Abstract
Alveolar hypoxia elicits increases in mitochondrial reactive oxygen species (ROS) signaling in pulmonary arterial (PA) smooth muscle cells (PASMCs), triggering hypoxic pulmonary vasoconstriction. Mice deficient in sirtuin (Sirt) 3, a nicotinamide adenine dinucleotide–dependent mitochondrial deacetylase, demonstrate enhanced left ventricular hypertrophy after aortic banding, whereas cells from these mice reportedly exhibit augmented hypoxia-induced ROS signaling and hypoxia-inducible factor (HIF)-1 activation. We therefore tested whether deletion of Sirt3 would augment hypoxia-induced ROS signaling in PASMCs, thereby exacerbating the development of pulmonary hypertension (PH) and right ventricular hypertrophy. In PASMCs from Sirt3 knockout (Sirt3−/−) mice in the C57BL/6 background, we observed that acute hypoxia (1.5% O2; 30 min)–induced changes in ROS signaling, detected using targeted redox-sensitive, ratiometric fluorescent protein sensors (roGFP) in the mitochondrial matrix, intermembrane space, and the cytosol, were indistinguishable from Sirt3+/+ cells. Acute hypoxia–induced cytosolic calcium signaling in Sirt3−/− PASMCs was also indistinguishable from Sirt3+/+ cells. During sustained hypoxia (1.5% O2; 16 h), Sirt3 deletion augmented mitochondrial matrix oxidant stress, but this did not correspond to an augmentation of intermembrane space or cytosolic oxidant signaling. Sirt3 deletion did not affect HIF-1α stabilization under normoxia, nor did it augment HIF-1α stabilization during sustained hypoxia (1.5% O2; 4 h). Sirt3−/− mice housed in chronic hypoxia (10% O2; 30 d) developed PH, PA wall remodeling, and right ventricular hypertrophy that was indistinguishable from Sirt3+/+ littermates. Thus, Sirt3 deletion does not augment hypoxia-induced ROS signaling or its consequences in the cytosol of PASMCs, or the development of PH. These findings suggest that Sirt3 responses may be cell type specific, or restricted to certain genetic backgrounds.
Keywords: sirtuins; hypoxia-inducible factor-1; reactive oxygen species; redox-sensitive, ratiometric fluorescent protein sensor; hypoxic pulmonary vasoconstriction
Clinical Relevance
Previous reports indicate that mice deficient in the nicotinamide adenine dinucleotide–dependent deacetylase, sirtuin (Sirt) 3, demonstrate increases in reactive oxygen species signaling, increases in hypoxia-inducible factor-1 stabilization, and augmented left ventricular hypertrophic responses. In this study, we find that Sirt3 deletion causes an increase in mitochondrial oxidant stress during sustained hypoxia, whereas oxidant signals in other compartments were not different from wild-type controls. Importantly, right ventricular remodeling and pulmonary hypertension responses to chronic hypoxia in the absence of Sirt3 were not different from controls. These findings suggest that Sirt3, in the C57/BL6 background, is not a significant regulator of the response to chronic hypoxia in the lung and right ventricle.
During alveolar hypoxia, small pulmonary arteries constrict in a response termed hypoxic pulmonary vasoconstriction (HPV). The HPV response is biphasic, consisting of an initial transient constriction (acute phase), followed by a slow, sustained constriction (chronic phase). The acute phase occurs within seconds after the onset of hypoxia, whereas the chronic phase develops after several minutes, and persists for the duration of alveolar hypoxia. The chronic alveolar hypoxia resulting from high-altitude exposure or hypoxic lung disease leads to sustained increases in pulmonary arterial (PA) pressure, pulmonary vascular remodeling, and right ventricular (RV) hypertrophy. The mitochondria of the PAs function as the oxygen sensors, and drive hypoxic responses by releasing reactive oxygen species (ROS) signals to the cytosol (1–13). During the acute phase, mitochondrial ROS trigger the release of calcium from intracellular stores, closure of voltage-dependent potassium channels, and opening of voltage-dependent and -independent calcium channels (14–18). During chronic hypoxia, mitochondrial ROS have been implicated in increased hypoxia-inducible factor (HIF)-1 stabilization, the principal transcription factor mediating O2-responsive expression of glycolytic enzymes, glucose transporters, and other genes (19–23). This results in PA vascular remodeling and the pulmonary hypertensive response observed during chronic hypoxia (24).
Sirtuins are nicotinamide adenine dinucleotide–dependent protein deacetylases involved in diverse cellular processes (25, 26). Mammalian sirtuins share significant homology with the yeast protein, silent information regulator 2, which is associated with increased life span in response to caloric restriction. Seven sirtuin proteins are currently known, with sirtuin (Sirt) -3, Sirt4, and Sirt5 primarily localizing to the mitochondria (25). Of these, Sirt3 is the only protein linked to lifespan in humans (27). Loss of Sirt3 results in hyperacetylation of mitochondrial proteins, including those involved in oxidative phosphorylation, the Krebs cycle, and maintenance of mitochondrial redox status, suggesting that it may function as a major regulatory element in the mitochondria (28). This is of interest to the study of hypoxia and ROS signaling, because Sirt3 alters proteins critical for mitochondrial function, including manganese superoxide dismutase (MnSOD) and catalase (29). Sirt3 deacetylates the transcription factor, FOXO3a, thereby promoting its nuclear localization, where it regulates to increase MnSOD expression (30). Therefore, a loss of Sirt3 causes a decrease in FOXO3a activity, resulting in a decrease in MnSOD expression and, therefore, a decrease in mitochondrial antioxidant levels (31). In accordance with that finding, Finley and colleagues (32) and Bell and colleagues (33) reported that the loss of Sirt3 enhances ROS signaling, which, in turn, enhances HIF-1α stabilization and activity in mouse embryonic fibroblasts (MEFs).
ROS signaling in cells is compartmentally regulated, but previous studies have not delineated which subcellular sites undergo redox shifts as a consequence of Sirt3 deletion. We sought to determine whether loss of Sirt3 augments hypoxia-induced increases in ROS signaling in the cytosol of PA smooth muscle cells (PASMCs), as these ROS signals have been implicated in the HPV response. Therefore, we measured the changes in oxidant signaling induced by acute hypoxia (1.5% O2; 30 min) and sustained hypoxia (1.5% O2; 16 h) in the cytosol, intermembrane space (IMS), and mitochondrial matrix of PASMCs isolated from Sirt3 wild-type (WT; Sirt3+/+) or knockout (KO; Sirt3−/−) mice. We also assessed the effects of Sirt3 depletion on HIF-1α stabilization under normoxic conditions, as well as during sustained hypoxia (1.5% O2; 4 h) in PASMCs. Finally, we assessed the effects of Sirt3 depletion on the development of pulmonary hypertension (PH) and RV hypertrophy during chronic hypoxia (10% O2; 30 d) in the whole mouse. As our previous studies of PH used mice in the C57BL/6 background, we crossed the Sirt3−/− mice into that genetic background for these studies.
Materials and Methods
Expanded Materials and Methods are contained in the online supplement. All animal protocols were reviewed and approved by the Northwestern University Institutional Animal Care and Use Committee.
Sirt3 Mice
Mice with genomic deletion of Sirt3 were generated by Dr. Frederick Alt (28). Heterozygous male Sirt3 mice (Sirt3+/−) (28) were bred into the C57BL/6 genetic background for at least eight generations. Sirt3+/− male and female offspring were bred to generate Sirt3 WT (Sirt3+/+) and Sirt3 KO (Sirt3−/−) littermates, which were used for experimentation.
Sirt3 Genotyping
Genotyping was performed by PCR using a REDExtract-N-Amp Tissue PCR Kit (Sigma-Aldrich, St. Louis, MO). Three Sirt3 primers were used: primer 1 (5′-TAC TGA ATA TCA GTG GGA ACG-3′), primer 2 (5′-TGC AAC AAG GCT TTA TCT TCC-3′), and primer 3 (5′-CCT CTG CGG CTC TTAT ACA CAG-3′), which yielded an RT-PCR product corresponding to the WT allele (∼560 bp [Sirt3+/+ or Sirt3+/−]) and/or the mutant allele (∼150 bp [Sirt3−/− or Sirt3+/−]) (28).
PASMC Isolation
PASMCs were isolated from mouse lungs as described previously (3) using a modification of the method of Marshall and colleagues (34) (see the online supplement).
Statistical Analysis
Acute hypoxia–induced changes in the redox-sensitive, ratiometric fluorescent protein sensor (roGFP) oxidation and Fura-2 were analyzed using a two-way ANOVA with repeated measures. A Newman-Keuls multiple-range test was used to evaluate significant differences between groups and times. Changes in sustained hypoxia–induced roGFP oxidation, HIF-1α expression, pulmonary acceleration time (PAT)/ejection time (ET), RV systolic pressure (RVSP), PA wall thickness, Fulton index, heart rate, and the acetylation status of MnSOD were evaluated by ANOVA. A Newman-Keuls multiple-range test was used to evaluate significant differences between experimental groups. To control for experimental differences in the hypoxic responses, experimental studies and control experiments were always performed on the same day. Statistical significance was set at P less than 0.05 (35).
Results
Effects of Sirt3 Deletion on Acute Hypoxia–Induced ROS and Ca2+ Signaling in PASMCs
Genotyping of the mice was performed by PCR (Figure 1A). Protein lysates from PASMCs isolated from Sirt3+/+ or Sirt3−/− mice were immunoblotted using Sirt3-specific antibody, and confirmed the expected absence of Sirt3 in the Sirt3−/− mice (Figure 1B).
Figure 1.
Genotyping sirtuin (Sirt) 3 mice. (A) Sirt3 RT-PCR genotyping, a roughly 560-bp product indicates a wild-type (Sirt3+/+) mouse, a roughly 150-bp product indicates a knockout (Sirt3−/−) mouse, and both products indicate a heterozygous (Sirt3+/−) mouse. (B) Western blot of lysates of pulmonary arterial (PA) smooth muscle cells (PASMCs) isolated from Sirt3+/+ or Sirt3−/− mice probed with antibodies against Sirt3 or β-actin.
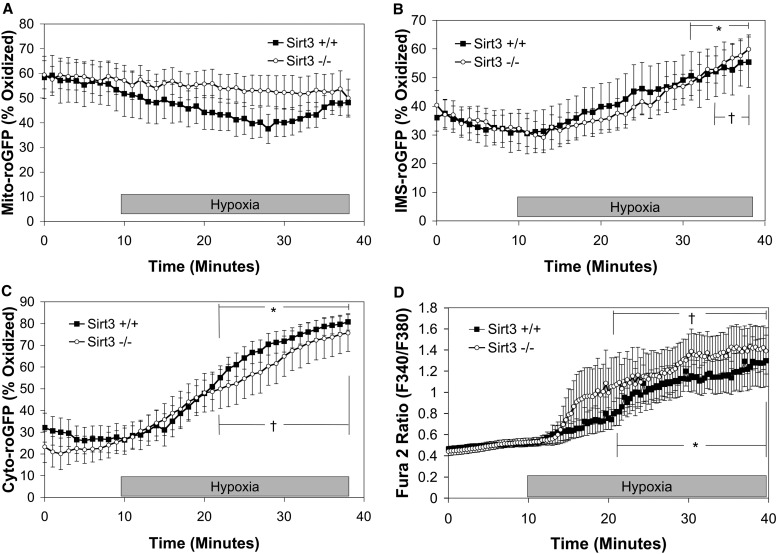
Previous studies demonstrated that acute hypoxia causes an increase in oxidation of roGFP expressed in the cytosol (Cyto-roGFP) and the IMS (IMS-roGFP) of PASMCs, whereas it decreases oxidation levels in the mitochondrial matrix (Mito-roGFP) (13). Because Sirt3 deletion results in a decrease MnSOD expression in the mitochondrial matrix (31), we sought to determine if Sirt3 deletion results in an increased oxidation of the mitochondrial matrix, IMS, and cytosol under normoxia (21% O2, 5% CO2, 74% N2), which is then augmented during acute hypoxia (1.5% O2, 5% CO2, 93.5% N2; 30 min). We also sought to determine if an increase in ROS signaling due to Sirt3 deletion resulted in an augmentation of the hypoxia-induced increases in [Ca2+]i. Interestingly, there was no difference in the oxidation of Mito-roGFP between Sirt3+/+ and Sirt3−/− PASMCs under normoxic baseline conditions (53.4 ± 8.6% and 57.7 ± 6.2%, respectively), nor was there a significant difference in hypoxia-induced decrease in oxidation of Mito-roGFP (Figure 2A). Oxidation of roGFP in the IMS under normoxia was also similar in Sirt3+/+ and Sirt3−/− PASMCs (30.7 ± 6.8% and 32.7 ± 4.9%, respectively). At the onset of hypoxia, IMS-roGFP steadily oxidized in Sirt3−/− PASMCs, which was indistinguishable from Sirt3+/+ cells (Figure 2B). Deletion of Sirt3 had no significant effect on the baseline oxidation of Cyto-roGFP (25.5 ± 5.3%) compared with Sirt3+/+ (27.8 ± 6.2%; Figure 2C) in normoxic PASMCs. Oxidation of Cyto-roGFP increased in Sirt3−/− PASMCs during acute hypoxia; however, this response was indistinguishable from hypoxia-induced oxidation of Cyto-roGFP in Sirt3+/+ PASMCs (Figure 2C). Deletion of Sirt3 also had no effect on the hypoxia-induced increase in [Ca2+]i compared with Sirt3+/+ PASMCs (Figure 2D). These results indicate that the absence of Sirt3 in PASMCs has no effect on the acute hypoxia–induced changes in ROS or calcium signaling.
Figure 2.
Effect of Sirt3 deletion on acute hypoxia–induced changes in reactive oxygen species (ROS) and calcium signaling in PASMCs. (A–D) PASMCs isolated from Sirt3+/+ or Sirt3−/− mice. PASMCs expressing the redox-sensitive, ratiometric fluorescent protein sensor (roGFP) in the mitochondrial matrix (Mito-roGFP) (A), intermembrane space (IMS)–roGFP (B), cytosolic roGFP (Cyto-roGFP) (C), or loaded with Fura-2 (D) were superfused with normoxic (21% O2) or hypoxic (1.5% O2) media for 30 minutes. Values are means (± SEM); n = 6 cover slips, 4–10 cells/cover slip. *P < 0.05 compared with normoxic baseline of Sirt3+/+ PASMCs; †P < 0.05 compared with normoxic baseline of Sirt3−/− PASMCs.
Sirt3 Deletion Augments Mitochondrial Matrix Oxidation in PASMCs
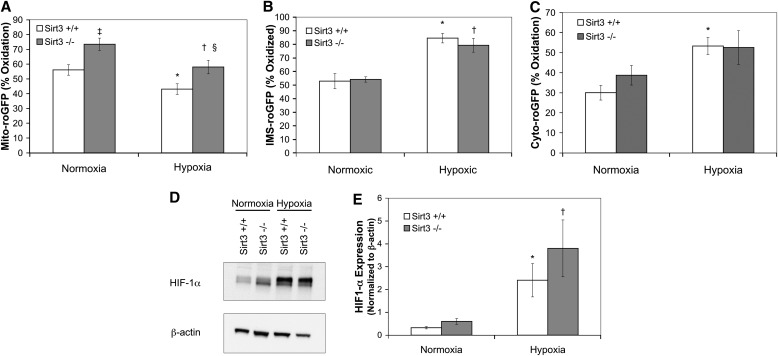
Because it was previously observed that Sirt3 depletion resulted in increased HIF-1α stabilization under normoxic conditions, which was augmented during sustained hypoxia (> 4 h) in MEFs (32, 33), we sought to determine if Sirt3 deletion in PASMCs also resulted in an increase in ROS signaling and HIF-1α stabilization during sustained hypoxia. Sirt3+/+ or Sirt3−/− PASMCs expressing one of the targeted roGFP probes were incubated in normoxia (21% O2, 5% CO2, 74% N2) or hypoxia (1.5% O2, 5% CO2, 93.5% N2) for 16 hours. Sirt3+/+ PASMCs incubated under hypoxia displayed a significant decrease in Mito-roGFP oxidation compared with Sirt3+/+ PASMCs incubated under normoxia (43.1 ± 3.6% and 56.1 ± 3.6%, respectively; Figure 3A), which was similar to the response observed in the acute hypoxia studies (Figure 2A). Interestingly, in Sirt3−/− PASMCs incubated under normoxia or hypoxia, oxidation levels of Mito-roGFP were increased (73.5 ± 4.2% and 58.0 ± 4.3%, respectively) compared with Sirt3+/+ PASMCs incubated at the same O2 tensions. However, deletion of Sirt3 did not alter the pattern of the hypoxia-induced decrease in Mito-roGFP oxidation. Oxidation of IMS-roGFP increased in Sirt3+/+ PASMCs incubated under hypoxia (84.5 ± 3.5%; Figure 3B) compared with Sirt3+/+ PASMCs incubated under normoxia (52.9 ± 5.6%). However, the increase in Mito-roGFP oxidation in Sirt3−/− PASMCs did not correspond to an increase in oxidation of IMS-roGFP in Sirt3−/− PASMCs incubated under normoxia (54.1 ± 2.0%), nor did it augment the oxidation of IMS-roGFP Sirt3−/− PASMCs incubated under hypoxia (79.3 ± 5.1%). Similarly, Sirt3 deletion did not increase the oxidation of Cyto-roGFP in Sirt3−/− PASMCs compared with Sirt3+/+ PASMCs incubated under normoxia (38.8 ± 4.9% and 30.0 ± 3.7%, respectively; Figure 3C), nor did it augment the hypoxia-induced oxidation of Cyto-roGFP in Sirt3−/− PASMCs compared with Sirt3+/+ PASMCs (52.6 ± 8.4% and 53.3 ± 4.3%, respectively). It should be noted that no significant differences were observed between the acute (Figures 2A–2C) and sustained (Figures 3A–3C) hypoxia studies with respect to the baseline, normoxia-induced oxidation of the roGFP probes in either the Sirt3+/+ or Sirt3−/− PASMCs. In separate studies, the effect of Sirt3 deletion on sustained hypoxia-induced HIF-1α stabilization was assessed (Figures 3D and 3E). Although sustained hypoxia (1.5% O2; 4 h) increased HIF-1α stabilization in Sirt3+/+ PASMCs (as assessed by the ratio of HIF-1α/β-actin; 2.4 ± 0.7) compared with normoxia (0.32 ± 0.05), Sirt3 deletion did not affect normoxic HIF-1α stabilization (0.6 ± 0.13), nor did it augment hypoxia-induced HIF-1α stabilization (3.8 ± 1.25; Figure 3E). This result paralleled the hypoxia-induced expression of a glycolytic target under HIF-1α regulation, glyceraldehyde-3-phosphate dehydrogenase (GAPDH; see Figure E1E in the online supplement). Taken together, these results indicate that, although Sirt3 deletion increases mitochondrial matrix oxidation during both normoxia and hypoxia, this increase does not result in an increase in IMS or cytosolic ROS signaling, nor is it associated with an increase in HIF-1α stabilization.
Figure 3.
Effect of Sirt3 deletion on sustained hypoxia–induced changes in ROS signaling and hypoxia-inducible factor (HIF)-1α expression in PASMCs. (A–E) PASMCs isolated from Sirt3+/+ or Sirt3−/− mice. PASMCs expressing Mito-roGFP (A), IMS-roGFP (B), or Cyto-roGFP (C) were incubated under normoxic (21% O2; Normoxia) or hypoxic (1.5% O2; Hypoxia) conditions for 16 hours. (D) Representative Western blot of lysates of PASMCs isolated from Sirt3+/+ or Sirt3−/− mice incubated under normoxic (21% O2; Normoxia) or hypoxic (1.5% O2; Hypoxia) conditions for 4 hours, probed with antibodies against HIF-1α or β-actin. (E) Quantitative analysis of multiple Western blots from (D). Values are means (± SEM); n = 6 cover slips, 4–10 cells/cover slip (A–C). Values are means (± SEM); n = 6 dishes of PASMCs (E). *P < 0.05 compared with Normoxia Sirt3+/+ PASMCs; †P < 0.05 compared with Normoxia Sirt3−/− PASMCs; ‡P < 0.05 compared with Normoxia Sirt3+/+ PASMCs; §P < 0.05 compared with Hypoxia Sirt3+/+ PASMCs.
Deletion of Sirt3 Does Not Augment Chronic Hypoxia–Induced PH
Because our in vitro studies suggested that Sirt3 deletion has no effect on ROS signaling in isolated PASMCs during acute hypoxia (30 min), nor does it have an effect on HIF1-α stabilization or ROS signaling during sustained hypoxia (4 or 16 h), we sought to determine if Sirt3 deletion has an effect on the development of PH in vivo during chronic hypoxia (30 d). Therefore, we assessed changes in PA pressure estimated from PAT/ET measured by echocardiography (Figure 4A) in Sirt3−/− mice and Sirt3+/+ littermates housed for 30 days under normoxic (room air) or hypoxic (10% O2) conditions. A decrease in the PAT/ET is inversely proportional to an increase in PA pressure (36). Whereas chronic hypoxia decreased PAT/ET in Sirt3+/+ mice (0.308 ± 0.008) from their normoxic Sirt3+/+ littermates (0.337 ± 0.006), the combination of Sirt3 deletion and chronic hypoxia did not further exacerbate PAT/ET in the Sirt3−/− mice (0.298 ± 0.007) compared with normoxic Sirt3−/− littermates (0.325 ± 0.014). The heart rates of the normoxic Sirt3+/+ and Sirt3−/− mice and the chronic hypoxic Sirt3+/+ and Sirt3−/− mice were not significantly different (412 ± 14 bpm, 367 ± 32 bpm, 402 ± 22 bpm, and 421 ± 11 bpm, respectively). Next, we directly measured RVSP using a micro-tip pressure transducer catheter inserted into the right ventricle and observed that loss of Sirt3 did not alter the chronic hypoxia–induced change in RVSP, as both Sirt3+/+ and Sirt3−/− RVSP values increased from normoxic levels (18.3 ± 0.9 mm Hg and 18.5 ± 1.1 mm Hg, respectively) to chronic hypoxia levels (22.1 ± 1.7 mm Hg and 22.1 ± 1.6 mm Hg, respectively; Figure 4B). We also assessed whether Sirt3 depletion affects the acute hypoxia–induced change in RVSP. When Sirt3+/+ and Sirt3−/− mice housed under normoxia were switched to hypoxic (5% O2, 95% N2) ventilation for 1 minute, RVSP increased by a similar amount (4.3 ± 0.9 mm Hg and 3.2 ± 0.7 mm Hg, respectively; Figure 4C). In the chronic hypoxia–housed mice, the response to 1 minute of acute hypoxia was significantly augmented compared with their normoxic counterparts, but there was no difference between the Sirt3+/+ and Sirt3−/− mice (8.2 ± 1.6 mm Hg and 8.7 ± 1.1 mm Hg, respectively). Vascular remodeling was also assessed by the change in PA wall thickness (Figures 4D and 4E). Chronic hypoxia increased PA wall thickness in both Sirt3+/+ and Sirt3−/− mice (45.0 ± 0.9% and 48.2 ± 1.8%, respectively) compared with their normoxic counterparts (40.4 ± 1.8% and 41.7 ± 3.5%, respectively), but no difference between the Sirt3+/+ and Sirt3−/− mice was detected. In addition, there was no difference in the development of RV hypertrophy detected from Fulton index assessments (Figure 4F), as chronic hypoxia increased the Fulton index to a similar level in both Sirt3+/+ and Sirt3−/− mice (0.37 ± 0.01 and 0.37 ± 0.007, respectively) from a similar normoxic level (0.26 ± 0.01 and 0.25 ± 0.01, respectively). To determine whether the lack of difference in the measured responses between WT and Sir3−/− mice was due to a down-regulation of Sirt3 in the WT mice during chronic hypoxia, we measured whole-lung Sirt3 expression. This analysis revealed that chronic hypoxia does not alter Sirt3 protein expression in the Sirt3+/+ mice (Figure E2E). Taken together, these results indicate that, while chronic hypoxia induces PH, Sirt3 deletion does not alter that response.
Figure 4.
Effect of Sirt3 deletion on chronic hypoxia–induced pulmonary hypertension. (A–F) Sirt3+/+ or Sirt3−/− mice housed under normoxic (room air; Normoxia) or hypoxic (10% O2; Hypoxia) conditions for 30 days. (A) Chronic hypoxia decreased pulmonary acceleration time (PAT)/ejection time (ET) in Sirt3−/− mice, but this response was not different from that in Sirt3+/+ animals. (B) Chronic hypoxia increased right ventricular (RV) systolic pressure (RVSP) in Sirt3−/− mice, but this response was not different from that in Sirt3+/+ animals. (C) Chronic hypoxia increased the acute hypoxia (5% O2; 1-min ventilation)–induced change in RVSP in Sirt3−/− mice, but this response was not different from that in Sirt3+/+ animals. (D) Inflation-fixed, hematoxylin and eosin–stained lung sections demonstrate chronic hypoxia–induced vascular remodeling. “PA” denotes small PAs. Left image denotes a lung section from a Normoxia Sirt3+/+ mouse; right image is from a Hypoxia Sirt3+/+ mouse. (E) Chronic hypoxia–induced vascular remodeling as assessed by PA wall thickness in both Sirt3+/+ and Sirt3−/− mice compared with their normoxic counterparts. (F) Chronic hypoxia–induced RV hypertrophy as assessed by the Fulton index in both Sirt3+/+ and Sirt3−/− mice compared with their normoxic counterparts. Values are means (± SEM); n = 6–10 mice per experimental group. *P < 0.05 compared with Normoxia Sirt3+/+ mice; †P < 0.05 compared with Normoxia Sirt3−/− mice. LV, left ventricular; S, septum.
MnSOD expression is regulated by the transcription factor, FOXO3a, the nuclear localization of which is regulated by Sirt3-mediated deacetylation (30). Sirt3 also regulates MnSOD directly by deacetylating the protein at lysine 122 (37). Deacetylation by Sirt3 promotes MnSOD activity, which suggests that the enhanced mitochondrial matrix oxidant status that we detected in the Sirt3−/− mice might be explained by a decrease in MnSOD activity mediated by its hyperacetylation. We therefore assessed the acetylation status of MnSOD in the Sirt3−/− mice. MnSOD was immunoprecipitated from lung tissue lysates and then probed with an antibody to detect lysine acetylation (Figure 5). These studies revealed no difference in MnSOD acetylation status between WT and Sirt3−/− mice. These findings suggest that the difference in mitochondrial oxidant status cannot be attributed to a difference in MnSOD acetylation in WT and Sirt3-deficient mice. Conceivably, MnSOD may be deacetylated by redundant mechanisms in these mice.
Figure 5.
Effect of Sirt3 deletion on the acetylation status of manganese superoxide dismutase (MnSOD). MnSOD was immunoprecipitated from lung tissue lysates from Sirt3+/+ and Sirt3−/− mice using a rabbit antibody against MnSOD, and the resulting product was assessed by immunoblotting for protein lysine acetylation. (A) Representative Western blot of the lung lysates probed with antibodies against acetylated lysine (acetylated MnSOD) or MnSOD. (B) Quantitative analysis of Western blots from (A). Values are means (± SEM); n = 3 mice per experimental condition.
Discussion
The rationale for this study was based on the observations of Bell and colleagues (33) and Finley and colleagues (32), who reported finding increases in ROS signaling and HIF-1α stabilization in MEFs from Sirt3−/− mice compared with control animals. In addition, Sundaresan and colleagues (31) reported that loss of Sirt3 augmented the mouse left ventricular hypertrophy in response to aortic constriction in association with increased ROS signaling in cardiac myocytes. These findings suggested that Sirt3 deletion augments stress responses through a shift in ROS-mediated signaling, potentially including the development of hypoxia-induced PH. Finley and colleagues (32) reported that Sirt3 deletion promotes a shift in glycolytic metabolism in tumor cells (Warburg effect) through an amplification of HIF-1α stabilization, whereas Bell and coworkers (33) reported that Sirt3 loss promotes tumor growth by enhancing HIF-1α stabilization through an increase in mitochondrial ROS production. HIF-1 has been implicated previously in the development of vascular remodeling, PH, and RV hypertrophy in mouse models of chronic hypoxia (24). In view of those findings, it is surprising that we did not detect any augmentation of hypoxia-induced ROS signaling or HIF-1α stabilization in Sirt3−/− PASMCs compared with controls.
Possible explanations for the differing results between the previous studies and the present study include the redox probes employed to measure oxidant signaling, the type of cell isolated for use in the in vitro studies, and the genetic background of Sirt3 KO mouse. For example, Bell and colleagues (33) used dihydroethidium, a probe oxidized by superoxide, whereas Finley and colleagues (32) used dichlorofluorescein diacetate, which is oxidized by H2O2. Although sensitive to ROS, those fluorescent probes lack specificity, and cannot distinguish the intracellular site of oxidation. To provide a more specific assessment of thiol redox status, we used roGFP, an H2O2-sensitive sensor targeted to subcellular compartments. Therefore, it is conceivable that differences in methods of ROS detection could explain the difference in ROS signaling reported by Bell and colleagues and Finley and colleagues and the present study. Another difference between our study and those of Bell and colleagues and Finley and colleagues is that they used MEFs isolated from Sirt3−/− mice, whereas we used isolated PASMCs. If the effects of Sirt3 are cell type specific, then the small effects in the PASMCs with respect to ROS signaling and HIF1-α stabilization could be explained. Although Sirt3 has been studied extensively, Sirt4 and Sirt5 are also localized to the mitochondria, and could impart overlapping properties with Sirt3 (25, 27). Such redundancy could potentially explain why KO of Sirt3 does not confound embryonic development. In view of the work of Sundaresan and coworkers (31), our finding of unchanged RV hypertrophy in the chronic hypoxia model is also surprising. An important distinction between the studies is that we evaluated RV hypertrophy, whereas they focused on the left ventricle. It is conceivable that hypertrophic responses in the two ventricles are regulated independently. However, a common difference between our study and those of Sundaresan and colleagues, Finley and colleagues, and Bell and colleagues relates to the genetic background. We bred the Sirt3−/− mice into the C57BL/6 background, because studies of mice in the parent background, 129Sv (28), would not have been comparable to our previous studies in the C57BL/6 background. It has been well documented that different mouse strains show different responses to various stimuli, including acute hypoxia, chronic hypoxia, and hypoxia–ischemia, as well as altered transcription factor signaling (39–43). Differences attributable to the background genetic strain might also explain why we failed to detect any difference in the acetylation status of MnSOD between the Sirt3+/+ and Sirt3−/− mice. Tao and colleagues (37) compared WT and Sirt3−/− MEFs and found differences in MnSOD acetylation. Their MEFs were obtained from mice in a mixed genetic background (R. Tao, personal communication), whereas ours were bred into the C57BL/6 background. To the extent that differences in genetic background affect physiological stress responses by altering redundancy of deacetylase activities, future mouse studies of sirtuin function should use fully inbred strains, where the effects of such redundancy can be defined.
Finally, although our findings suggest that Sirt3 is not required for the development of PH in the mouse model of chronic hypoxia, this does not exclude the possibility that Sirt3 may contribute to the regulation of pulmonary vascular remodeling in other experimental models, or in human PH. To the extent that Sirt3 depletion has been found to exacerbate tissue remodeling in other stress models, future studies of PH should test whether overexpression or activation of Sirt3 can confer protection.
Acknowledgments
Acknowledgments
The authors thank Dr. David Gius, Dr. Yueming Zhu, and Constance Runyan (Northwestern University, Chicago, IL) for their assistance with the immunoprecipitation studies.
Footnotes
This work was supported by National Institutes of Health grants HL66315, HL35440, HL079650 (P.T.S), and NS056313 (J.D.M.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0191OC on September 18, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 2.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 3.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 4.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species–dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol. 2008;28:718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastukh V, Ruchko M, Gorodnya O, Wilson GL, Gillespie MN. Sequence-specific oxidative base modifications in hypoxia-inducible genes. Free Radic Biol Med. 2007;43:1616–1626. doi: 10.1016/j.freeradbiomed.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Lavani R, Chang WT, Anderson T, Shao ZH, Wojcik KR, Li CQ, Pietrowski R, Beiser DG, Idris AH, Hamann KJ, et al. Altering CO2 during reperfusion of ischemic cardiomyocytes modifies mitochondrial oxidant injury. Crit Care Med. 2007;35:1709–1716. doi: 10.1097/01.CCM.0000269209.53450.EC. [DOI] [PubMed] [Google Scholar]
- 8.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMP–activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korde AS, Yadav VR, Zheng YM, Wang YX. Primary role of mitochondrial Rieske iron–sulfur protein in hypoxic ROS production in pulmonary artery myocytes. Free Radic Biol Med. 2011;50:945–952. doi: 10.1016/j.freeradbiomed.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Leach RM, Hill HM, Snetkov VA, Robertson TP, Ward JP. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol. 2001;536:211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. 2010;106:526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu W, Wang J, Peng G, Shimoda LA, Sylvester JT. Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297:L17–L25. doi: 10.1152/ajplung.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L104–L113. doi: 10.1152/ajplung.00058.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morio Y, McMurtry IF. Ca(2+) release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol. 2002;92:527–534. doi: 10.1152/jappl.2002.92.2.527. [DOI] [PubMed] [Google Scholar]
- 19.Chandel NS, Schumacker PT. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS Lett. 1999;454:173–176. doi: 10.1016/s0014-5793(99)00783-8. [DOI] [PubMed] [Google Scholar]
- 20.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 21.Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 2000;106:809–812. doi: 10.1172/JCI11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 24.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guarente L. Mitochondria—a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J. 2012;444:1–10. doi: 10.1042/BJ20120030. [DOI] [PubMed] [Google Scholar]
- 27.Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38:1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 28.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs KM, Pennington JD, Bisht KS, Aykin-Burns N, Kim HS, Mishra M, Sun L, Nguyen P, Ahn BH, Leclerc J, et al. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int J Biol Sci. 2008;4:291–299. doi: 10.7150/ijbs.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–2996. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 35.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Thibault HB, Kurtz B, Raher MJ, Shaik RS, Waxman A, Derumeaux G, Halpern EF, Bloch KD, Scherrer-Crosbie M. Noninvasive assessment of murine pulmonary arterial pressure: validation and application to models of pulmonary hypertension. Circ Cardiovasc Imaging. 2010;3:157–163. doi: 10.1161/CIRCIMAGING.109.887109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 39.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia–ischemia. Brain Res. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda S, Hawes NL, Chang B, Avery CS, Smith RS, Nishina PM. Severe ocular abnormalities in C57BL/6 but not in 129/Sv p53-deficient mice. Invest Ophthalmol Vis Sci. 1999;40:1874–1878. [PubMed] [Google Scholar]
- 41.Ward NL, Moore E, Noon K, Spassil N, Keenan E, Ivanco TL, LaManna JC. Cerebral angiogenic factors, angiogenesis, and physiological response to chronic hypoxia differ among four commonly used mouse strains. J Appl Physiol. 2007;102:1927–1935. doi: 10.1152/japplphysiol.00909.2006. [DOI] [PubMed] [Google Scholar]
- 42.Adachi T, Ogawa H, Okabe S, Kitamuro T, Kikuchi Y, Shibahara S, Shirato K, Hida W. Mice with blunted hypoxic ventilatory response are susceptible to respiratory disturbance during hypoxia. Tohoku J Exp Med. 2006;209:125–134. doi: 10.1620/tjem.209.125. [DOI] [PubMed] [Google Scholar]
- 43.Zwemer CF, Song MY, Carello KA, D’Alecy LG. Strain differences in response to acute hypoxia: CD-1 versus C57BL/6J mice. J Appl Physiol. 2007;102:286–293. doi: 10.1152/japplphysiol.00536.2006. [DOI] [PubMed] [Google Scholar]