue was demonstrated using Gomori trichrome stain as a standard measure for collagen deposition. Fibrotic areas were observed as early as Day 14 in BLM-treated mice. Fibrotic regions were substantially larger by Day 28, and showed a complete obliteration of the alveolar compartment (Figure E1C). Interstitial collagens (Col1a1, Col1a2, Col3a1, Col5a1, and Col5a2) were quantified by quantitative PCR (Figure E1D). All collagen chains were found to be significantly increased at both 2 and 4 weeks. As a confirmation of the profibrotic environment of BLM lungs, the mRNA expressions of profibrotic mediators PAI-1, CTGF, and TGF-β were analyzed. The expression of PAI-1 was marginally increased in the total tissue, showing a significant effect at 2 weeks (Figure E1E). Changes in CTGF were not observed in BLM mice after 4 weeks (Figure E1F), whereas TGF-β1 was moderately increased at 4 weeks in total tissue (Figure E1G; P = 0.066). These results confirm the presence of pulmonary inflammation and fibrosis in a chronic model of lung injury after systemic BLM administration.
Macrophages Are Recruited to Tissue by 2 Weeks in Subcutaneous BLM Lung Injury
Fibrosis is believed to be the result of a response to injury, with macrophage recruitment as a central cell type in inflammation-driven fibrosis (15). We investigated the infiltration of macrophages to the lung after injury by BLM, and aimed to determine the role of endothelial cells in their recruitment. Chemokine (C-C motif) ligand 2 (CCL2)/monocyte chemotactic protein–1 (MCP-1) is a main chemotactic factor for macrophage recruitment (19), and was significantly increased at the mRNA level in tissue from BLM-injured lungs, 2 weeks after treatment (Figure 1A). CCL2/MCP-1 expression steadily increased from the first week, reaching the highest induction during the fourth week. As an additional measure, we quantified the gene expression of a marker expressed on macrophages, EGF-like module containing, mucin-like, hormone receptor-like sequence 1 (EMR1), which was significantly elevated in total lung homogenate from BLM-treated mice during the fourth week (Figure 1B), suggesting macrophage infiltration. Immunohistochemistry for the Mac3 antigen, a general macrophage marker, was used to observe the presence and localization of macrophages in tissue (20). Macrophages were observed within the alveolar and interstitial spaces of BLM-treated mice by the second week, and macrophages increased further in number by the fourth week (Figure 1C). As a quantitative measure of macrophage infiltration, total cell counts from a single-cell suspension revealed changes in immune cell populations within the lung by FACS. A significant increase was evident in absolute numbers (Figure 1D) and percentages (Figure 1E) of CD11b+ CD11c− cells (interstitial macrophages) and CD11b+ CD11c+ cells (dendritic cells) after 2 and 4 weeks of BLM treatment. Conversely, CD11b− CD11c+ cells (alveolar macrophages) were decreased at both time points (Figures 1D and 1E and Table 2). Together, the quantitative RT-PCR of macrophage markers, immunohistochemistry, and FACS data show that there is an increase in macrophage numbers in BLM-injured lungs.
Figure 1.
Subcutaneous bleomycin (BLM) injection induces the infiltration of macrophages. The presence of macrophages in the lung was analyzed by the quantitative RT-PCR of total tissue extract for monocyte chemotactic protein–1 (MCP-1)/chemokine (C-C motif) ligand 2 (CCL2) (A) and EGF-like module containing, mucin-like, hormone receptor-like sequence 1 (EMR1) (B). Relative expression was normalized to normal (saline-injected) lung tissue. Macrophage numbers were increased in tissue in 2- and 4-week BLM mice, as indicated by the immunohistochemistry of macrophage antigen-3 (MAC3) (C) shown at high magnification, with nuclei stained by hematoxylin (×200; scale bar = 50 μm). Flow-cytometric counts of cell populations from lungs of PBS, and 2- and 4-week BLM mice were determined by the FACS sorting of alveolar macrophages (CD11b−CD11c+), interstitial macrophages (CD11b+CD11c−), and dendritic cells (CD11b+CD11c+). Cell counts represent a total count from one full lung (D), and representative dot plots show cell populations in percentages, taken from a sampling of 10,000 cells (E). A two-tailed, unpaired Student t test compared BLM samples to saline-injected samples at the same time-point (n = 3–4). *P ≤ 0.05, **P ≤ 0.01. Bleo, bleomycin; wk, weeks.
TABLE 2.
SUMMARY OF CELL POPULATIONS IN FACS SORTING
| CD11b− CD11c+ | CD11b+ CD11c− | CD11b+ CD11c+ | |
|---|---|---|---|
| PBS 2 wk | 6.78 ± 1.29 | 10.47 ± 2.47 | 1.84 ± 0.73 |
| PBS 4 wk | 4.02 ± 2.86 | 5.17 ± 1.95 | 0.54 ± 0.36 |
| BLM 2 wk | 2.00 ± 0.27‡ | 25.98 ± 13.82* | 2.89 ± 1.09 |
| BLM 4 wk | 0.53 ± 0.19* | 22.90 ± 7.85† | 4.76 ± 1.24† |
Definition of abbreviation: BLM, bleomycin.
Data are represented as percentages (n = 3–4).
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
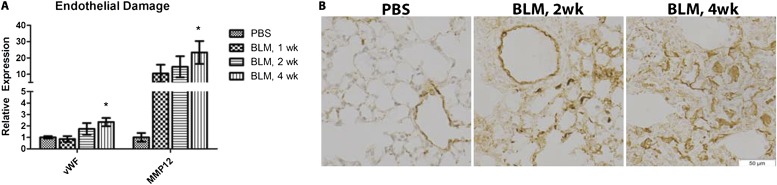
Endothelial Cells Are Damaged in Response to the Subcutaneous Administration of BLM
Endothelial injury and apoptosis are believed to contribute to the pathogenesis of fibrotic skin and lung disease (2, 21). Much of what is known about the role of endothelial cells in fibrotic disease comes from in vitro studies or from serum markers in human fibrotic diseases. The role of endothelial cells in the commonly used BLM mouse model of pulmonary fibrosis has not been extensively characterized. We hypothesized that subcutaneously administered BLM would target endothelial cells, leading to damage. To determine this, mice were injected with BLM or PBS for 1 week, 2 weeks, or 4 weeks, and endothelial cells were collected from the lung by FACS cell sorting, using the markers CD31 and CD45. The mRNA was isolated for gene expression analysis of damage markers. vWF has long been implicated as a marker for endothelial damage, and is up-regulated in the serum of patients with SSc (22). In endothelial cells from BLM lungs, the expression of vWF was elevated at 2 weeks, reaching statistical significance at 4 weeks (Figure 2A). To better understand the distribution of increased vWF expression at the protein level in endothelial cells, lung tissue was stained according to immunohistochemistry (Figure 2B). The expression of vWF was predominantly localized around the blood vessels of lungs from saline-injected mice, with minor interstitial staining observed in some samples. After 2 weeks of BLM treatment, vWF expression was enhanced around the blood vessels and within the interstitium. As a second marker of endothelial injury, we analyzed matrix metallopeptidase (MMP)–12. MMP12 is increased in the skin and serum during SSc, and correlates with vascular damage and pulmonary fibrosis (23). MMP12 is most highly expressed in macrophages, but was shown to be induced in many different cell types, including endothelial cells, during SSc-ILD (23). In isolated endothelial cells from the lungs of BLM-injected mice, we observed a marked up-regulation in MMP12, which was significant at 4 weeks (Figure 2A). These data suggest that endothelial cells are damaged in this model of chronic lung injury, similar to the endothelial damage associated with SSc (22, 23).
Figure 2.
Evidence of endothelial injury by BLM injection in mice. mRNA was isolated from FACS-sorted pulmonary endothelial cells (CD31+CD45−) of 1-, 2-, and 4-week BLM-injected or saline-injected animals. (A) The gene expressions of vascular injury markers von Willebrand factor (vWF) and matrix metalloproteinase–12 (MMP12) were quantified by quantitative RT-PCR. Data showing fold differences were normalized to endothelial cells from normal (saline-injected) lungs. (B) Vascular injury was confirmed by the immunohistochemistry of vWF in saline-injected and 2- and 4-week BLM lung sections. Representative images are shown at high magnification, with nuclei stained by hematoxylin (×200; scale bar = 50 μm). A two-tailed, unpaired Student t test compared endothelial cells from BLM lungs with endothelial cells from saline-injected lungs (n = 4). *P ≤ 0.05.
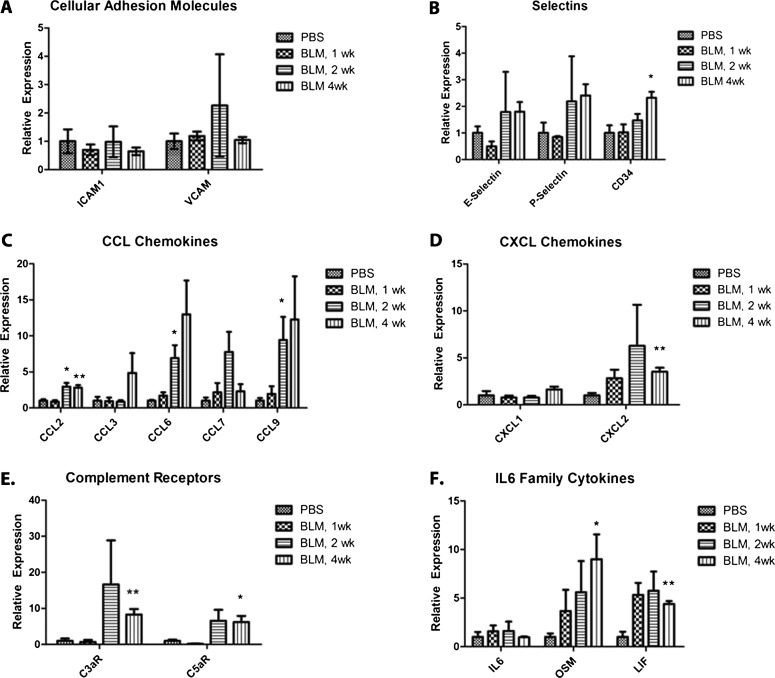
Activation of Endothelium Contributes to Inflammation and Macrophage Recruitment in Subcutaneous BLM Lung Injury
The activation of endothelial cells plays an important role in the recruitment of immune cells to sites of tissue injury. The recruitment of immune cells involves a process of rolling, binding, and extravasation through the vascular wall. This process is mediated by adhesion molecules on the vascular wall. To determine whether endothelial cells contributed to the infiltration of macrophages (Figure 1), the expression of chemokines and adhesion molecules was quantified in isolated pulmonary endothelial cells from 1-, 2-, and 4-week BLM-injected or PBS-injected animals, using quantitative RT-PCR. Previous reports have shown that selectins are up-regulated in vitro and acutely in vivo (11, 13, 14) in response to BLM. We show an increased trend in the selectins, with CD34, a ligand for L-selectin, significantly increased after 4 weeks of treatment (Figure 3B). Interestingly, the transcriptional level of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM1) were both unchanged after BLM treatment (Figure 3A), despite previous reports (10, 12). Along with adhesion molecules, chemokines provide important signals to recruit leukocytes to sites of inflammation (24).
Figure 3.
Endothelial cells recruit monocytes and perpetuate inflammation. mRNA isolated from FACS-sorted pulmonary endothelial cells (CD31+CD45−) of 1-, 2-, and 4-week BLM-injected or saline-injected animals was used to compare the gene expression of inflammatory mediators. The endothelial expression of mediators important for monocyte recruitment was quantified in terms of cellular adhesion molecules (A), selectins (B), CCL chemokines (C), and CXCL chemokines (D). The endothelial cell contribution to the inflammatory response was shown by the up-regulated expression of complement receptors (E) and the IL-6 family of cytokines (F). Relative expression is quantified by the ΔΔCt method, and represented as fold change, normalized to endothelial cells from normal a (saline-injected) lung. A two-tailed, unpaired Student t test compared endothelial cells from BLM lungs with endothelial cells from saline-injected lungs (n = 4). *P ≤ 0.05, **P ≤ 0.01. C3aR, complement 3a receptor; C5aR, complement 5a receptor; ICAM1, intercellular adhesion molecule 1; LIF, leukemia inhibitory factor; OSM, oncostatin-M; VCAM, vascular cell adhesion molecule 1.
The expression of chemokines has been noted in the serum of patients with SSc, including CCL2, CCL3, CCL4, and CXCL8 (25). In a separate study, the expression of CCL2 was correlated with pulmonary fibrosis in SSc (26). We observed a significant induction of CCL2, a strong chemotactic factor for macrophages, at both 2 weeks and 4 weeks. Other CCL chemokines (CCL3, CCL6, CCL7, and CCL9) were all elevated at 4 weeks, whereas CCL6 and CCL9 were significantly increased at 2 weeks (Figure 3C). Although chemokine expression is considered redundant, the CCL family is importantly involved in monocyte recruitment, whereas the CXC family is typically important in neutrophil recruitment (27). CXCL1 and CXCL2 have also been shown to be increased in BLM lung fibrosis (28). Although CXCL2 was elevated between 1 and 4 weeks, and significantly increased at 4 weeks in endothelial cells from the lungs of BLM-treated mice (Figure 3D), the fold-change was much lower than that of the CCL chemokine family.
To investigate further the inflammatory properties of endothelial cells after BLM injury, we investigated the regulation of receptors for complement 3a (C3a) and complement 5a (C5a) anaphylatoxins, which have been shown to up-regulate chemotactic factors synergistically in response to an inflammatory stimulus such as IL-6 (29). After BLM lung injury, complement 3a receptor (C3aR) and complement 5a receptor (C5aR) were elevated at 2 weeks, reaching statistical significance during the fourth week (Figure 3E). In vitro studies have shown synergistic activation of the anaphylatoxin receptors with IL-6 activation (29), although IL-6 was not elevated in the endothelial cells in our system (Figure 3F). Within the IL-6 family, we observed an increased trend in oncostatin-M (OSM) and leukemia inhibitory factor (LIF), which were both significant during the fourth week. Together, the elevation of adhesion molecules, chemokines, anaphylatoxin receptors, and cytokines show the participation of endothelial cells in inflammation during the progression of BLM-induced lung injury.
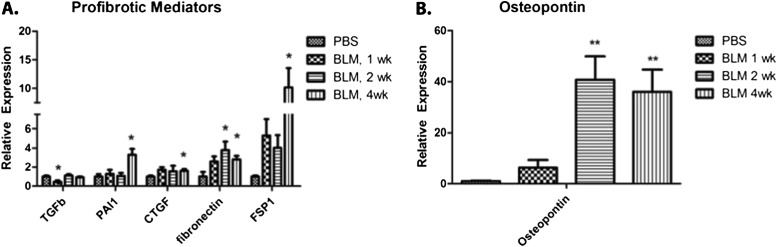
Endothelial Cells Contribute to a Sustained Profibrotic Environment in Chronic Lung Injury
In vitro, endothelial cells have been shown to secrete profibrotic mediators such as TGF-β, PAI-1, and CTGF, which induce fibroblast growth, differentiation, and the synthesis of collagen (7, 8). Recent evidence has also shown that endothelial cells may become fibroblast-like and secrete collagen through the process of EndMT. To determine the extent to which endothelial cells contribute to the fibrotic process, we analyzed the gene expression of fibrotic mediators and EndMT markers in isolated pulmonary endothelial cells from BLM-treated mice. The expression of TGF-β1 mRNA was reduced at 1 week, but no difference in expression was evident at 2 and 4 weeks, compared with endothelial cells from PBS-injected mice. The gene expression of PAI-1 in endothelial cells was significantly increased at 4 weeks in the lungs of BLM-treated mice. CTGF was elevated during the first week, reaching significance during the fourth week (Figure 4A). Consistent with previous reports (9), markers for EndMT, fibronectin and fibroblast specific protein–1 (FSP-1), were both elevated in endothelial cells after BLM injury (Figure 4A). Fibronectin was elevated early, reaching significance during the second and fourth weeks, and FSP-1 was also elevated early, achieving significance during the fourth week. Interestingly, osteopontin (OPN), a profibrotic and proinflammatory molecule, was highly induced during the second and fourth weeks after BLM injury (Figure 4B). Together, increases in CTGF, PAI-1, OPN, and EndMT markers in endothelial cells from BLM-treated lungs suggest that endothelial cells contribute to the profibrotic milieu and sustain a local profibrotic environment.
Figure 4.
Endothelial expression of profibrotic mediators in response to BLM in vivo. mRNA isolated from FACS-sorted pulmonary endothelial cells (CD31+CD45−) of 1-, 2-, and 4-week BLM-injected or saline-injected animals was used to compare the gene expression of profibrotic mediators. The expression of secreted mediators (CTGF, connective tissue growth factor; PAI-1, plasminogen activator inhibitor–1; and TGF-β1, transforming growth factor–β), endothelial-to-mesenchymal transition markers (Fn, fibronectin; and FSP-1, fibroblast-specific protein–1) (A), and the profibrotic and proinflammatory mediator osteopontin (B) were quantified by the ΔΔCt method, and are represented as fold change, normalized to endothelial cells from a saline-injected lung. A two-tailed, unpaired Student t test compared endothelial cells from BLM lungs with endothelial cells from saline-injected lungs (n = 4). *P ≤ 0.05, **P ≤ 0.01.
Discussion
Although skin fibrosis in SSc is generally believed to be caused by injury to the endothelium, little is known about the involvement of endothelial cells in SSc-ILD, as well as in IPF. Our goal was to characterize the response of pulmonary endothelial cells to the subcutaneous administration of BLM, a model for both SSc-ILD and IPF. We found that endothelial cells expressed elevated levels of vWF and MMP12, indicating sustained cell injury in response to BLM administration. The activation of endothelial cells was evidenced by increases in the endothelial expression of selectins and chemokines, which correlated with an increased number of interstitial macrophages in lung tissue. Endothelial cells further contributed to the inflammatory process through the elevated expression of complement receptors and IL-6 family cytokines. In addition, the elevated expression of profibrotic mediators and EndMT markers indicated the direct involvement of endothelial cells in the fibrotic response.
Currently, little is known about the in vivo role of endothelial cells during BLM lung injury. Reports have cited changes in adhesion molecules (11, 13, 14), cytokines (10, 11, 30), vascular mediators (31), and profibrotic molecules (7, 8) after the in vitro treatment of endothelial cells with BLM, but many of these mediators were not verified in vivo. Furthermore, the presence of cytokines and chemokines in bronchiolar lavage fluid and serum has been reported in patients and in animals treated with BLM, but the cellular origin of these mediators is either unknown or assumed. In our study, we used a methodology that can accurately detect the regulation of genes in an isolated cell population. Although the confirmation of endothelial localization in human lung biopsies would be beneficial for the markers described, our method would not be possible in human samples. Human samples often present difficulties in obtaining proper controls, and samples are often not suitable for cell isolation, which must be performed on fresh tissues. Both of these problems are overcome by using animal models of disease, providing insights into the early manifestations of a disease process, mechanistic data, and the identification of novel targets. An area of future exploration will include the confirmation of novel targets in fixed human tissue samples from a library of healthy and fibrotic tissues.
Although endothelial cells undergo apoptosis when treated with BLM in vitro (6), the role of apoptosis has been debated in scleroderma (2). In our model, we did not detect a significant amount of apoptosis, either via the expression of apoptotic markers in isolated endothelial cells, or by cleaved caspase 3 staining in the lung (data not shown). On the other hand, endothelial injury was evident. Endothelial injury has been indicated by increased circulating vWF in patients with scleroderma (32) and by increases in angiotensin-converting enzyme in either the serum or BAL of mice exposed to BLM (31). Because vWF initiates thrombus formation in response to denudation of the vascular wall, its expression is induced upon injury to endothelial cells, and is likely a protective mechanism (33). We observed an increase of vWF expression in endothelial cells, suggesting injury to the endothelium. In a recent report by Manetti and colleagues (23), increased MMP12 in the serum of patients with scleroderma correlated with the severity of pulmonary fibrosis and vascular injury. Consistent with this, we found that MMP12 was up-regulated in endothelial cells from mice treated with BLM. Thus, our data suggest that in this subcutaneous BLM model, inflammation and fibrosis are primarily driven by endothelial injury.
In addition to skin and organ fibrosis, a major characteristic of scleroderma involves vascular dysfunction (2, 3, 34). The overexpression of endothelin-1 and the down-regulation of endothelial nitric oxide synthase (eNOS) in endothelial cells have been suggested to trigger abnormal vasoconstriction, contributing to Raynaud’s phenomenon and pulmonary hypertension in patients with SSc. Park and colleagues (35) reported an increase in total levels of endothelin-1 (ET-1), localized to the airway epithelium and inflammatory cells, but not endothelial cells. Endothelin-converting enzyme–1 (ECE-1) was also reportedly increased in the airway epithelium, Type II pneumocytes, and endothelium, but not in immune cells. In our study, we found a slight decrease in ECE-1 and no changes in the expression of ET-1 or eNos in endothelial cells from BLM lungs (data not shown). Also observed during scleroderma vascular dysfunction are the loss of vascular endothelial (VE)-cadherin, gaps between endothelial cells, and the loss of vascular integrity (2, 36). Contrary to these changes in the vasculature of humans with SSc, we found minimal changes in adhesion molecules known to maintain vascular integrity, including Claudin 5, VE-cadherin, platelet/endothelial cell adhesion molecule 1 (PECAM1)/CD31, and junctional adhesion molecule 1 (JAM1) (data not shown). We conclude that little evidence existed for changes in the expression of genes regulating the integrity of the vascular barrier or in vascular tone, although we cannot exclude possible changes in the expression or subcellular localization of the corresponding proteins.
As a measure of endothelial cell activation and interaction with the immune system, we measured the expression of adhesion molecules and chemokines in endothelial cells. The overexpression of adhesion molecules (E-selectin, P-selectin, and CD34) and chemokines (CXCL1, CXCL2, CCL2, CCL3, CCL6, CCL7, and CCL9) suggests that endothelial cells are activated and contribute to the homing of immune cells to sites of injury. Although others have reported increases in E-selectin and P-selectin after BLM injury (11, 13, 14), the induction of selectins is typically shown to be induced early. Serrano-Mollar and colleagues (14) showed P-selectin induction after 1 hour, and the P-selectin returned to basal levels after 3 hours. Our study showed a prolonged induction of P-selectin, E-selectin, and CD34. Further evidence of endothelial activation and immune system involvement is found in the induction of members of the IL-6 family of cytokines, LIF and OSM. Although evidence indicates that IL-6 is elevated in SSc (37), our experiments found no evidence of its induction in endothelial cells. Interestingly, OSM has been shown to contribute to the prolonged activation of P-selectin in endothelial cells in vitro (38), and was reportedly increased in the serum and BAL from patients with ILD (39). Together, the prolonged expression of the selectin family of adhesion molecules and CC chemokines, which typically recruit monocytes (19), suggests an important role of endothelial cells in monocyte recruitment after BLM.
The up-regulation of complement receptors further suggests that endothelial cells are activated after BLM treatment in vivo. Endothelial cells have been shown to constitutively express low levels of C5aR, which is induced further after the treatment of endothelial cells in vitro with proinflammatory cytokines, including LPS, IL-6, or IFN-γ (29). The activation of complement has been noted in many autoimmune and inflammatory diseases (40), and the depletion of complement has been shown to attenuate BLM-induced pulmonary fibrosis in mice (41). In SSc, activated complement and the increased expression of C5aR have been shown in skin biopsies (42), and an increased expression of complement components has been shown in serum (43). Evidence from Laudes and colleagues (29) suggests that the activation of C5aR in endothelial cells provides a proinflammatory signal, working synergistically with proinflammatory cytokines such as IL-6. The costimulation of endothelial cells in culture with IL-6 and C5a led to an enhanced production of MCP-1/CCL2 and macrophage inflammatory protein–2/CXCL2. In our study, we also observed the increased expression of many CC chemokines, including MCP-1/CCL2, suggesting that the up-regulation and engagement of C5aR and C3aR may enhance the inflammatory response.
In SSc, endothelial cells are believed to contribute to the development of skin fibrosis, but little is known about the role of endothelial cells in pulmonary fibrosis. In vitro studies have shown that endothelial cells secrete many profibrotic mediators, and among these, TGF-β (7, 8), CTGF (8), and PAI-1 (44) are up-regulated after the stimulation of endothelial cells with BLM in vitro. According to our experiments, CTGF was elevated in endothelial cells between the first and fourth weeks after BLM stimulation, and was significantly elevated during the fourth week. In addition, Pai-1 was also significantly increased during the fourth week. PAI-1 is the main inhibitor of fibrinolysis, and is speculated to play an additional role in tissue fibrosis through the inhibition of MMP activity and modulation of the inflammatory response (45). These data suggest that endothelial cells may contribute to the proliferation and activation of nearby fibroblasts or pericytes in vivo.
We found that OPN was markedly elevated within 1 week in pulmonary endothelial cells exposed to BLM. OPN is a profibrotic cytokine with many functions in fibrosis and inflammation. It is up-regulated in the serum of patients with IPF (46) and SSc (47) and in fibroblasts, macrophages, and epithelial cells of SSc dermal biopsies (46, 47). OPN−/− mice treated with BLM were resistant to skin (47) and lung (48) fibrosis. Reduced fibrosis has been attributed to the effects of OPN on fibroblasts or macrophages. Macrophages secrete large amounts of TGF-β during fibrosis, an effect that is greatly attenuated in OPN-deficient macrophages (47). Furthermore, OPN induces migration and proliferation in fibroblasts (46, 47). Although a profibrotic role of OPN in endothelial cells has not been described, the secretion of OPN may exert a significant effect on surrounding cell types.
As an additional mechanism of endothelial cell contributions to fibrosis, endothelial cells may take on a fibroblast-like phenotype through EndMT, and contribute to the secretion and deposition of collagen in tissue (9). To test this, we measured the expression of fibronectin (Fn) and FSP-1, both markers for EndMT. The increased expression of these markers supports the conclusions of Hashimoto and colleagues (9), who used lineage tracing studies to determine the role of EndMT during pulmonary fibrosis. Fibronectin is also a component of the extracellular matrix, and is secreted at areas of injury. Increased Fn expression has been shown to precede the development of pulmonary fibrosis in rats treated with BLM (49). In our system, Fn was elevated in endothelial cells early, and was significantly increased by the second week. Interestingly, fibronectin has a well-known function in wound healing and fibrosis, and may contribute to fibroblast adhesion, contraction, and motility (50). This suggests that a local change in the profibrotic milieu of endothelial cells after injury contributes directly to the fibrotic process, possibly accounting for a sustained and self-perpetuating fibrotic response.
In conclusion, these results suggest that endothelial cells facilitate the development of BLM-induced fibrosis through several different mechanisms, contributing to both inflammatory and fibrotic processes, continuing into late-stage disease and potentially perpetuating injury and repair. The up-regulation of genes involved in vascular injury, activation, inflammation, and fibrosis suggests that the crosstalk of endothelial cells, immune cells, and fibroblasts is important in the development of tissue injury and fibrosis.
Footnotes
This work was supported by National Institutes of Health grants AR042334 (M.T.) and T32 AR007598 (R.H.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0152OC on July 25, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Varga JA, Trojanowska M. Fibrosis in systemic sclerosis. Rheum Dis Clin North Am. 2008;34:115–143. doi: 10.1016/j.rdc.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pattanaik D, Brown M, Postlethwaite AE. Vascular involvement in systemic sclerosis (scleroderma) J Inflamm Res. 2011;4:105–125. doi: 10.2147/JIR.S18145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manetti M, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M. Mechanisms in the loss of capillaries in systemic sclerosis: angiogenesis versus vasculogenesis. J Cell Mol Med. 2010;14:1241–1254. doi: 10.1111/j.1582-4934.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison JH, Jr, Lazo JS. High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther. 1987;243:1185–1194. [PubMed] [Google Scholar]
- 5.Mungunsukh O, Griffin AJ, Lee YH, Day RM. Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L696–L703. doi: 10.1152/ajplung.00322.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamson IY. Pulmonary toxicity of bleomycin. Environ Health Perspect. 1976;16:119–126. doi: 10.1289/ehp.7616119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phan SH, Gharaee-Kermani M, Wolber F, Ryan US. Stimulation of rat endothelial cell transforming growth factor–beta production by bleomycin. J Clin Invest. 1991;87:148–154. doi: 10.1172/JCI114964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Q, Nan HY, Zhang WH, Yan LF, Cui GB, Huang XF, Wei JG. Pulmonary microvascular endothelial cells from bleomycin-induced rats promote the transformation and collagen synthesis of fibroblasts. J Cell Physiol. 2011;226:2091–2102. doi: 10.1002/jcp.22545. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, Hasegawa Y. Endothelial–mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43:161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fichtner F, Koslowski R, Augstein A, Hempel U, Rohlecke C, Kasper M. Bleomycin induces IL-8 and ICAM-1 expression in microvascular pulmonary endothelial cells. Exp Toxicol Pathol. 2004;55:497–503. doi: 10.1078/0940-2993-00345. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto H, Sugawara I, Azuma A, Saito Y, Kohno N, Kudoh S. Differential secretion of cytokines and adhesion molecules by HUVEC stimulated with low concentrations of bleomycin. Cell Immunol. 2002;219:73–81. doi: 10.1016/s0008-8749(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 12.Sato N, Suzuki Y, Nishio K, Suzuki K, Naoki K, Takeshita K, Kudo H, Miyao N, Tsumura H, Serizawa H, et al. Roles of ICAM-1 for abnormal leukocyte recruitment in the microcirculation of bleomycin-induced fibrotic lung injury. Am J Respir Crit Care Med. 2000;161:1681–1688. doi: 10.1164/ajrccm.161.5.9907104. [DOI] [PubMed] [Google Scholar]
- 13.Azuma A, Takahashi S, Nose M, Araki K, Araki M, Takahashi T, Hirose M, Kawashima H, Miyasaka M, Kudoh S. Role of E-selectin in bleomycin induced lung fibrosis in mice. Thorax. 2000;55:147–152. doi: 10.1136/thorax.55.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano-Mollar A, Closa D, Cortijo J, Morcillo EJ, Prats N, Gironella M, Panes J, Rosello-Catafau J, Bulbena O. P-selectin upregulation in bleomycin induced lung injury in rats: effect of N-acetyl-l-cysteine. Thorax. 2002;57:629–634. doi: 10.1136/thorax.57.7.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleret A, Quesnel-Hellmann A, Mathieu J, Vidal D, Tournier JN. Resident CD11c+ lung cells are impaired by anthrax toxins after spore infection. J Infect Dis. 2006;194:86–94. doi: 10.1086/504686. [DOI] [PubMed] [Google Scholar]
- 18.Rydell-Tormanen K, Andreasson K, Hesselstrand R, Risteli J, Heinegard D, Saxne T, Westergren-Thorsson G. Extracellular matrix alterations and acute inflammation: developing in parallel during early induction of pulmonary fibrosis. Lab Invest. 2012;92:917–925. doi: 10.1038/labinvest.2012.57. [DOI] [PubMed] [Google Scholar]
- 19.Bandinelli F, Del Rosso A, Gabrielli A, Giacomelli R, Bartoli F, Guiducci S, Matucci Cerinic M. CCL2, CCL3 and CCL5 chemokines in systemic sclerosis: the correlation with SSc clinical features and the effect of prostaglandin E1 treatment. Clin Exp Rheumatol. 2012;30(Suppl. 71):S44–S49. [PubMed] [Google Scholar]
- 20.Ho MK, Springer TA. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem. 1983;258:636–642. [PubMed] [Google Scholar]
- 21.Magro CM, Waldman WJ, Knight DA, Allen JN, Nadasdy T, Frambach GE, Ross P, Marsh CB. Idiopathic pulmonary fibrosis related to endothelial injury and antiendothelial cell antibodies. Hum Immunol. 2006;67:284–297. doi: 10.1016/j.humimm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Kahaleh MB, Osborn I, LeRoy EC. Increased Factor VIII/von Willebrand factor antigen and von Willebrand factor activity in scleroderma and in Raynaud’s phenomenon. Ann Intern Med. 1981;94:482–484. doi: 10.7326/0003-4819-94-4-482. [DOI] [PubMed] [Google Scholar]
- 23.Manetti M, Guiducci S, Romano E, Bellando-Randone S, Conforti ML, Ibba-Manneschi L, Matucci-Cerinic M. Increased serum levels and tissue expression of matrix metalloproteinase–12 in patients with systemic sclerosis: correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann Rheum Dis. 2012;71:1064–1072. doi: 10.1136/annrheumdis-2011-200837. [DOI] [PubMed] [Google Scholar]
- 24.Dixit N, Simon SI. Chemokines, selectins and intracellular calcium flux: temporal and spatial cues for leukocyte arrest. Front Immunol. 2012;3:188. doi: 10.3389/fimmu.2012.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codullo V, Baldwin HM, Singh MD, Fraser AR, Wilson C, Gilmour A, Hueber AJ, Bonino C, McInnes IB, Montecucco C, et al. An investigation of the inflammatory cytokine and chemokine network in systemic sclerosis. Ann Rheum Dis. 2011;70:1115–1121. doi: 10.1136/ard.2010.137349. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa M, Sato S, Takehara K. Augmented production of chemokines (monocyte chemotactic protein–1 (MCP-1), macrophage inflammatory protein–1alpha (MIP-1alpha) and MIP-1beta) in patients with systemic sclerosis: MCP-1 and MIP-1alpha may be involved in the development of pulmonary fibrosis. Clin Exp Immunol. 1999;117:159–165. doi: 10.1046/j.1365-2249.1999.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wynn TA. Fibrotic disease and the T(h)1/T(h)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J, Jung Y, Tighe RM, Xie T, Liu N, Leonard M, Gunn MD, Jiang D, Noble PW. A macrophage subpopulation recruited by CC chemokine ligand–2 clears apoptotic cells in noninfectious lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L933–L940. doi: 10.1152/ajplung.00256.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laudes IJ, Chu JC, Huber-Lang M, Guo RF, Riedemann NC, Sarma JV, Mahdi F, Murphy HS, Speyer C, Lu KT, et al. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol. 2002;169:5962–5970. doi: 10.4049/jimmunol.169.10.5962. [DOI] [PubMed] [Google Scholar]
- 30.Karmiol S, Remick DG, Kunkel SL, Phan SH. Regulation of rat pulmonary endothelial cell interleukin-6 production by bleomycin: effects of cellular fatty acid composition. Am J Respir Cell Mol Biol. 1993;9:628–636. doi: 10.1165/ajrcmb/9.6.628. [DOI] [PubMed] [Google Scholar]
- 31.Day RM, Yang Y, Suzuki YJ, Stevens J, Pathi R, Perlmutter A, Fanburg BL, Lanzillo JJ. Bleomycin upregulates gene expression of angiotensin-converting enzyme via mitogen-activated protein kinase and early growth response 1 transcription factor. Am J Respir Cell Mol Biol. 2001;25:613–619. doi: 10.1165/ajrcmb.25.5.4521. [DOI] [PubMed] [Google Scholar]
- 32.Blann AD, Illingworth K, Jayson MI. Mechanisms of endothelial cell damage in systemic sclerosis and Raynaud’s phenomenon. J Rheumatol. 1993;20:1325–1330. [PubMed] [Google Scholar]
- 33.Lip GY, Blann A. Von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34:255–265. doi: 10.1016/s0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 34.Guiducci S, Giacomelli R, Cerinic MM. Vascular complications of scleroderma. Autoimmun Rev. 2007;6:520–523. doi: 10.1016/j.autrev.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Park SH, Saleh D, Giaid A, Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156:600–608. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 36.Asano Y, Stawski L, Hant F, Highland K, Silver R, Szalai G, Watson DK, Trojanowska M. Endothelial FLI1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am J Pathol. 2010;176:1983–1998. doi: 10.2353/ajpath.2010.090593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes TC, Anderson ME, Moots RJ.The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis Int J Rheumatol 2011. 2011721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao L, Pan J, Setiadi H, Patel KD, McEver RP. Interleukin 4 or oncostatin M induces a prolonged increase in P-selectin mRNA and protein in human endothelial cells. J Exp Med. 1996;184:81–92. doi: 10.1084/jem.184.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atamas SP, White B. Cytokine regulation of pulmonary fibrosis in scleroderma. Cytokine Growth Factor Rev. 2003;14:537–550. doi: 10.1016/s1359-6101(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 40.Addis-Lieser E, Kohl J, Chiaramonte MG. Opposing regulatory roles of complement factor 5 in the development of bleomycin-induced pulmonary fibrosis. J Immunol. 2005;175:1894–1902. doi: 10.4049/jimmunol.175.3.1894. [DOI] [PubMed] [Google Scholar]
- 41.Phan SH, Thrall RS. Inhibition of bleomycin-induced pulmonary fibrosis by cobra venom factor. Am J Pathol. 1982;107:25–28. [PMC free article] [PubMed] [Google Scholar]
- 42.Sprott H, Muller-Ladner U, Distler O, Gay RE, Barnum SR, Landthaler M, Scholmerich J, Lang B, Gay S. Detection of activated complement complex C5b-9 and complement receptor C5a in skin biopsies of patients with systemic sclerosis (scleroderma) J Rheumatol. 2000;27:402–404. [PubMed] [Google Scholar]
- 43.Benbassat C, Schlesinger M, Luderschmidt C, Valentini G, Tirri G, Shoenfeld Y. The complement system and systemic sclerosis. Immunol Res. 1993;12:312–316. doi: 10.1007/BF02918260. [DOI] [PubMed] [Google Scholar]
- 44.Nuver J, De Haas EC, Van Zweeden M, Gietema JA, Meijer C. Vascular damage in testicular cancer patients: a study on endothelial activation by bleomycin and cisplatin in vitro. Oncol Rep. 2010;23:247–253. [PubMed] [Google Scholar]
- 45.Sisson TH, Simon RH. The plasminogen activation system in lung disease. Curr Drug Targets. 2007;8:1016–1029. doi: 10.2174/138945007781662319. [DOI] [PubMed] [Google Scholar]
- 46.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M, Schneider DJ, Mayes MD, Assassi S, Arnett FC, Tan FK, Blackburn MR, Agarwal SK. Osteopontin in systemic sclerosis and its role in dermal fibrosis. J Invest Dermatol. 2012;132:1605–1614. doi: 10.1038/jid.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berman JS, Serlin D, Li X, Whitley G, Hayes J, Rishikof DC, Ricupero DA, Liaw L, Goetschkes M, O’Regan AW. Altered bleomycin-induced lung fibrosis in osteopontin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1311–L1318. doi: 10.1152/ajplung.00394.2003. [DOI] [PubMed] [Google Scholar]
- 49.Hernnas J, Nettelbladt O, Bjermer L, Sarnstrand B, Malmstrom A, Hallgren R. Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur Respir J. 1992;5:404–410. [PubMed] [Google Scholar]
- 50.Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998;18:1363–1370. doi: 10.1161/01.atv.18.9.1363. [DOI] [PubMed] [Google Scholar]