Abstract
Second-hand smoke (SHS) exposure in utero exacerbates adult responses to environmental irritants. We tested the hypothesis that effects of in utero SHS exposure on modulating physiological and transcriptome responses in BALB/c mouse lungs after ovalbumin (OVA) challenge extend well into adulthood, and that the responses show a sex bias. We exposed BALB/c mice in utero to SHS or filtered air (AIR), then sensitized and challenged all offspring with OVA from 19 to 23 weeks of age. At the end of the adult OVA challenge, we evaluated pulmonary function, examined histopathology, analyzed bronchoalveolar lavage fluid (BALF), and assessed gene expression changes in the lung samples. All groups exhibited lung inflammation and inflammatory cell infiltration. Pulmonary function testing (airway hyperresponsiveness [AHR], breathing frequency [f]) and BALF (cell differentials, Th1/Th2 cytokines) assessments showed significantly more pronounced lung responses in the SHS-OVA groups than in AIR-OVA groups (AHR, f; eosinophils, neutrophils; IFN-γ, IL-1b, IL-4, IL-5, IL-10, IL-13, KC/CXCL1, TNF-α), with the majority of responses being more pronounced in males than in females. SHS exposure in utero also significantly altered lung gene expression profiles, primarily of genes associated with inflammatory responses and respiratory diseases, including lung cancer and lung fibrosis. Altered expression profiles of chemokines (Cxcl2, Cxcl5, Ccl8, Ccl24), cytokines (Il1b, Il6, Il13) and acute phase response genes (Saa1, Saa3) were confirmed by qRT-PCR. In conclusion, in utero exposure to SHS exacerbates adult lung responses to OVA challenge and promotes a pro-asthmatic milieu in adult lungs; further, males are generally more affected by SHS-OVA than are females.
Keywords: second-hand smoke, in utero exposure, mouse asthma model, inflammation, gene regulation
Clinical Relevance
Male and female BALB/c mice were exposed in utero to diluted second-hand smoke (SHS) and then from 19 to 23 weeks of age to ovalbumin (OVA). In utero SHS (1) aggravates airway hyperresponsiveness; (2) increases expression of chemokines, cytokines, and acute phase response genes; and (3) promotes a pro-asthmatic milieu in OVA-exposed adult mice. Furthermore, males are more affected by SHS-OVA than are females.
Second-hand smoke (SHS) exposure in utero aggravates responses to subsequent post-natal exposures to environmental irritants, including house dust mites (1), Aspergillus fumigatus (2) and SHS (3). The most common observation among these studies is increased airway hyperresponsiveness (AHR), a hallmark of allergic asthma.
In the murine model of asthma featuring ovalbumin (OVA) sensitization and challenge, OVA-induced lymphocyte infiltration, elevated Th2 cytokine production, and increased AHR mirror several key characteristics of asthma (4–7). Whether and how in utero exposure to SHS affects responses of adults treated with OVA, and whether there is a sex bias in the responses, are the subjects of the present investigation.
Just as sex differences in response to irritants exist among human patients with asthma (8), studies in animal models have revealed some sex differences in lung responses, particularly those associated with AHR. Male C57BL/6 mice are innately more responsive than females to aerosolized methacholine (9). In BALB/c mice, however, females were reported to be more responsive than males after OVA challenge (10). Whether sex-associated differences also exist among OVA-challenged mice that have been exposed in utero to SHS has not been investigated rigorously. An earlier study in our laboratory showed that in utero SHS exposure mildly aggravated asthma-associated lung responses to OVA exposure in 15-week-old female BALB/c mice (11). We designed the present study to:
-
•
Test whether the effects of in utero SHS exposure were sustained in older mice exposed to OVA;
-
•
Examine in greater detail than previously determined (11) the range and extent of responses to in utero SHS-adult OVA exposure; and
-
•
Determine whether sex differences exist in responses of mice to SHS-OVA.
We exposed pregnant BALB/c mice to HEPA-filtered air (AIR) or to SHS diluted with AIR daily from gestation Days 6–19 and exposed all offspring to an OVA sensitization/challenge protocol from 19 to 23 weeks of age. We assessed the following outcomes: pulmonary function (AHR, breathing frequency [f]), lung histopathology (inflammation, structural changes), bronchoalveolar lavage fluid (BALF) cytokine levels, and lung gene expression patterns, primarily the identification of SHS-related gene pathways.
Materials and Methods
Animal Protocols, SHS, and OVA Exposures
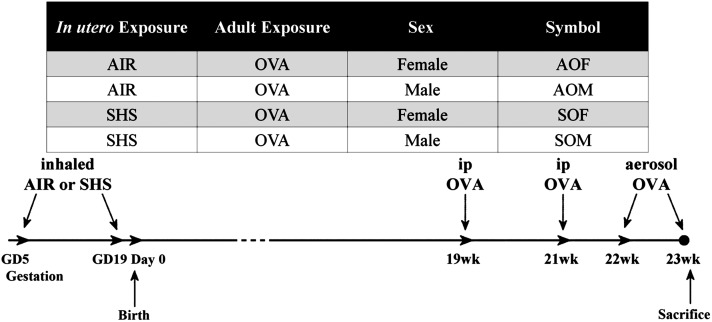
We conducted SHS exposures on BALB/c mice (Harlan, Indianapolis, IN), as described previously (3, 11). Briefly, half of the mated females, randomly selected, were exposed to SHS generated from 3R4F filtered research cigarettes (10 mg/m3; University of Kentucky, Lexington, KY) mixed with AIR daily, from Days 6 to 19 of gestation. The remaining mated females received 100% AIR exposures instead. All offspring were sensitized by injections of OVA (Grade V > 98% pure, 80 µg in 2 ml alum; Sigma-Aldrich, St. Louis, MO) at 19 and 21 weeks of age, followed by OVA aerosol challenge every other day in Weeks 22 and 23, before they were killed. Mice were classified in one of four groups, depending on in utero exposure to SHS (S) or AIR (A), and their sex. All offspring were exposed to OVA. The group designations and exposure timeline are presented in Figure 1. The numbers of animals in each group assessed in each assay are listed with each figure. Mice were housed and handled in accord with the NIH Guide for the Care and Use of Laboratory Animals. All procedures and protocols were approved by the LSU Institutional Animal Care and Use Committee.
Figure 1.
Group designation and exposure timeline. The three-letter symbols were assigned to four experimental groups, depending on the exposure period in utero and adult, plus sex of the mice. Times shown in the exposure timeline are expressed relative to birthdate of offspring. GD, gestation day.
Pulmonary Function Testing
This was performed as described previously (3, 11). For each mouse at each methacholine dose level (3–50 mg/ml), readings over 5 minutes were averaged for Penh and f. Penh values represent the degree of AHR in each animal.
Histopathologic Analysis of Lungs
We followed previously published procedures (3, 11) for BALF collection, fixation, sectioning, staining, and scoring of 3- to 4-µm lung sections, and for lung morphometric analysis (3). Goblet cells and mucin were stained with the routine Periodic acid-Schiff (PAS) stain on 5-µm paraffin-embedded tissue sections from the same blocks as were used in the hematoxylin and eosin stains. Twelve males each from the treatment and control groups were evaluated. Slides were scored for estimated percent of linear mucosa that was occupied by goblet cells and given the following scores: 0 for less than 10%, one for 10% to 33%, two for 34% to 67%, and three for greater than 67%. In addition, lungs were evaluated for the presence (+) or absence (–) of goblet cells in the distal two generations of bronchioles in the peripheral lung.
Cytokine Quantitation in BALF
Mouse Th1/Th2 9-plex kits (MSD; Meso Scale Discovery, Gaithersburg, MD) and ELISA plates for IL-13 were used to measure 10 major Th1/Th2 cytokines (IFN-γ, IL-1b, IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, KC/CXCL1, TNF-α) in BALF.
Statistical Analysis
We used the SAS statistical package (version 9.3; SAS Institute, Inc., Cary, NC) for data analyses. We performed one-way ANOVA and post hoc Tukey’s HSD (honest significant difference) test for multiple pairwise comparisons, including SHS-OVA versus AIR-OVA responses in males and females (* in the figures) as well as SHS-OVA responses in males versus females († in the figures). Statistical differences between SHS-OVA and AIR-OVA with pooled M and F responses (‡ in the figures) were calculated by a t test. In all cases, we considered comparisons significant at P < 0.05. All error bars in the figures indicate means ± SEMs.
Lung Harvest and mRNA Extraction
We followed previously described procedures (3, 11), including RNA sample quantity and purity assessment with a NanoDrop ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE), and further assayed 1:5 dilutions of RNA samples with an Agilent 2100 BioAnalyzer and Agilent RNA 6000 Nano Series II Kits (Agilent Technologies, Palo Alto, CA). All samples fell into the following ranges: 260/280 ratio: 2.14–2.19; 260/230 ratio: 1.91–2.34; concentration: 970–2845 ng/μl; 28S/18S ratio: 1.6–2.0, RNA integrity number: 9.2–10.0.
Microarray Analysis and Ingenuity Pathway Analysis
We assessed global gene expression in lungs of individual 23-week-old mice (four mice per group) on mouse 430.2 genome arrays (Affymetrix, Santa Clara, CA). The arrays were processed at the Research Core Facility of Louisiana State University Health Science Center-Shreveport. We performed two pairwise comparisons on “SOF versus AOF” and “SOM versus AOM” with the limma package (12) in the R/bioconductor platform (www.r-project.org; www.bioconductor.org). Gene probes with at least 2-fold up-/down-regulation and false discovery rate < 0.05 were considered differentially expressed. The microarray data have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO, accession number GSE38409). We performed gene-set functional analyses and generated gene networks with Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, Redwood City, CA; www.ingenuity.com).
Quantitative RT-PCR
Total RNA was reverse-transcribed with the High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Expression levels of selected genes were measured with TaqMan universal PCR master mix (Applied Biosystems) and predesigned Taqman probes for mouse genes (assay ID: Hprt1, Mm00446968_m1; Cxcl2, Mm00436450_m1; Cxcl5, Mm00436451_g1; Ccl8, Mm01297183_m1; Ccl24, Mm00444701_m1; Il1b, Mm00434228_m1; Il6, Mm00446190_m1; Il13, Mm00434204_m1; Il17b, Mm01258783_m1; Saa1, Mm00656927_g1; Saa3, Mm00441203_m1; Timp1, Mm00441818_m1). The gene expression levels were normalized to Hprt1 levels.
Results
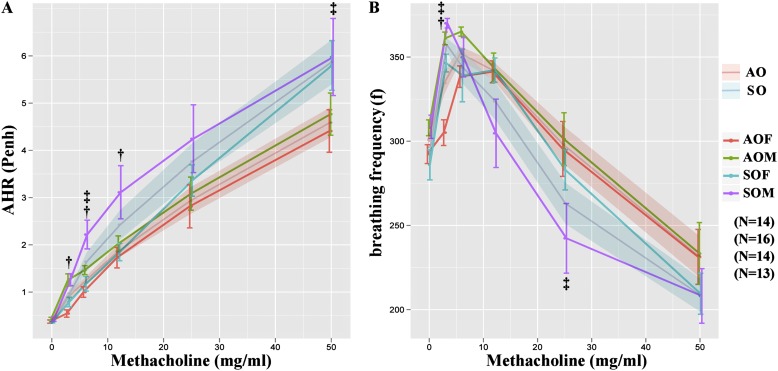
Pulmonary function testing revealed significantly increased AHR and decreased f in the male and female SHS-OVA groups after methacholine challenge, compared with those in AIR-OVA groups (Figure 2). Among all mice exposed in utero to SHS, male offspring exhibited significantly aggravated AHR compared with SHS-exposed females at methacholine doses of 3 to 12 mg/ml (Figure 2A).
Figure 2.
Whole-body plethysmography revealed significantly (A) increased airway hyperresponsiveness (AHR;Penh) and (B) decreased breathing frequency (f) in secondhand smoke–ovalbumin (OVA) mice, compared with all mice from filtered air–OVA groups (‡when we pooled data for females plus males in each exposure set). In addition, there was significantly increased AHR in SOM versus all other groups especially at low methacholine doses (†SOM vs. SOF).
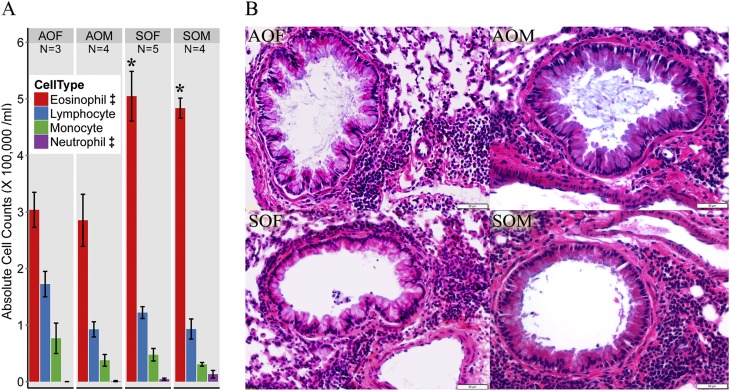
Eosinophil counts in BALF were high for all four groups (Figure 3A). Significant differences were found between SHS-OVA and AIR-OVA for eosinophils and neutrophils. There was a pronounced lung inflammatory response in all four groups (Figure 3B). The high levels of inflammation made it difficult to reliably quantitate the mean linear intercept and radial alveolar count values, which we have used previously to assess lung structural changes (3).
Figure 3.
(A) The eosinophil is the major cell type found in bronchoalveolar lavage fluid for all groups. There were significantly increased eosinophils and neutrophils in the combined SHS-OVA groups compared with the combined filtered air–ovalbumin groups (‡). Significant differences within either sex (SOF vs. AOF and SOM vs. AOM) were also found for eosinophils (*). (B) Lung histology of all four groups revealed severe inflammation with accumulated inflammatory cells in the airways around bronchioles. Scale bar, 50 µm.
We also evaluated percent linear mucosa occupied by goblet cells and presence/absence of goblet cells in the distal two generations of bronchioles in the peripheral lung. We found no differences for either set of measurements between males and females or between SHS- and AIR-exposed mice (see the online supplement).
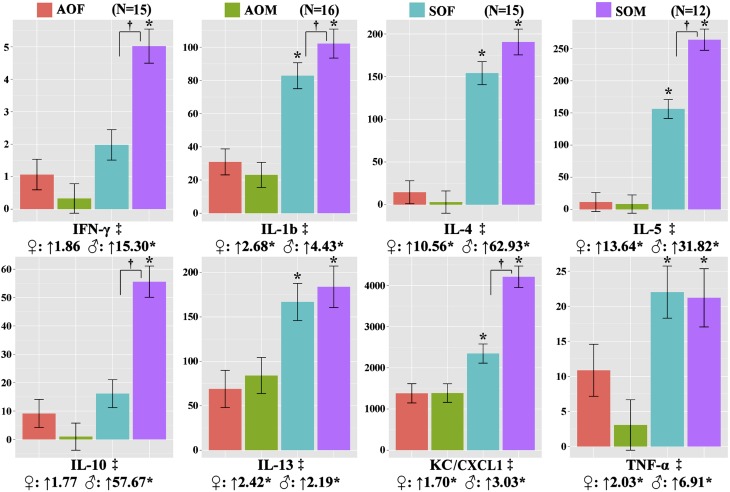
We found significantly elevated cytokine levels in SHS-OVA versus AIR-OVA for eight of the 10 BALF Th1/Th2 cytokines we assessed (both sexes pooled, Figure 4): IFN-γ, IL-1b, IL-4, IL-5, IL-10, IL-13, KC/CXCL1, and TNF-α. IL-2 and IL-12 also were detected but were not significantly different between AIR-OVA and SHS-OVA groups (data not shown). The post-hoc Tukey’s test among all four groups further confirmed the significant differences between males (SOM vs. AOM) for all 8 cytokines and between females (SOF vs. AOF) for five cytokines (IL-1b, IL-4, IL-5, IL-13, KC/CXCL1). Cytokine levels in BALF from the male SHS-OVA offspring were even higher than corresponding levels in SHS-OVA females and significant differences were found for IFN-γ, IL-1b, IL-5, IL-10, and KC/CXCL1.
Figure 4.
Bronchoalveolar lavage fluid cytokine levels determined by ELISA for the eight cytokines listed were increased significantly in secondhand smoke (SHS)-ovalbumin (OVA) groups compared with filtered air–OVA groups (‡). This was also true for all eight cytokine comparisons between the males (*, ♂, SOM vs. AOM) and six out of eight cytokines for females (*, ♀, SOF vs. AOF). In the cases of IFN-γ, IL-1b, IL-5, IL-10, and TNF-α, we found significantly elevated responses in the males compared with the females for mice exposed in utero to SHS (†SOM vs. SOF). Units on y-axes are pg/ml.
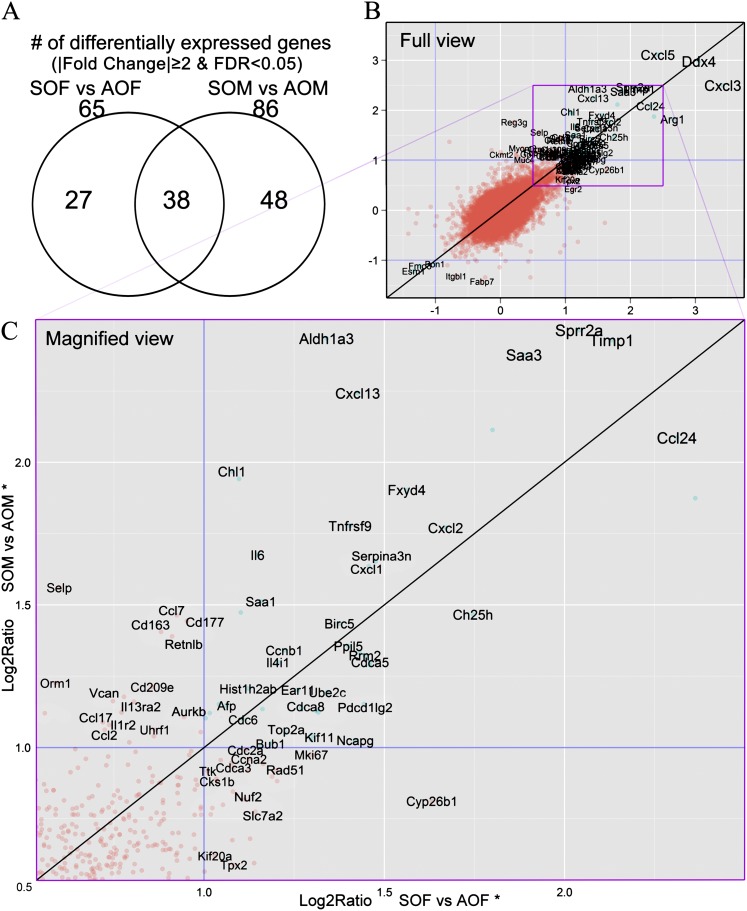
With the differential expression threshold for genes in lung homogenates set to a fold change of 2 and false discovery rate < 0.05, 65 genes in the females and 86 in the males were differentially expressed between SHS-OVA and AIR-OVA mice (see the online supplement), whereas 38 genes were shared by both sexes (Figure 5A). Although the ranges of fold change values are similar for both sexes (fold change, ↓4.0 to ↑11.3; Log2Ratio, −2.0 to +3.5, Figure 5B), not only were there more differentially expressed genes for males than for females (86 vs. 65), the majority of the 38 shared genes exhibited higher fold change values in the males than the females (Figures 5 and 6). This indicated that, on a transcriptional level, males are more responsive than females to OVA as adults after in utero exposure to SHS. This is also evident from Figure 5C (in magnified view, Log2Ratios within 0.5–2.5 range), where there are more genes located above the “y = x” line that delineates the same degree of up-regulation in both males and females. A few down-regulated genes also were identified, including β1-integrin (Itgb1, ♀: ↓1.60; ♂: ↓2.49) and paraoxonase (Pon1, ♀: ↓2.01; ♂: ↓2.10).
Figure 5.
(A) Venn diagram shows numbers of differentially expressed genes in two pairwise comparisons. (B) Transcriptome screening revealed differentially expressed genes by comparing secondhand smoke–ovalbumin (OVA) and filtered air–OVA responses in mice of each sex. We plotted each gene’s Log2 ratios from “SOF versus AOF” and “SOM versus AOM” onto the x-axis and y-axis, respectively. The “y = x” line indicates the same degree of expression changes in both females and males. All gene symbols shown are differentially expressed in either male or female mice (|fold change|≥2, false discovery rate < 0.05; n = 4 mice in each group). (C) Magnified view of box outlined in B.
Figure 6.
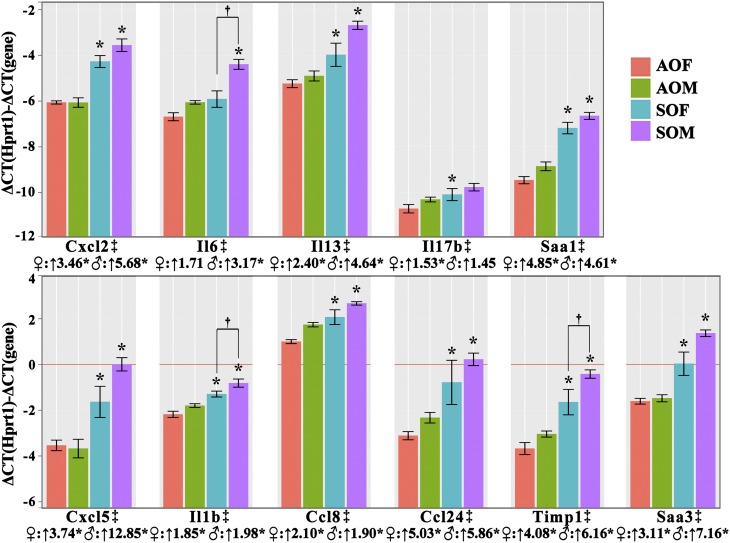
qRT-PCR confirmed differential expression of all 11 genes identified previously by bronchoalveolar lavage fluid cytokine assessment or microarray analysis. The y-axis is –ΔΔCT, so the higher the value, the higher the expression level for each group. The line “y = 0” represents the level of housekeeping gene (Hprt1) expression. Because the y-axis is calculated from cycle number differences, a difference of one on the y axis = a 2-fold difference in expression. Beneath each gene symbol, the fold change differences between SHS-OVA and AIR-OVA in either sex are shown, together with asterisks to indicate statistical differences (P < 0.05). We performed qRT-PCR in each of the four groups (six mice/group) except for Ccl24 (N ≥ 5) and Timp1 (N ≥ 4). In addition, three genes (Il1b, Il6, and Timp1) exhibited significantly increased expression levels (†SOM vs. SOF). Expression levels for all 11 genes were significantly increased in second-hand smoke (SHS)-ovalbumin (OVA) groups compared with filtered air–OVA groups (‡).
We used IPA to illuminate biological responses and signaling pathways consistent with in utero SHS-associated gene expression changes in adult mouse lungs. We focused on the 113 differentially expressed genes found in either or both sexes (Figure 5A, 27 + 38 + 48 = 113). We found the most statistically significant diseases and disorders pathways to be “inflammatory response,” “cancer,” and “respiratory disease” (P ⩽ 7.05E-03). The IL-17 signaling pathways (P ⩽ 8.32E-04) were among the most significant canonical signaling pathways (see the online supplement).
We found significant differences by qRT-PCR (Figure 6) between SHS-OVA and AIR-OVA for all 11 genes initially identified by BALF cytokine assessment (ELISA) or by microarray analysis. For each of the 11 genes, relative expression levels were significantly higher for SOF versus AOF and for SOM versus AOM. These 11 genes include chemokines (Ccl8, Ccl24, Cxcl2, Cxcl5), cytokines (Il1b, Il6, Il13, Il17b) and acute phase response genes (Saa1, Saa3) associated with inflammatory responses. In addition, for 3 of the 11 genes (Il1b, Il6 and Timp1), there were significantly increased expression levels in SOM versus SOF. The ΔΔCT values (compared with the housekeeping gene Hprt1) are seen in Figure 6. A ΔΔCT difference of one represents a 2-fold difference in expression.
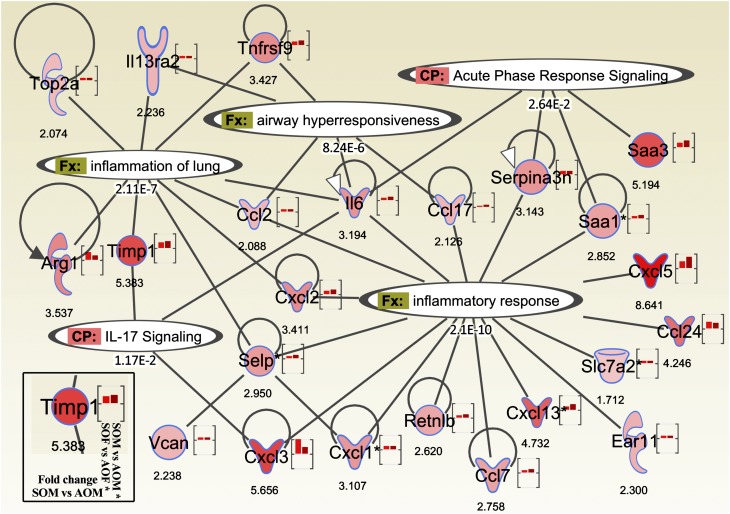
We then created a gene network in IPA by combining the most differentially expressed genes with the most significant biological functions and canonical pathways, according to the Ingenuity Knowledge Base. These functions and pathways were directly associated with inflammatory responses, AHR, acute phase response signaling and IL-17 signaling (Figure 7). The network indicated key information about the gene of interest, including degree of up/down-regulation for either sex (for the two bars in the brackets: females on the left, males on the right), fold change values in males, and each gene’s functional association. With very few exceptions, the left-hand bar (female) is shorter than the right-hand bar (male), further supporting the conclusion that males are more sensitive than females in this SHS-OVA model.
Figure 7.
Ingenuity Pathway Analysis on microarray results identified several significant biological functions (Fx) and canonical pathways (CP) associated with in utero exposure to secondhand smoke in either sex. The color intensity inside each gene symbol and in the right-hand bar inside each bracket is associated with relative fold changes between SOM and AOM (more saturated red = higher degree of up-regulation). The left-hand bar represents relative level of gene up-regulation in SOF versus AOF. Gene abbreviations: Arg1, arginase (liver); Ear11, eosinophil-associated RNase 11; Retnlb, resistin like β; Saa 1/3, serum amyloid A; Selp, selectin (platelet); Serpina3n, serine (or cysteine) peptidase inhibitor, clade A, member 3N; Slc7a2, solute carrier family 7 member 2; Timp1, tissue inhibitor of metalloproteinase 1; Top2a, DNA topoisomerase 2-α; Vcan, versican.
Discussion
This study was designed to investigate the effects of in utero SHS exposure and of sex differences on adult responses of mice under identical OVA challenge conditions in a mouse asthma model. We used BALB/c mice for three reasons: (1) BALB/c mice are a well-accepted strain for asthma studies (4–7, 13); (2) a high correlation has been reported for these mice between Penh values and lung resistance (14–16), and (3) we have established laboratory protocols in BALB/c mice for investigating the effects of in utero SHS exposure on adult lung responses to environmental irritants (3, 11). Control groups without OVA challenge were not included in the study because our previous studies revealed that adult mice exposed in utero to SHS, but not exposed as adults to environmental stressors, do not exhibit signs of inflammation compared with the AIR mice (3, 11).
One consequence of having all groups of adult mice exposed to OVA was that the resulting inflammation masked airway structural changes to which the in utero SHS exposure undoubtedly contributed (3). The large numbers of infiltrated inflammatory cells present in the lungs of both male and female mice in all groups made accurate morphometric assessment unachievable (Figure 3). In contrast, the results from lung function testing, BALF analysis, and gene expression profiling all demonstrated that in utero SHS exposures aggravate responses to adult OVA exposures. In addition, results are more pronounced in male mice than in females.
Lung function testing revealed that there was increased AHR and decreased f in SHS-OVA mice, compared with AIR-OVA mice, regardless of sex, at multiple methacholine doses. The males exposed to SHS in utero were more responsive than females who were exposed similarly (Figure 2). These findings indicate that both SHS in utero and sex differentially affect adult lung responses to inhaled environmental stressors.
The prominent lung responses of OVA-exposed adult males that had been exposed in utero to SHS were supported by the elevated BALF cytokine levels (Figure 4). SHS exposure in utero markedly increased the levels both of pro-inflammatory cytokines (IFN-γ, IL-1b, KC/CXCL1, and TNF-α) and of Th2 cytokines (IL-4, IL-5, IL-10, and IL-13). This is consistent with the OVA-induced mouse model of asthma. The sex-associated differences described here are associated with two other findings: (1) the baseline BALF cytokine levels of male mice were not significantly higher than females; on the contrary, for 6/8 cytokines–IFN-γ, IL-1b, IL-4, IL-5, IL-10, and TNF-α–female mice exhibited slightly higher baseline BALF cytokine levels than the males, although the difference for each of the cytokines did not reach the level of statistical significance; and (2) male mice exposed in utero to SHS had much higher BALF cytokine levels than the females.
Results from transcriptome screening supported the findings from lung function testing and BALF cytokine measurements. We performed transcriptome screening on individual whole lung homogenates collected from four mice/group for each of the four groups, and searched for the differentially expressed genes between SHS-OVA and AIR-OVA in either sex. As shown in Figure 5A, we found more differentially expressed genes for the males (n = 86 genes, SOM vs. AOM) than for the females (n = 65 genes, SOF vs. AOF). Notably, a large portion of the most differentially expressed genes were chemokines and cytokines involved in inflammatory responses. With only a few exceptions, they exhibited a higher degree of up-regulation in the males than in the females.
To study the biological functions and signaling pathways associated with these differentially expressed genes identified by transcriptome screening, we performed several analyses in IPA of the 113 genes we found differentially expressed in either males or females. “Inflammatory response,” “cancer,” and “respiratory disease” are the top three diseases/disorders that are significantly associated with these genes. As shown in Figure 7, in addition to inflammation pathways, significantly associated pathways include “airway hyperresponsiveness,” “acute phase response signaling,” and “IL-17 signaling.” There is no doubt that the OVA challenge triggers acute phase response signaling, causes severe inflammation, and increases AHR. However, all mice received identical OVA exposures, indicating that changes in gene expression were due to SHS exposure in utero and supporting in utero SHS exposure as a potential factor in aggravated adult lung responses.
Despite the fact that most genes affected by in utero SHS were up-regulated on microarrays, we identified a few down-regulated genes as well. These genes, including Itgb1 and Pon1, were consistent with data from some human studies. B1-integrin (Itgb1, ♀: ↓1.60; ♂: ↓2.49), initially activated by P-selectin, has been reported to increase during early stages of asthma but to diminish in subjects with severe asthma (17). Pon1 (♀: ↓2.01; ♂: ↓2.10) is decreased in children with asthma versus children without asthma (18). The decreases in expression of Itgb1 and Pon1 found during asthma exacerbation suggest a protective role for these genes against asthma progression. For instance, integrin α9β1 suppresses exaggerated contraction of the airway smooth muscle through inhibition of IP3-dependent pathways (19). Pon1, on the other hand, may attenuate asthmatic responses via its antioxidative properties (20). The down-regulation of these genes resulting from in utero exposure to SHS would further impair the defenses against severe asthma.
We observed increased production of Th2 cytokines both transcriptionally (whole lung) and translationally (BALF). The Th2 cytokines, IL-4, IL-5, and IL-13, are believed to mediate isotype switching of allergen-specific B cells into IgE production, promoting airway eosinophilia and inducing AHR (21), the latter two of which were also observed in this study. Although Th2 responses and eosinophilic inflammation largely explained the observed responses in our earlier study, here the increased BALF neutrophil counts and up-regulation of neutrophil surface antigen Cd177 (♀: ↑1.93; ♂: ↑2.72), suggested early signs of airway neutrophilia in OVA-challenged adult mice that were exposed in utero to SHS. As neutrophils accumulate in the airway, which is common in severe allergic asthma (22), the presence of both eosinophils and neutrophils in the airways have been reported to be associated with lowest lung function, poorest asthma control, and increased exacerbations of patients with asthma (23). Recent reports have suggested the possible involvement of IL-17 in neutrophilic inflammation (24–26). IL-17 can induce lung structural cells to secrete proinflammatory cytokines, as was found here for TNF-α, IL-1b, IL-6, and chemokines, including CXCL1 (KC), CXCL2, and CXCL8, thereby attracting neutrophils (27). According to IPA, the IL-17 signaling pathway is closely associated with Il6, Cxcl3, and Timp1, all of which we found to be significantly up-regulated on microarrays. Microarray analysis also revealed that IL-17B, among all the cytokines in the IL-17 family, showed increased expression in the SHS-OVA groups. In our previous study (3) where we studied recurring SHS exposure to mice that had been to SHS in utero, IL-17 signaling also was one of the most significant canonical pathways associated with in utero exposure to SHS. Findings from both studies demonstrate that the IL-17 signaling pathway is persistently modulated by in utero SHS exposures, independent of the adult irritant. In addition, Th2 cytokines, particularly IL-4 and IL-13, also affect macrophage polarization and induce alternatively activated M2 macrophages (28). Although clear evidence was not found for M2 macrophages in the lungs, we found elevated expression of genes that are primarily expressed in M2 macrophages (29–31): Arg1 (♀: ↑6.27; ♂: ↑3.54), Ccl17 (♀: ↑1.65; ♂: ↑2.13) and Cd163 (♀: ↑1.84; ♂: ↑2.65). Thus, SHS exposure in utero appears to aggravate adult asthmatic responses mediated by a variety of cell types, including eosinophils, neutrophils, and macrophages.
The most highly responsive group, SOM, is of particular interest. The sex bias we report was not apparent unless mice were exposed to SHS in utero, which means (1) that the in utero environment selectively modified the male offspring more significantly than the females, and (2) that the differential effects of in utero exposure are prolonged to at least 23 weeks of age. Sex-specific effects induced by smoke exposures in utero have been shown by other researchers, as well. In a study by Ng and colleagues (32), in utero exposure to mainstream cigarette smoke significantly reduced cytotoxic T-lymphocyte activity in 5- and 10-week-old male offspring, whereas the females were not affected. In another study, Murphy and colleagues (33) reported in utero cigarette smoke–related increases in DNA methylation of insulin-like growth factor 2, which is more pronounced in the male offspring than the females. Epigenetic alterations induced by in utero smoke exposures have been revealed (34, 35), with the potential for these alterations to persist into adulthood and modulate adult responses (36, 37). We are currently investigating the epigenetic changes associated with SHS exposure in utero to gain more insights into adult disease susceptibility.
Acknowledgments
Acknowledgments
The authors thank Lindsey Clemones of LSU SVM and Paula Polk of the Research Core Facility of LSU Health Science Center-Shreveport for excellent technical assistance.
Footnotes
This research was supported by the Louisiana Governor’s Biotechnology Initiative (A.L.P.) and National Institutes of Health grant HL092906 (N.A.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0164OC on July 30, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raherison C, Pénard-Morand C, Moreau D, Caillaud D, Charpin D, Kopferschmitt C, Lavaud F, Taytard A, Maesano IA. Smoking exposure and allergic sensitization in children according to maternal allergies. Ann Allergy Asthma Immunol. 2008;100:351–357. doi: 10.1016/S1081-1206(10)60598-4. [DOI] [PubMed] [Google Scholar]
- 2.Singh SP, Mishra NC, Rir-Sima-Ah J, Campen M, Kurup V, Razani-Boroujerdi S, Sopori ML. Maternal exposure to secondhand cigarette smoke primes the lung for induction of phosphodiesterase-4D5 isozyme and exacerbated Th2 responses: rolipram attenuates the airway hyperreactivity and muscarinic receptor expression but not lung inflammation and atopy. J Immunol. 2009;183:2115–2121. doi: 10.4049/jimmunol.0900826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao R, Perveen Z, Paulsen D, Rouse RL, Ambalavanan N, Kearney MT, Penn AL. In utero exposure to second-hand smoke aggravates adult responses to irritants: adult second-hand smoke. Am J Respir Cell Mol Biol. 2012;47:843–851. doi: 10.1165/rcmb.2012-0241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax. 1998;53:849–856. doi: 10.1136/thx.53.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kips JC, Anderson GP, Fredberg JJ, Herz U, Inman MD, Jordana M, Kemeny DM, Lötvall J, Pauwels RA, Plopper CG, et al. Murine models of asthma. Eur Respir J. 2003;22:374–382. doi: 10.1183/09031936.03.00026403. [DOI] [PubMed] [Google Scholar]
- 6.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1:213–220. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd CM. Building better mouse models of asthma. Curr Allergy Asthma Rep. 2007;7:231–236. doi: 10.1007/s11882-007-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tantisira KG, Colvin R, Tonascia J, Strunk RC, Weiss ST, Fuhlbrigge AL Childhood Asthma Management Program Research Group. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am J Respir Crit Care Med. 2008;178:325–331. doi: 10.1164/rccm.200708-1174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177:621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 2005;35:1496–1503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 11.Penn AL, Rouse RL, Horohov DW, Kearney MT, Paulsen DB, Lomax L. In utero exposure to environmental tobacco smoke potentiates adult responses to allergen in BALB/c mice. Environ Health Perspect. 2007;115:548–555. doi: 10.1289/ehp.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smyth GK.Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol2004. 3:Article3. [DOI] [PubMed]
- 13.Boyce JA, Austen KF. No audible wheezing: nuggets and conundrums from mouse asthma models. J Exp Med. 2005;201:1869–1873. doi: 10.1084/jem.20050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 15.DeLorme MP, Moss OR. Pulmonary function assessment by whole-body plethysmography in restrained versus unrestrained mice. J Pharmacol Toxicol Methods. 2002;47:1–10. doi: 10.1016/s1056-8719(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 16.Singh SP, Barrett EG, Kalra R, Razani-Boroujerdi S, Langley RJ, Kurup V, Tesfaigzi Y, Sopori ML. Prenatal cigarette smoke decreases lung cAMP and increases airway hyperresponsiveness. Am J Respir Crit Care Med. 2003;168:342–347. doi: 10.1164/rccm.200211-1262OC. [DOI] [PubMed] [Google Scholar]
- 17.Johansson MW, Han ST, Gunderson KA, Busse WW, Jarjour NN, Mosher DF. Platelet activation, P-selectin, and eosinophil β1-integrin activation in asthma. Am J Respir Crit Care Med. 2012;185:498–507. doi: 10.1164/rccm.201109-1712OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cakmak A, Zeyrek D, Atas A, Selek S, Erel O. Oxidative status and paraoxonase activity in children with asthma. Clin Invest Med. 2009;32:E327–E334. doi: 10.25011/cim.v32i5.6920. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Kudo M, Rutaganira F, Takano H, Lee C, Atakilit A, Robinett KS, Uede T, Wolters PJ, Shokat KM, et al. Integrin α9β1 in airway smooth muscle suppresses exaggerated airway narrowing. J Clin Invest. 2012;122:2916–2927. doi: 10.1172/JCI60387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tölgyesi G, Molnár V, Semsei AF, Kiszel P, Ungvári I, Pócza P, Wiener Z, Komlósi ZI, Kunos L, Gálffy G, et al. Gene expression profiling of experimental asthma reveals a possible role of paraoxonase-1 in the disease. Int Immunol. 2009;21:967–975. doi: 10.1093/intimm/dxp063. [DOI] [PubMed] [Google Scholar]
- 21.Epstein MM. Targeting memory Th2 cells for the treatment of allergic asthma. Pharmacol Ther. 2006;109:107–136. doi: 10.1016/j.pharmthera.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Wang YH, Wills-Karp M. The potential role of interleukin-17 in severe asthma. Curr Allergy Asthma Rep. 2011;11:388–394. doi: 10.1007/s11882-011-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER.Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol2010. 125:1028–1036. [DOI] [PMC free article] [PubMed]
- 24.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 25.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki S, Kokubu F, Kawaguchi M, Homma T, Odaka M, Watanabe S, Ieki K, Matsukura S, Kurokawa M, Takeuchi H, et al. Expression of interleukin-17F in a mouse model of allergic asthma. Int Arch Allergy Immunol. 2007;143:89–94. doi: 10.1159/000101413. [DOI] [PubMed] [Google Scholar]
- 27.Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC, Skoogh BE, Lindén A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 28.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 29.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staples KJ, Hinks TS, Ward JA, Gunn V, Smith C, Djukanović R. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCL17. J Allergy Clin Immunol. 2012;130:1404–1412. doi: 10.1016/j.jaci.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen TT, Schwartz EJ, West RB, Warnke RA, Arber DA, Natkunam Y. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol. 2005;29:617–624. doi: 10.1097/01.pas.0000157940.80538.ec. [DOI] [PubMed] [Google Scholar]
- 32.Ng SP, Silverstone AE, Lai ZW, Zelikoff JT. Effects of prenatal exposure to cigarette smoke on offspring tumor susceptibility and associated immune mechanisms. Toxicol Sci. 2006;89:135–144. doi: 10.1093/toxsci/kfj006. [DOI] [PubMed] [Google Scholar]
- 33.Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL, Schildkraut JM, Murtha AP, Iversen ES, Hoyo C. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene. 2012;494:36–43. doi: 10.1016/j.gene.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerrero-Preston R, Goldman LR, Brebi-Mieville P, Ili-Gangas C, Lebron C, Witter FR, Apelberg BJ, Hernández-Roystacher M, Jaffe A, Halden RU, et al. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5:539–546. doi: 10.4161/epi.5.6.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang WY, Ho SM. Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord. 2007;8:173–182. doi: 10.1007/s11154-007-9042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]