Abstract
Purpose
Photon therapy has been reported to induce resets of implanted cardiac devices, but the clinical sequelae of treating patients with such devices with proton beam therapy (PBT) are not well known. We reviewed the incidence of device malfunctions among patients undergoing PBT.
Methods
From March 2009 through July 2012, 42 patients with implanted cardiac implantable electronic devices (CIEDs) (28 pacemakers and 14 cardioverter-defillibrators) underwent 42 courses of PBT for thoracic (23 [55%]), prostate (15 [36%]), liver (3[7%]), or base of skull (1 [2%]) tumors at a single institution. The median prescribed dose was 74 Gy(RBE) [range 46.8–87.5 Gy(RBE)], and the median distance from the treatment field to the CIED was 10 cm (range 0.8–40 cm). Maximum proton and neutron doses were estimated for each treatment course. All CIEDs were checked before radiation delivery and monitored throughout treatment.
Results
Median estimated peak proton and neutron doses to the CIED in all patients were 0.8 Gy (range 0.13–21 Gy) and 346 Sv (range 11–1100 mSv). Six CIED malfunctions occurred in five patients (2 pacemakers and 3 defibrillators). Five of these malfunctions were CIED resets, and one patient with a defibrillator (in a patient with a liver tumor) had an elective replacement indicator (ERI) after therapy that was not influenced by radiation. The mean distance from the proton beam to the CIED among devices that reset was 7.0 cm (range 0.9–8 cm), and the mean maximum neutron dose was 655 mSv (range 330–1100 mSv). All resets occurred in patients receiving thoracic PBT and were corrected without clinical incident. The generator for the defibrillator with the ERI message was replaced uneventfully after treatment.
Conclusions
The incidence of CIED resets was about 20% among patients receiving PBT to the thorax. We recommend that PBT be avoided in pacing-dependent patients and that patients with any type of CIED receiving thoracic PBT be followed closely.
INTRODUCTION
Implantable cardiac electronic devices (CIEDs) are used to manage several types of cardiac morbidity, and the demographics of patients who require such devices can be similar to those with common adult malignancies (lung, prostate, or breast cancer). Thus some percentage of patients who require radiation therapy for such malignancies will have CIEDs in place, which further complicates management of already-complex clinical situations. Photon radiation has been amply demonstrated to induce device resets, with higher-energy photons showing higher risks of device malfunctions 1–5. However, data regarding the incidence of CIED resets in patients receiving proton beam therapy (PBT), which poses a greater risk of neutron scatter than high-energy photons, are sparse 6.
The purpose of this study was to determine the incidence of cardiac-device resets and other malfunctions among patients receiving PBT at a single institution. Specifically, we assessed the incidence of malfunctions according to tumor site and distance from the CIED and estimated the neutron and proton doses to each CIED, with the goal of deriving recommendations regarding the safety of PBT for individual patients. Our hypothesis was that the incidence of resets would be low overall but higher among patients with thoracic target volumes and among CIEDs exposed to high neutron or proton doses.
METHODS
Data Sources
The study was approved by the appropriate institutional review board, and patient confidentiality was provided in accordance with the Health Insurance Portability and Accountability Act. Patients in this retrospective analysis were selected from a database of all patients treated with PBT at a single institution. Information was extracted on prescribed radiation dose and fractionation, tumor site (including details of the radiation treatment plan), the presence of a CIED, whether the patient was considered pacemaker-dependent or pacemaker-independent, and information generated by the CIED.
Participants and Pre-Treatment CIED Evaluation
Patients were included in this study if they received PBT and had an implanted CIED, regardless of tumor location or pacing-dependence or -independence. At our institution, all patients with such devices in place undergo a thorough evaluation before PBT, including assessment of the underlying native rhythm and the dependence of the patient on pacing and other features of the CIED. For the purposes of this study, pacing-dependence was defined as the lack of an intrinsic rhythm >30 bpm or as hemodynamic instability in the native rhythm. Dependence on other CIED features (e.g., cardiac resynchronization and anti-tachycardia) was also considered. The robustness of the CIED systems was evaluated in terms of pacing and sensing thresholds, leads, and battery usage. A CIED-management plan was also devised during the PBT, which typically included setting alerts for malfunction, programming changes as needed, and daily pulse checks by the PBT team. Any deviations from the recommended pulse levels were communicated immediately to the Department of Cardiology’s device clinic staff for further evaluation. Device-clinic visits were arranged during PBT as needed, and afterward all patients underwent full CIED interrogation, with reprogramming as needed.
The manufacturer’s recommendation for radiation exposure was reviewed in the context of the PBT plan, as was the feasibility of relocating the CIED to a site more distant from the treatment field. All attempts were made to adhere to the guidelines set by the manufacturer, and if delivering an appropriate PBT plan was not feasible without exceeding these constraints, the risks of proceeding with PBT were discussed with the patient and among the cardiology/ radiation oncology teams. In particular, if it was expected that the dose to the CIED would exceed the manufacturer’s recommendations, high-risk patients (i.e. pacing –dependent, moving the CIED not feasible without a substantial risk of toxicity) were not treated with PBT and were instead were referred for IMRT. If the dose to the CIED exceeded manufacturer’s recommendations but the patient was not classified as high-risk, the patient was informed of the potential consequence of changing the device after treatment, and was monitored closely as described above.
Proton Beam Therapy Delivery
PBT was delivered by passive scattering to thoracic or abdominal locations and by either passive scattering or pencil beam scanning to the pelvis (e.g., for prostate cancer). No patient was treated with the CIED within the radiation field, and great care was taken during treatment planning to avoid selecting beam angles that would intersect the CIEDs. Dose constraints to the CIED’s power generator were maintained within manufacturer-recommended limits and typically involved a maximum dose of 2 Gy(RBE) or less 7. All patients were treated in a facility in which cardiac resuscitation equipment was immediately available, and patients were monitored throughout each PBT fraction with an in-room video system for any clinical changes.
Estimation of Proton and Neutron Dose
The maximum proton dose to the CIED was estimated from lateral dose profiles obtained during commissioning of the machine. The shortest distance between the device generator and the aperture outline was used to calculate the dose levels for both protons and neutrons. We used the approach of Wang et al. 8 to calculate the neutron dose equivalent as a function of proton energy, aperture distance, field size, and width of the spread-out Bragg peak:
H/D (E,A,W,S) = H0 × Fa × Fw × Fs
Where Fa is the aperture-to-isocenter distance factor, Fw is the is the SOBP width factor, and Fs is the field size factor. To obtain the H0 at a distance d, which is located between the in-field point (d=0cm) and the out-of-field point (30cm), we used the following formula:
H0 (d) = H0 (d = 0) – (d/30 cm) × [H0 (d = 0) – H0 (d = 30)],
Where H0 (d = 0) is the neutron dose (in mSv) relative to the prescribed proton dose for in field Neutrak measurements and H0 (d = 30) is the neutron dose (in mSv) relative to the prescribed proton dose for out-of-field.
Assessment of Device Malfunctions
Each CIED was programmed in advance such that a reset would be evident from the pulse rate. The CIED was then monitored for resets after each treatment by checking the pulse rate. Formal interrogation occurred at the beginning and end of the course of radiation, or more frequently based on the risk level for each patient, as assessed by the managing cardiologist.
Two types of CIED malfunctions were assessed during this study, a CIED reset and an elective replacement indicator, or ERI. A CIED reset is a software reversion to backup mode of function as a result of electromagnetic interference. It is recognized by changes in the pacing function and/or rate on an electrocardiogram, or by alerts at the time of formal interrogation. The response of each device during a reset is proprietary and may vary with the extent of damage to a silicon chip. In contrast, elective replacement indication is a device alert as to declining battery voltage. It signals that generator replacement should be electively scheduled within the next 2–3 months, during which time the device can be expected to provide continuing service, but after which function cannot be guaranteed.
All CIED malfunctions were verified by cardiology clinic personnel. In the event of reset or malfunction, the device was interrogated and reprogrammed to the prior settings if possible and recommendations made as to whether PBT should be continued or not. A CIED reset was defined as a non-programmed change in pacing or sensing parameters during PBT. All devices were also analyzed for intercurrent cardiac events, lead impedance, and sensing and pacing thresholds before, during, and after the full course of PBT.
RESULTS
Proton and Neutron Dose to the Implanted Cardiac Devices
Patient and treatment characteristics are shown in Table 1. Treatment in most cases was delivered by passive scattering to tumors in the thorax (esophagus, lung, or thymus). Ten men were treated with pencil beam scanning to the prostate. The median estimated maximum proton and neutron doses to the CIED in all patients were 0.80 Gy(RBE) [range 0.13–21 Gy(RBE)] and 3.46 Sv (range 0.11–11 Sv). One patient had the CIED moved prior to radiation therapy, and the doses to this device after pacemaker migration were 0.13 Gy(RBE) and 741 mSv.
Table 1.
Patient Characteristics
| Characteristic | No. (%) or Value |
|---|---|
| Tumor Location | |
| Thorax | 25 (59) |
| Prostate | 14 (33) |
| Liver | 2 (5) |
| Base of skull | 1 (3) |
| Prescribed Radiation Dose | |
| Median | 74 Gy(RBE) |
| Range | 46.8–87.5 Gy(RBE) |
| No. of Fractions | |
| Median | 35 |
| Range | 15–39 |
| Type of Device | |
| Pacemaker | 28 (68) |
| Defibrillator | 14 (32) |
| Pacemaker-Dependent | |
| No | 39 (93) |
| Yes | 3 (7) |
| Shortest Distance from Treatment Field to CIED | |
| Median | 10 cm |
| Range | 0.8–40 cm |
| Estimated Maximum Proton Dose | |
| Median | 0.8 Gy(RBE) |
| Range | 0.13–21 Gy(RBE) |
| Estimated Maximum Neutron Dose | |
| Median | 346 mSv |
| Range | 11–1100 mSv |
| Planning Technique | |
| Passive Scattering | 32 (76) |
| Scanning Beam | 10 (24) |
Total number of treatment courses=42
Abbreviations: RT=radiation therapy, CIED=cardiac implantable electronic device
Incidence and Sequelae of Cardiac Device Malfunctions During Proton Beam Therapy
Six CIED malfunctions in five patients were observed, five resets and one ERI (Table 2). All four of the patients who had device resets were receiving passive scattering therapy to the thorax, and none were pacing-dependent. The elective replacement indication occurred in a patient receiving PBT to the liver. The mean maximum proton and neutron doses to the CIED generator in the four patients with resets were 0.745 Gy(RBE) and 655 mSv (means are reported here because of the small numbers of patients). Malfunctions occurred at a wide variety of cumulative delivered doses, ranging from 4 Gy(RBE) to 67.5 Gy(RBE) near the end of the PBT. Normal functioning was restored in all cases of CIED reset without adverse clinical effects; only the device with the elective replacement indicator required replacement of the generator. Notably, the pre-proton therapy interrogation in the patient with the ERI had demonstrated an expected decline in battery status, and the timing of the ERI signal was consistent with that predicted before treatment.
Table 2.
Characteristics of Device Malfunctions
| Patient ID |
Tumor Site |
Device Type |
Dose, Gy(RBE) / No. of Fractions |
Distance from Device to Treatment Field, cm |
Estimated Maximum Proton Dose±1σ(d), Gy(RBE) |
Estimated Maximum Neutron Dose±1σ(e), Sv |
Dose at Malfunction / Total Dose, Gy(RBE) |
Nature of Malfunction |
|---|---|---|---|---|---|---|---|---|
| 1 | Thorax | ICD | 74/37 | 5a | 0.87±0.08 | 1.10±0.55 | 40/74 | Resetb |
| 2 | Liver | ICD | 67.5/15 | 18 | 0.17±0.05 | 0.33±0.17 | 67.5/67.5 | ERIc |
| 3 | Thorax | Pacemaker | 50.4/28 | 0.9 | 1.80±0.18 | 0.54±0.27 | 16.2/50.4 | Resetb |
| 4 | Thorax | Pacemaker | 60/30 | 3 | 0.21±0.02 | 0.48±0.24 | 4/60 | Resetb |
| 5 | Thorax | ICD | 87.5/35 | 8 | 0.10±0.02 | 0.50±0.25 | 32.5/87.5, 47.5/87.5 | Resetb |
This patient was unique because one of the beams that was utilized in the treatment plan was directed towards the CIED. To minimize dose to the device, distal blocking of this beam was used.
Device reset resulting from radiation-induced change in pacing or sensing parameters (or both); reprogrammed by device clinic/manufacturer.
Elective replacement indicator when the battery voltage dropped to 2.62 V, prompting a change of generator.
Uncertainty, 1σ confidence level, was estimated based on the calibrated reading of the ion chamber detector array (doses obtained at very low dose levels)
Uncertainty, 1σ confidence level, was estimated based on the largest error bars of the figures published by Wang etal.
Abbreviations: ICD=implantable cardioverter-defibrillator
The locations of two CIEDs (both pacemakers) requiring resets relative to the radiation fields are shown in Figures 1 and 2. The ranges of proton and neutron doses to the CIED at which device malfunctions occurred in the entire group of 40 patients are illustrated in Figure 3. Notably, all CIED resets occurred at neutron doses to the device of at least 300 mSv, and the device with the replacement message had been exposed to low doses of both neutrons and protons.
Figure 1.
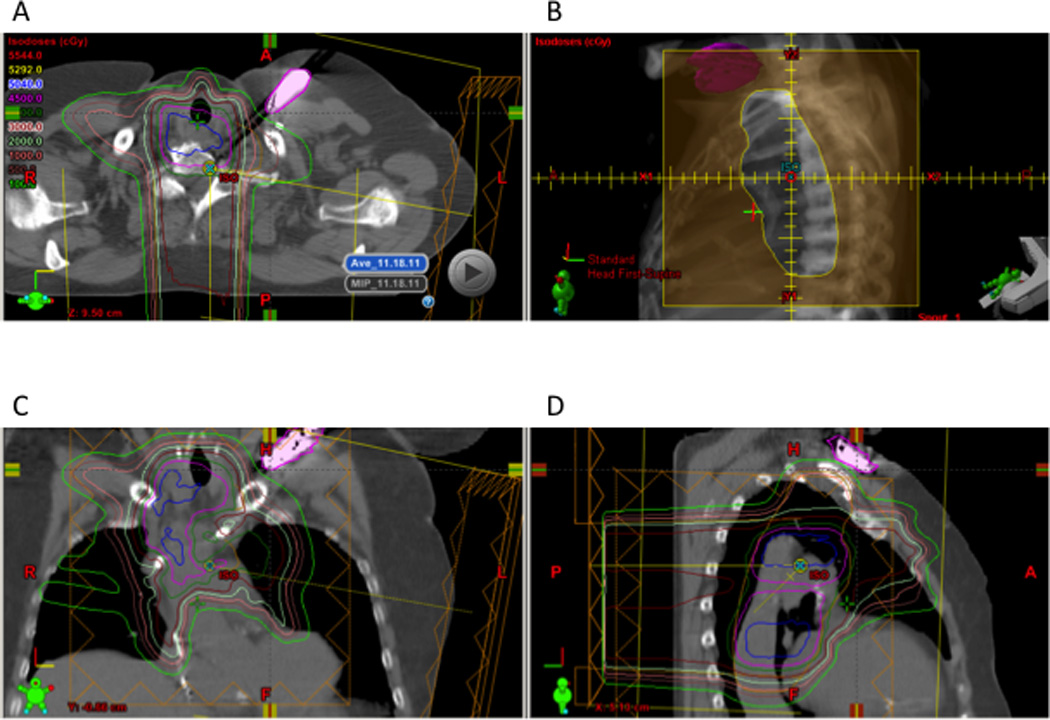
Patient 3 experienced a device reset of a pacemaker at delivery of 16.4 Gy(RBE) of a total 50.4 Gy(RBE) dose. The pacemaker (in pink) is shown in relationship to the treatment fields in the axial (A), coronal (C), and sagittal (D) planes and in relation to the aperture (B). The device was outside the radiation field and no beam range intersected the device. Estimated maximum proton and neutron doses were 1.82 Gy(RBE) and 536 mSv.
Figure 2.
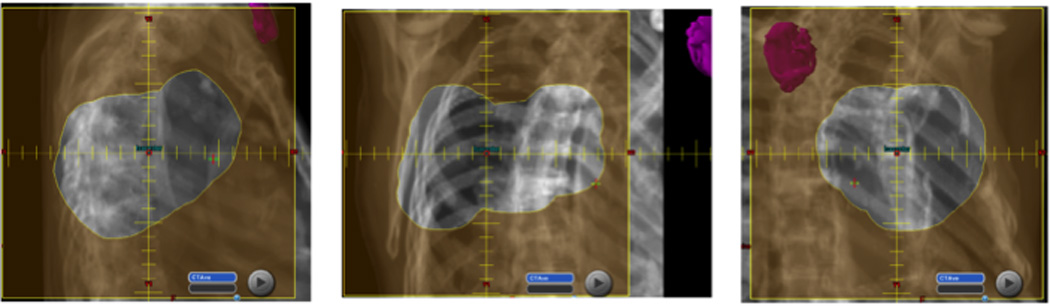
Patient 4 experienced a device reset of a pacemaker at 4 Gy(RBE) of a total 60 Gy(RBE) dose. The apertures from each field demonstrate the position of the pacemaker (in pink) in relation to the radiation field. The distance from the edge of the field to the device was 3 cm, but the estimated maximum neutron dose was 484 mSv.
Figure 3.
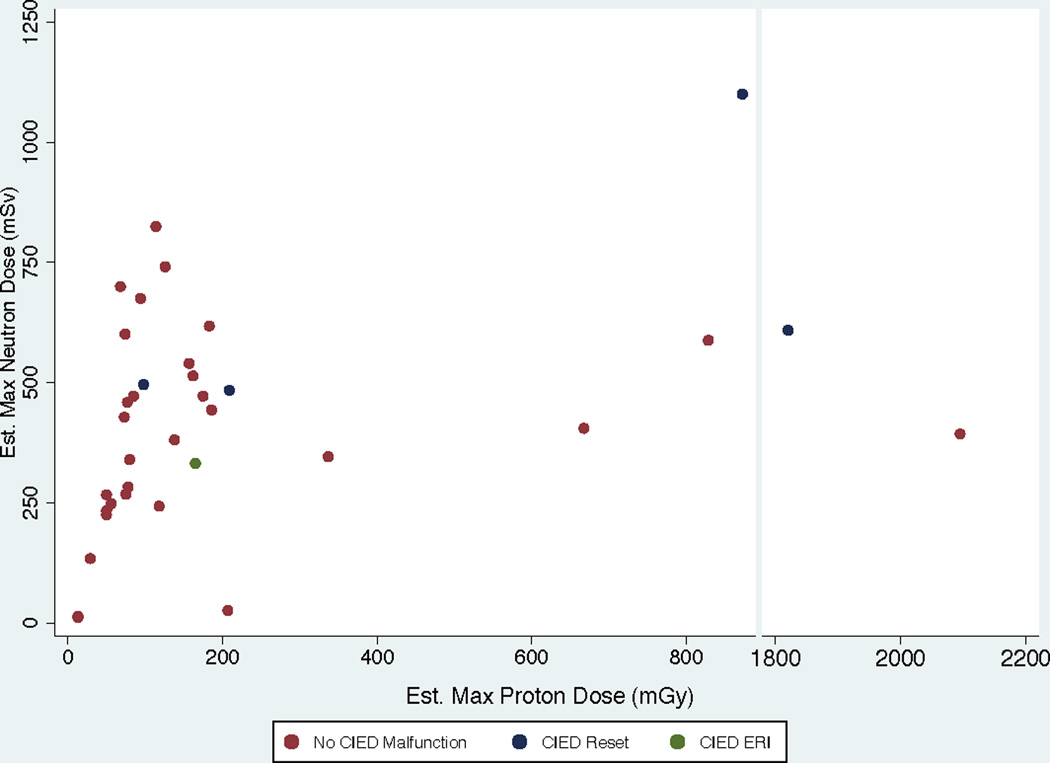
Scatter plot of neutron and proton doses for all 40 patients. Notably, no patient experienced a reset of a cardiac implantable electronic device (CIED) at a neutron dose of less than approximately 500 mSv. One patient with a liver tumor experienced an elective replacement indicator (ERI) message at 330 mSv that was predicted prior to radiation. In contrast, the variation in proton doses associated with resets was broad.
DISCUSSION
To our knowledge, this is the largest series reported to date on implantable CIED malfunctions in patients undergoing PBT. Our pertinent findings can be summarized as follows. First, the probability of CIED resets in patients being treated for pelvic tumors (e.g., prostate cancer) seems quite low. In contrast, all CIED resets occurred in patients being treated for thoracic malignancies, and one elective replacement indication occurred in a patient being treated for a liver tumor. However, it should be emphasized that the ERI was predicted prior to treatment and therefore was not influenced by radiation dose. Third, all malfunctions were addressed without symptomatic cardiac events through close monitoring, although patient selection procedures (such as excluding patients with thoracic tumors who were pacing-dependent) probably also minimized the sequelae of malfunctions. Finally, resets occurred at a range of delivered doses, suggesting that the probability of this event does not correlate directly with total delivered dose but rather is a random occurrence related to proton or neutron exposure from each fraction.
Several reports have been published on the effects of photon-based radiation therapy on implantable CIEDs1,2,5, but until now little clinical information has been available as to the specific consequences of PBT. Notably, the risk associated with treating patients with thoracic tumors and implanted CIEDs differs substantially for protons vs. photons because of the production of neutrons from protons; photons of lower energies (e.g., 6 MV) do not produce neutrons. Indeed, prior CIED studies have suggested that neutron therapy can cause CIED errors 1, and therefore it is reasonable to conclude that recommendations for radiation therapy for patients with implanted cardiac CIEDs should be individualized according to treatment modality.
The published experience of CIEDs with PBT has been limited primarily to a small number of phantom studies and very early clinical results6,9. In one study, leads were placed within the treatment field and a CIED was placed within 30 cm of the field isocenter; neutron scatter was found to have no effect on the function of the CIEDs. However, when 8 patients were assessed for resets, all of whom had pacemakers outside the treatment field (liver), 2 of 8 patients experienced a reset, leading the authors to conclude that risks to the patient could be significant if minor damage accumulated and therefore that PBT should be used only selectively for patients with CIEDs 6. In a second analysis, investigators from the same institution delivered 2 Gy/minute to a field size of 10 × 10 cm2 to 4 implanted defibrillators, and quantified the rate of CIED resets as a function of dose, in order to determine the influence of secondary neutrons as measured by Monte Carlo simulation.9
In our clinical analysis, we found a low overall reset rate (5 resets in 1352 total fractions, or 0.36%). Indeed, it is not surprising that conclusions in the clinical setting may differ from those in phantom studies. For instance, while it is useful to know mechanistically that the frequency of resets was 1 per approximately 50 Gy when delivered at 50 Gy per minute in a phantom field, as was reported in Hashimoto et al.9, our data complements these results by reporting the rate of reset with CIEDs at various distances from the treatment field in a clinical context, a scenario that is made more complex through tissue heterogeneity, the presence of device leads, and the timing of fractionated treatment. When reporting future results, we believe that discussing the risks of reset independent of tumor site or estimated proton and neutron doses can be misleading, because the CIED-reset rate for abdominopelvic tumors seems almost negligible, whereas the risk for thoracic malignancies is non-negligible and related to neutron dose. These findings are also in agreement with those of Tayama et al., who found that the neutron dose from PBT delivered by passive scattering was 0.02 Sv/Gy outside the treatment field but was reduced with increasing distance and aperture size 10.
We did not find a difference in the rate of resets for pacemakers vs. defibrillators. Although modern CIEDs may be more sensitive to radiation than older devices because of differences in internal circuitry11, studies that have included both categories of CIEDs have produced no definitive evidence exists to indicate that one device is more radiosensitive than the other 4,12,13. Therefore, we do not advocate the use of different guidelines for patients treated with PBT based on the type of CIED. Rather, we recommend that each institution generate a specific algorithm to allow careful selection of patients based on radiation dose, tumor and cardiac-device location, and dependence on pacing and other cardiac-device functions, and implement a vigilant monitoring plan such as that described here. The management algorithm for PBT used at our institution is similar to one presented by investigators at the University of Michigan 4, and a robust and broad approach of risk classification of CIEDs, with a combined set of management procedures, has also been published by the American Association of Physicists in Medicine14 and by investigators in the Netherlands15. Such guidelines are crucial in the multidisciplinary coordination of care for these complex cases.
Other than the constraints of any retrospective analysis, our study had the following limitations. First, the small number of events precluded our ability to make specific recommendations on quantitative threshold distances or doses that place patients at a higher risk of adverse events, other than the site-specific recommendations noted above; indeed, the effect of neutron scatter on CIEDs seems to be minimal for patients with treatment fields farther than 30 cm from the CIED. Establishing more precise guidelines will likely require in vitro phantom studies in which both pacemaker and defibrillators are placed at various distances from treatment fields; use of a spectrum of delivered doses may allow the dose or distance (or both) that substantially increases the probability of reset for a given CIED to be defined. Such studies are currently underway at our institution.
Second, our analysis did not address the potential for dose perturbations associated with generators or leads of implanted CIEDs, as has been demonstrated elsewhere16,17. Disturbances such as these could significantly reduce the overall delivered dose of PBT, possibly reducing the overall effectiveness of PBT in some patients. Although this topic is beyond the scope of the current analysis, the effect of CIED generators and leads on PBT dose perturbations should be considered when establishing treatment guidelines, particularly for patients with thoracic tumors.
Finally, the method we used to estimate proton and neutron doses, although easily applied across institutions, is nonetheless an estimate. This method of estimation differs from others presented across other institutions, such as the University of Tsukuba.2,9, who utilized methods such as an optically stimulated luminescence dosimeter (OSL), a CR39 dosimeter, and Monte Carlo simulations to assess photon/neutron dose directly. And in another study by the same institution, in which the authors assessed the influence of secondary neutrons on implantable defibrillators in an in vitro setting with four devices, Monte Carlo simulations were used to estimate the secondary neutron dose9. In contrast, using our methods of estimation, the error rate ranges from 5% for doses close to the field edge, to 20% in longer distances, and neutron dose estimation has a larger degree of uncertainty, up to 50%.8 However, in this retrospective clinical setting, we believe that it is reasonable to use this estimation approach, as it has been published as a valid estimation tool and can be applied in a straightforward, reproducible manner to future studies assessing similar questions.
Summary.
We examined the incidence of cardiac-device resets in patients undergoing proton beam therapy at our institution in 2009–2012. The percentage of resets was low overall but was more common among patients with thoracic tumors and those receiving a high estimated neutron dose to the device. We recommend that proton beam therapy be avoided for patients who are “pacing-dependent” and those with tumors in close proximity to the device.
Acknowledgments
Funding was provided in part by Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center
REFERENCES
- 1.Elders J, Kunze-Busch M, Jan Smeenk R, et al. High incidence of implantable cardioverter defibrillator malfunctions during radiation therapy: neutrons as a probable cause of soft errors. Europace. 2013;15:60–65. doi: 10.1093/europace/eus197. [DOI] [PubMed] [Google Scholar]
- 2.Hashii H, Hashimoto T, Okawa A, et al. Comparison of the Effects of High-Energy Photon Beam Irradiation (10 and 18 MV) on 2 Types of Implantable Cardioverter-Defibrillators. Int J Radiat Oncol Biol Phys. 2013;85:840–845. doi: 10.1016/j.ijrobp.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Kapa S, Fong L, Blackwell CR, et al. Effects of scatter radiation on ICD and CRT function. Pacing Clin Electrophysiol. 2008;31:727–732. doi: 10.1111/j.1540-8159.2008.01077.x. [DOI] [PubMed] [Google Scholar]
- 4.Makkar A, Prisciandaro J, Agarwal S, et al. Effect of radiation therapy on permanent pacemaker and implantable cardioverter-defibrillator function. Heart Rhythm. 2012;9:1964–1968. doi: 10.1016/j.hrthm.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Solan AN, Solan MJ, Bednarz G, et al. Treatment of patients with cardiac pacemakers and implantable cardioverter-defibrillators during radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:897–904. doi: 10.1016/j.ijrobp.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Oshiro Y, Sugahara S, Noma M, et al. Proton beam therapy interference with implanted cardiac pacemakers. Int J Radiat Oncol Biol Phys. 2008;72:723–727. doi: 10.1016/j.ijrobp.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 7.Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management this document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS) Heart Rhythm. 2011;8:1114–1154. doi: 10.1016/j.hrthm.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Sahoo N, Zhu RX, et al. Measurement of neutron dose equivalent and its dependence on beam configuration for a passive scattering proton delivery system. Int J Radiat Oncol Biol Phys. 2010;76:1563–1570. doi: 10.1016/j.ijrobp.2009.07.1732. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto T, Isobe T, Hashii H, et al. Influence of secondary neutrons induced by proton radiotherapy for cancer patients with implantable cardioverter defibrillators. Radiat Oncol. 2012;7:10. doi: 10.1186/1748-717X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tayama RFY, Tadokoro M, Fujimaki H, Sakae T, Toshiyuki T. Measurement of neutron dose distribution for a passive scattering nozzle at the Proton Medical Research Center (PMRC) Nucl Instr Methods. 2006;564:532–536. [Google Scholar]
- 11.Sundar S, Symonds RP, Deehan C. Radiotherapy to patients with artificial cardiac pacemakers. Cancer Treat Rev. 2005;31:474–486. doi: 10.1016/j.ctrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Soejima T, Yoden E, Y NI, et al. Radiation therapy in patients with implanted cardiac pacemakers and implantable cardioverter defibrillators: a prospective survey in Japan. J Radiat Res. 2011;52:516–521. doi: 10.1269/jrr.10143. [DOI] [PubMed] [Google Scholar]
- 13.Hudson F, Coulshed D, D'Souza E, et al. Effect of radiation therapy on the latest generation of pacemakers and implantable cardioverter defibrillators: A systematic review. J Med Imaging Radiat Oncol. 2010;54:53–61. doi: 10.1111/j.1754-9485.2010.02138.x. [DOI] [PubMed] [Google Scholar]
- 14.Marbach JR, Sontag MR, Van Dyk J, et al. Management of radiation oncology patients with implanted cardiac pacemakers: report of AAPM Task Group No. 34. American Association of Physicists in Medicine. Med Phys. 1994;21:85–90. doi: 10.1118/1.597259. [DOI] [PubMed] [Google Scholar]
- 15.Hurkmans CW, Knegjens JL, Oei BS, et al. Management of radiation oncology patients with a pacemaker or ICD: a new comprehensive practical guideline in The Netherlands. Dutch Society of Radiotherapy and Oncology (NVRO) Radiat Oncol. 2012;7:198. doi: 10.1186/1748-717X-7-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gossman MS, Blohm CM. Beam profile disturbances from interactions with implantable pacemakers and implantable cardioverter-defibrillators. Med Dosim. 2013 doi: 10.1016/j.meddos.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Wootton LS, Polf JC, Peterson S, et al. Proton dose perturbations caused by high-voltage leads from implanted cardioverter defibrillators. J Appl Clin Med Phys. 2012;13:3813. doi: 10.1120/jacmp.v13i4.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]


