Abstract
Background:
Sox9 is an operon that positively regulates the transcription of type II collagen. The generation of type II collagen plays a critical role in the healing process of the bone-tendon junction (BTJ).
Materials and Methods:
Sox9 was injected into an established bone-tendon healing model in order to observe its effect on the healing by determining the biomechanical properties of the BTJ. In addition, the recombinant adenovirus Sox9 was used to transduce the animal model samples and in vivo observations of the effect of the adenovirus-mediated Sox9 transduction as well as its promotion of the healing properties were made.
Results:
Sox9 was not only able to promote the healing, but also increased the biomechanical strength. The recombinant Sox9 delivered by adenoviral vector can be expressed at a high level in the damaged tissues of the bone-tendon junction, which can stimulate the production of type II collagen and improve the healing of the BTJ.
Conclusions:
Based on the results of this study, we considered that gene therapy may be applicable in the healing process of the bone-tendon junction.
Keywords: Bone-tendon junction healing, histological examination, recombinant adenovirus Sox9
INTRODUCTION
Bone-tendon junction injuries are common problem in athletes. Due to a change in lifestyle, there has been an increase in the occurrence of bone-tendon junction (BTJ) injuries like patellar tendinitis, lateral epicondylitis, rotator cuff injury and the rupture of the Achilles tendon in recent years.1,2,3 As a result, there has been a rising interest in the research of BTJ-related injuries. The BTJ generally consists of four groups of tissue, namely fibrous tissue, uncalcified fibrocartilage (UF), calcified fibrocartilage (CF), and bone. Based on the properties of the bone-tendon tissues, it can also be categorized into fibro-tissues and bone, with a spacing known as the fibrocartilage zone.4 The short transition distance between the soft and hard tissues results in a slower rate of recovery. Furthermore, the recovery of the fibrocartilage zone has to be taken into consideration.
The fibrocartilage zone connecting the bone to the tendon at the BTJ plays an important role in the repair and reconstruction of the BTJ. The successful reconstruction of the fibrocartilage zone will allow for an improvement in the histological and mechanical recovery of the BTJ. However, related studies have shown that the reconstruction of the fibrocartilage zone is extremely slow and difficult.5,6 There are several traditional treatments for BTJ injuries. In recent years, non-invasive techniques or therapies like low intensity pulsed ultrasound (LIPUS), extracorporeal shock-wave therapy, and electrical stimulation have gained recognition as well.2
The Sox9 gene, which belongs to a family of genes known as SOX and can be found in the chromosome region 17q24II-25,7 is crucial to the embryonic development in the early stages.8 Sox9 is a transcription factor that has C-terminal high-mobility group (HMG) domain and N-terminal transactivation domain. It participates in various embryonic developmental processes such as chondrogenesis.9 Translocations upstream of the coding sequence suggested that the altered expression of Sox9 is capable of severely impacting chondrogenesis and thus lead to abnormal skeletal development. As a transcriptional activator, the Sox9 gene is able to co-regulate the proliferation and differentiation of the chondrocytes together with other signal pathways through direct or indirect means.10,11 This can be achieved through the combination with the chondrocytes-specific enhancer type II collagen gene (Col2al) and L-Sox5, Sox6.
Currently, main treatments for bone-tendon healing include surgical treatment and conservative therapy. However, there are obvious limitations to these treatments.12 Gene therapy involves the use of gene transfer techniques to replace a mutated gene with an exogenous gene that encodes a therapeutic protein in order to treat certain diseases13 Previous studies have confirmed that the target genes of Sox9 promote the healing of bone and cartilage damage.13,14 Therefore, the synthesis of various matrix components can be regulated by the insertion or transformation of the therapeutic gene Sox9. The gene therapy should ideally provide a precise transference of the target gene to target cells to achieve an efficient, specific, and controllable expression.15 However, at present, the transfers of such genes have poor tissue specificity, which leads to problems like low transfer efficiency and difficulties in gene expression. There is an extensive use of adenovirus in current gene therapies due to advantages such as susceptibility to infection, effective expression, high stability, high genome capacity, propensity to proliferate and purification and high viral titer.16
In this study, we injected Sox9 into an established bone-tendon healing model and aimed to observe its effect on the healing of the BTJ by determining the biomechanical properties of the junction.
MATERIALS AND METHODS
48 adult New Zealand white rabbits (18 weeks old), weighing between 2.0 and 2.9 kg, were randomly divided into four equal groups: A, B, C, and D. The research protocol was approved in accordance with the institutional guidelines of the Animal Care and Use Committee at Wuhan University.
Establishment of animal model and grouping
The animals received an intravenous injection of 2.5% pentobarbital sodium (0.5 mL) and 10% ketamine hydrochloride (0.5 mL/kg) into the marginal ear vein before the surgery. Establishment of animal model was reported in previous report.17 During our study, we found that simply injecting Sox9 gene resulted in low transcript and using adenovirus vector could improve this situation to a certain level. In our study, we constructed recombinant adenovirus vector Sox9, AdSox9, and it was used to treat BTJ. Sox9 was injected into the BTJ of rabbits in group A and AdSox9 was transfected into the BTJ of rabbits in group C. Rabbits in group B served as control and did not receive any injection during surgery. Rabbits in group D were transfected with adenovirus and served as a control for group C. For the rabbits in group A, the seam between the bone and tendon was injected with a concentration of 200 ng/ml Sox9 that was dissolved in 0.9% sodium chloride (Sigma, MDL number: MFCD03097288, Product number: S4444). The BTJs of the rabbits in group C were injected with AdSox9 that had been previously diluted to a titer of 3.0 × 106 pfu/mL. The epidermis of the right lower limb of the rabbits in all groups was sutured after the incision and they served as false-positive groups. The operated limbs were fixed with plaster casts and the rabbits received 400,000 U/kg/d of penicillin via intramuscular injection 3 days after surgery. The surgical incision and blood supply in the limbs were observed. The plaster casts were removed 4 weeks later and the rabbits were allowed to roam freely within the cage.
Four groups of rabbits were sacrificed 4 weeks, 8 weeks, and 12 weeks after surgery. After the rabbits in group A were sacrificed, two were selected for biomechanical testing and two for histological examinations. After the biomechanical testing, samples were used for the histological examination. The BTJ of the rabbits in group C were divided into two parts: One part was subjected to biomechanical testing (two samples) and the other part was subjected to histological examinations (two samples). After the biomechanical testing was conducted, the samples were collected and used for mRNA detection.
Histological examination
The experimental samples were collected from different groups of animals. The experimental limb was extracted whole from the quadriceps femoris to the tibia while muscles and other tissues from the attachment points were detached. Next, the distal patellar tendon was removed from the tibial tubercle and the patella and patellar ligament were kept intact. The structure, comprising of the patellar ligament and the patella with some quadriceps femoris attached, was sliced along the middle in a vertical axis. We then conducted a preliminary observation of the specimen's healing progress. The structure was later fixed with 10% formaldehyde for 24 hours and decalcified with 10% formic acid for 4 weeks. It was subsequently dehydrated with progressively concentrated amounts of ethanol, treated with clearing and infiltration agents and then embedded in paraffin. A 5μm thick incision in the sagittal plane of the specimen was made. The specimen was stained using the hematoxylin and eosin stain (H and E stain) and the morphology of the BTJ osteocytes, chondrocytes, and tendon tissue ground substance were observed under a light microscope.
Biomechanical testing
The connective tissues and sutures from the surgery surrounding the knee of the specimen were first removed. Using a vernier caliper, triplicate measurements of the thickness, width, and cross section of the healing surface from the end of the quadriceps femoris to the proximal tibial osteotomy were made to obtain mean readings for statistical analysis. The end of the distal quadriceps femoris and the proximal tibial osteotomy were secured on a fixture and a 10° anteversion angle between the tibia and the load bearing axis was created. The structure was then pulled at a velocity of 20 mm/min using a dynamometer with the force of 2 KN and the breaking load was recorded. The rupture surface created during experiment was examined.
RT-PCR analysis of mRNA expression
Total RNA was extracted from 100 mg of BTJ tissues and the RT-PCR was used to identify the Sox9 gene expression. Each sample was tested in triplicates.
Statistical analysis
All data were expressed as mean ± SD. Statistical analysis was performed with 4 × 2 factorial analysis of variance between the treatment and control groups using the SPSS 13.0 statistical software. Statistical significance was set at the P < 0.05 level.
RESULTS
The healing effect of Sox9 on BTJ was observed through the histological changes and detection of the biomechanical properties of the junction. After the surgery, varying degrees of swelling to the surgical incisions were observed in the animals. They were given symptomatic treatments like antibiotics and none of them succumbed to infection.
General examination
According to observations made on the samples collected from group A 4 weeks after surgery, fibrous tissue hyperplasia could be seen on the dorsal surface of the patella with no obvious adhesions on the skin. In addition, the cut surface of the surgery was unclear and the surface of the bone-tendon showed trends of fusion. The dorsal surface of the patella of group B, which was treated with 0.9% sodium chloride, had smaller amounts of fibrous tissue hyperplasia than that of group A. A certain degree of adhesion could be seen between the incision tissues and the skin. The cut surface in group B was clearer in comparison to that of group A. There was a notable increase of fibrous tissue hyperplasia in group A animals that were sacrificed 8 weeks after the surgery, compared to those sacrificed after 4 weeks. The articular surface was flexible and there was a loss of glossiness. There was an increase in the fusion in the BTJ region and the cut surface was quite unclear. For the corresponding group B, fibrous tissue hyperplasia could be seen and there were fewer adhesions between the incision tissues and the skin. However, the cut surface was clear and animals in group A still healed faster than those in group B. In the case of the specimens from Group A that were collected 12 weeks after the surgery, most of the BTJ had fused, the granulation tissues were clearer and neatly arranged, and the cut surface could not be distinguished. In contrast, the patella and the dorsal surface of the patellar tendon of the corresponding control group had a little fibrous tissue hyperplasia, the granulation tissues were unclear and arranged irregularly, and the cut surface was a little unclear.
Results of histological observation
The morphological changes of the osteocytes, chondrocytes, and tendon tissue ground substance of the BTJ at different times were observed under the light microscope after H and E staining [Figure 1]. Large amounts of fibroblasts and chondrocyte-like cells could be found in the Sox9 group 4 weeks after the surgery. There was an obvious increase in fibroblasts and part of the cells and collagen were aligned along the vertical axis of the bone-tendon. There was a disorder in the arrangement of a large portion of the cells and newly formed cancellous bones that grew from the cut surface toward the distal patellar tendon. Some chondrocyte-like cells that displayed a focal distribution could be seen in the bone-tendon contact area. A large amount of fibrous connective tissue cover was seen on the dorsal surface of the patella and patellar tendon [Figure 1a]. In the corresponding group B, there was a lower proliferation rate in chondrocyte-like cell and fibroblasts. The cut surface of the patellar and patellar tendon was fairly clear and new bone growth could still be found in the BTJ [Figure 1b].
Figure 1.
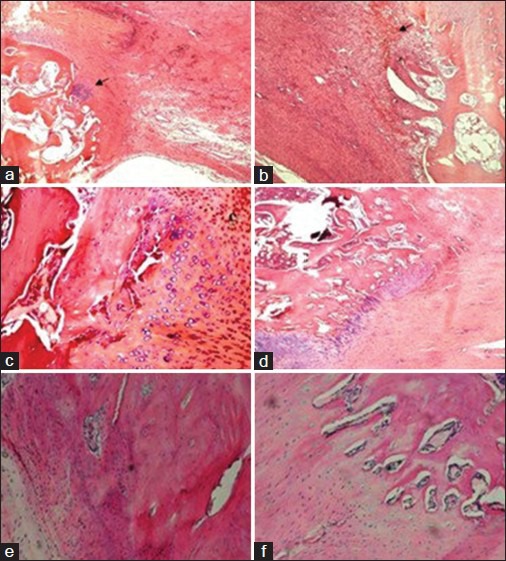
Histological observations of bone tendon junction healing in group A and B treated with Sox9; a and b (4 weeks); c and d (8 weeks); e and f (12 weeks). The arrow in a shows the fibrous connective tissue, while the arrow in Figure 1b shows the chondrocyte-like cells, (H and E, ×10).
Fibroblast proliferation could also be seen in samples collected from group A 8 weeks after the surgery. The formation of the transition zone of the chondrocytes on the contact surfaces of the patellar tendon and cancellous bone was not obvious. Chondrocyte-like cell proliferation with a regular arrangement could be found. New cancellous bone could be seen growing towards the inside of the patellar tendon at the distal end of the BTJ. Part of the patellar tendon and cancellous bone showed a fusion trend and the cut surface was unclear [Figure 1c]. Fibroblast proliferation could be seen and the formation of the transition zone of the chondrocytes on the contact surfaces of the patellar tendon and cancellous bone was not obvious in the corresponding control group. The chondrocyte-like cells showed a regular arrangement and new cancellous bone in the residual distal patella was seen growing toward the inside of the patellar tendon. Part of the patellar tendon and cancellous bone showed a fusion trend and the cut surface was unclear [Figure 1d].
The cut surface of samples in group A collected 12 weeks after the surgery was found to be almost completely fused and a large amount of new and actively proliferating cancellous bone could be seen. There was a notable increase in collagen fibers, which had an orderly arrangement along the vertical axis [Figure 1e]. Part of the cut surface had fused for samples in group B that were collected 12 weeks after the surgery. There were fewer chondrocyte-like cells in a bead-like arrangement and collagen fibers had a more regular pattern compared to that of samples from 4 weeks after surgery. The tidemark line was yet to form on the cells and collagen fibers that were arranged along the vertical axis [Figure 1f].
Results of biomechanical test
The results of comparison of the cross-sectional area, breaking load, and ultimate tensile strength of the BTJ of group A and B are shown in Table 1 and Figure 2. The cross-sectional areas of group A at 4, 8, and 12 weeks after the surgery are 24.38 ± 4.53, 18.65 ± 3.49, and 14.82 ± 1.57 mm2, respectively. The cross-sectional areas for control group are 33.42 ± 7.25, 22.12 ± 4.52, and 20.81 ± 4.14 mm2, respectively. The results of the comparison of the cross-sectional areas between group A and B for 4 and 12 weeks after the surgery had a statistical significance (P < 0.05). The breaking load of group A at 4, 8, and 12 weeks after the surgery were found to be 112.23 ± 21.29, 165.31 ± 18.32, and 359.25 ± 28.31 N, respectively. In contrast, the breaking load of group B at 4, 8, and 12 weeks after the surgery were much smaller than in group A, at 64.76 ± 12.84, 114.92 ± 18.46, and 268.32 ± 23.29 N, respectively. Results showed that the breaking load of the three samples in group A are significantly higher than those of samples from group B (P < 0.05). The results showed that there were significant differences (P < 0.05) between group A and B at 4, 8, and 12 weeks after the surgery. The ultimate tensile strength values (MPa) of group B at 4, 8, and 12 weeks after the surgery were shown to be 2.72 ± 2.43, 4.53 ± 3.21, and 14.54 ± 4.65 MPa, respectively. These values are much lower than the corresponding values of group A at 9.79 ± 4.66, 13.89 ± 5.78, and 26.72 ± 12.54 MPa, respectively.
Table 1.
Results of biomechanical test of group A and group B at different time after the surgery
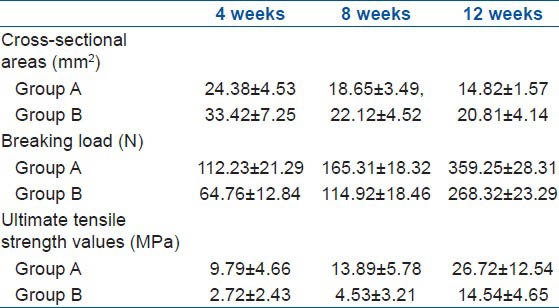
Figure 2.
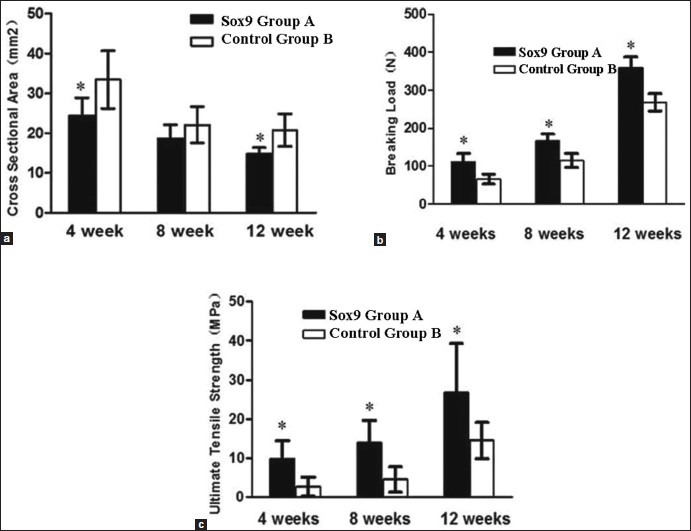
A comparison of the cross-sectional area (a) breaking load (b) and ultimate tensile strength (c) of the samples in group A (Sox9) and group B (control) at 4, 8, and 12 weeks after the surgery. *Indicates a significant difference at P < 0.05
Results of general observations in groups treated with AdTrack-Sox9 at different times
In comparison to the adenovirus group D, observations of the samples in group C at 4 weeks after the surgery showed fibrous tissue hyperplasia on the dorsal surface of the patella, with no obvious adhesions on the skin. The articular surface was glossy and the cut surface was unclear. There was a notable increase in the fibrous tissue in group C samples collected 8 weeks after the surgery compared to those collected after 4 weeks. The articular surface was flexible and there was a loss of glossiness. In addition, there was an increase in the fusion in the BTJ region and the cut surface was quite unclear. In the case of the samples of Group C collected 12 weeks after the surgery, most of the BTJ had fused, the granulation tissues were clearer and neatly arranged, and the cut surface could not be distinguished.
Results of H and E, staining of specimen in groups treated with AdTrack-Sox9 at different times
Large amounts of fibroblasts and chondrocyte-like cells could be found in the AdTrack-Sox9 group 4 weeks after surgery. Part of the cells and collagen were aligned along the vertical axis of the bone-tendon and most of the cells had a disordered arrangement while a portion was arranged in a regular pattern. Newly formed cancellous bone was seen and some chondrocyte-like cells that displayed a focal distribution were also seen in the bone-tendon contact area.
There was a notable increase in the fibrous tissue hyperplasia in group C specimens collected 8 weeks after the surgery. The generation of the transition zone of the chondrocytes on the contact surfaces of the patellar tendon and cancellous bone was visible. There was also a tremendous growth of chondrocyte-like cells with a relatively regular arrangement. New cancellous bone could be seen growing toward the inside of the patellar tendon at the distal end of the BTJ. Part of the patellar tendon and cancellous bone showed a fusion trend.
In the case of samples collected 12 weeks after surgery, the cut surface had fused and a large amount of new cancellous bone had formed.
Results of mRNA expression of specimen in groups treated with AdTrack-Sox9 at different times
The nucleus pulposus of the animals which was injected with AdTrack-Sox9 after 1 week was used for PCR analysis. The PCR results showed that the product size of the transcription of the Sox9 gene was between 400 and 500 bp (base-pairs), which is in accordance with the length of the design.
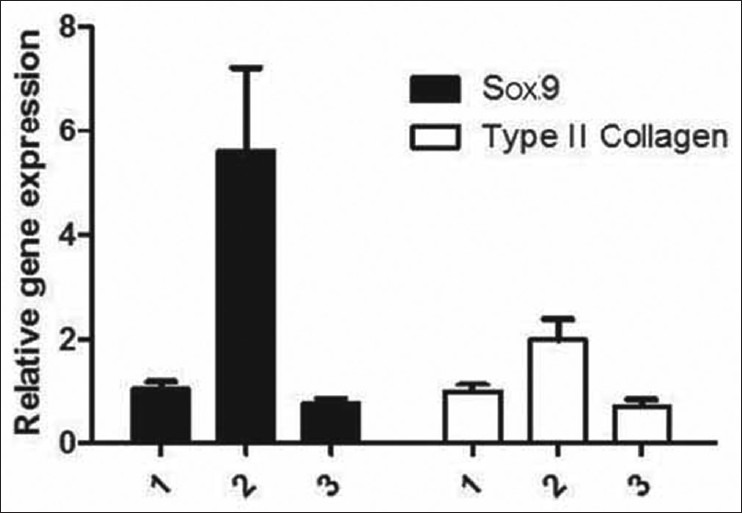
The relative level of the mRNA expression of the Sox9 gene was measured through a quantitative analysis of the PCR product 4 weeks after the surgery. After injecting the Sox9 vector that was transfected with the recombinant adenovirus 8 weeks after the surgery for group C, there was an obvious improvement of 532% and 205% in the expression level for Sox9 and type II collagen mRNA, respectively (P < 0.05). The corresponding values for group D were lower in comparison to that of group C, at 76% for Sox9 and 69% for type II collagen mRNA [Table 2 and Figures 3 and 4].
Table 2.
The gene expression in the nucleus pulposus tissues after the BTJ was injected with Sox9 that was transduced with the recombinant adenovirus vector after Surgery
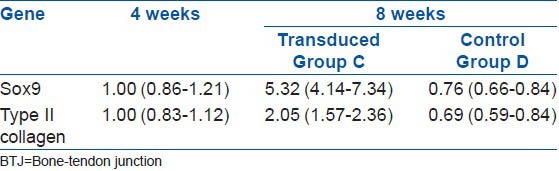
Figure 3.
A comparison of the cross-sectional area, breaking load and ultimate tensile strength of group A and B at 4, 8, and 12 weeks after the surgery. *Indicates a significant difference at P < 0.05
Figure 4.
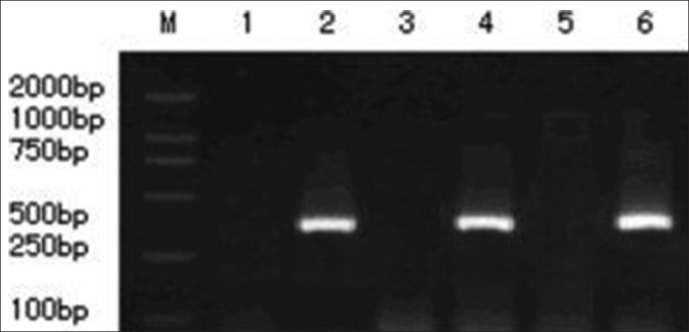
The level of mRNA expression of the Sox9 gene that was transduced with recombinant adenovirus vector in the nucleus pulposus is shown. Lines 1, 3, and 5 represent the mRNA expression of the Sox9 gene that was transduced with adenovirus in group D at 1, 4, and 8 weeks, respectively. PCR results showed that there was no mRNA expression during the treatment period; lines 2, 4, and 6 represent the mRNA expression of the Sox9 gene that was transduced with AdSox9. PCR results showed that there was a gradual increase in the expression of Sox9 in group C with time
Results of the biomechanics of specimen in groups treated with AdTrack-Sox9 at different times
The cross-sectional area, breaking load, and ultimate tensile strength of the samples in groups C and D were determined [Table 3]. The results showed a significant difference (P < 0.05) in the cross-sectional area of group C compared to that of group D. The cross-sectional areas of samples in group C collected at 4, 8, and 12 weeks after the surgery were found to be 20.62 ± 4.17, 15.43 ± 2.87, and 13.56 ± 1.92 mm2, respectively.
Table 3.
Results of biomechanical test of group C at different time after the surgery
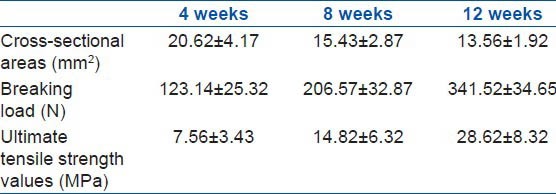
The breaking load values of the samples in group C that were treated with AdTrack-Sox9 at 4, 8, and 12 weeks after the surgery were 123.14 ± 25.32, 206.57 ± 32.87, and 341.52 ± 34.65 N, respectively. There was a statistical significance (P < 0.05) in relation to the values collected from the control group. The ultimate tensile strengths of the samples in group C that were treated with AdTrack-Sox9 at 4, 8, and 12 weeks after the surgery were found to be 7.56 ± 3.43, 14.82 ± 6.32, and 28.62 ± 8.32 MPa, respectively. There was also a statistical significance (P < 0.05) in relation to the values from the control group [Figure 5].
Figure 5.
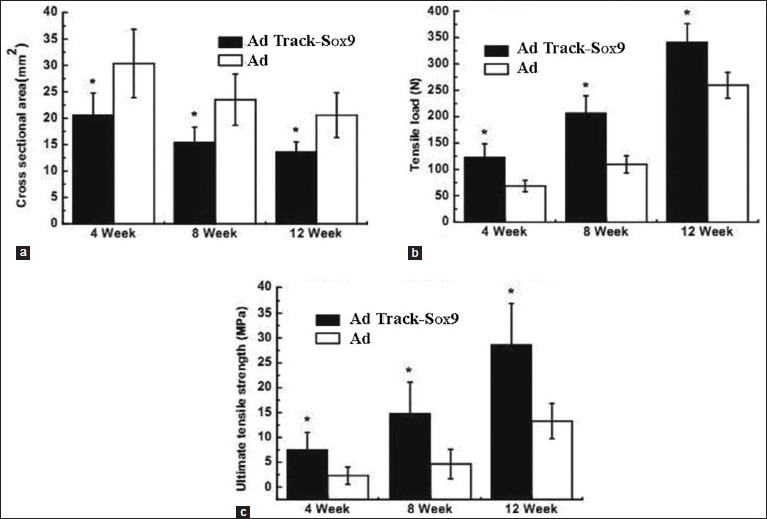
A comparison of the cross-sectional area (a), breaking load (b), and ultimate tensile strength (c) of the samples in group C and group D at 4, 8, and 12 weeks after the surgery (), n=4. *Indicates a significant difference at P < 0.05
DISCUSSION
Although ongoing researches on the improvement to these techniques are underway, most of them are focussed on the development of the tissue structure. The joint has to be immobilized after surgery as the BTJ is situated near the joint. Long term immobilization gives rise to a range of problems like joint stiffness, which negatively impacts patient's daily work and life.18,19 Therefore, it is important to have early treatment programs that can provide a quicker healing of BTJ injuries. Previous research has reported that the injection of osteoprotegerin into the bone-tendon interface in a BTJ damage repair model of rabbits can suppress the formation, differentiation, activation, and resorption of osteoclasts, which ultimately improves the healing of BTJ injuries.20 Researchers used liposomal clodronate on the damage repair model of Sprague-Dawley rats to diminish macrophage-induced TGF-β production, which can promote the rejuvenation of the BTJ.21 Although such literature is relatively abundant, most of it is focused on the histology of bone-tendon healing.
The main causes of tendon adhesion formation are thought to be the extrinsic healing mechanism, which is dependent on the blood supply and repair cells, which originate from the peritenon tissue adhesions. Biomechanical-based researches, on the other hand, are less common. Soft tissues such as tendons and ligaments in BTJ can turn into hard tissues (like bone) through a range of tissue types in a short distance, the stress requirements for the BTJ are relatively large.22,23 A simple histology-based treatment of the BTJ cannot satisfy the body's needs as the choice of treatment program not only depends on the histology but also on the patient's tolerance to stress.
Results have shown that the animals in Sox9-treated groups had a higher cross-sectional area, breaking load, and ultimate tensile strength than those in the control groups. Taking into consideration that the fourth week after surgery is the inflammatory phase, a large amount of granulation tissue had filled the BTJ, resulting in an increase in the cross-sectional area for all groups. However, as the healing of the BTJ in the control group is slower than that of the Sox9 group, the cross-sectional area of the Sox9 group is still larger. As a result of the new bone formation stage that accompanies the structural formation of the fibrocartilage transition zone, the regeneration of the woven bone, maturation of the fibrocartilage node as well as the bone remodeling phase,24,25 the cross-sectional area of the BTJ of the Sox9 group gradually became smaller. The breaking load and ultimate tensile strength for all Sox9 group samples collected over the three periods were higher than that of the control group. These indicate that the Sox9 can not only improve the healing of the BTJ, but also increase the biomechanical strength, which in turn results in the acceleration of the healing process.
The RT-PCR results in the present study demonstrated that Sox9 can be expressed at a high level in BTJ that had been injected with AdSox9, thus enhancing the healing process in the BTJ. The subsequent biomechanical tests showed that the cross-sectional area, breaking load, and ultimate tensile strength for the Sox9-treated group samples collected 4, 8, and 12 weeks after the surgery were significantly higher (P < 0.05) than that of the control group, proving that AdSox9 can improve the healing of the BTJ.
In this study, we used recombinant adenovirus Sox9 (AdSox9) in this research, the transgenic technology involved insertion of the Sox9 coding gene into specific target cells and transfection of the animal model. And then the healing effect of Sox9 on BTJ was detected. In summary, based on our findings we considered that gene therapy may be applicable in the healing process of the bone-tendon junction.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Gao JZ, Messner K. Quantitative comparison of soft tissue-bone interface at chondral ligament insertion in the rabbit knee joint. J Anat. 1996;188:367–73. [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H, Hu J, Qin L, Chan KM, Li G, Li K. Area, length and mineralization content of new bone at bone-tendon junction predict its repair quality. J Orthop Res. 2011;29:672–7. doi: 10.1002/jor.21292. [DOI] [PubMed] [Google Scholar]
- 3.Nourissat G, Diop A, Maurel N, Salvat C, Dumont S, Pigenet A, et al. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS One. 2010;18(5):e12248. doi: 10.1371/journal.pone.0012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong MW, Qin L, Tai JK, Lee SK, Leung KS, Chan KM. Engineered allogeneic chondrocyte pellet for reconstruction of fibrocartilage zone at bone-tendon junction: A preliminary histological observation. J Biomed Mater Res B Appl Biomater. 2004;70:362–7. doi: 10.1002/jbm.b.30049. [DOI] [PubMed] [Google Scholar]
- 5.Silva MJ, Boyer MI, Ditsios K, Burns ME, Harwood FL, Amiel D, Gelberman RH. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury andsuture repair. Orthop Res. 2002;20:447–453. doi: 10.1016/S0736-0266(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/ormechanical load. J Anat. 2006;208:471–90. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dilling CF, Wada AM, Lazard ZW, Salisbury EA, Gannon FH, Vadakkan TJ, et al. Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification. J Bone Miner Res. 2010;25:1147–56. doi: 10.1359/jbmr.091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu MH, Zheng YP, Huang QH, Lu HB, Qin L. Low intensity pulsed ultrasound increases the mechanical properties of the healing tissues at bone-tendon junction. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2141–4. doi: 10.1109/IEMBS.2009.5333960. [DOI] [PubMed] [Google Scholar]
- 9.Munirah S, Samsudin OC, Aminuddin BS, Ruszymah BH. Expansion of human articular chondrocytes and formation of tissue-engineered cartilage: A step towards exploring a potential use of matrix-induced cell therapy. Tissue Cell. 2010;42:282–92. doi: 10.1016/j.tice.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Jung SN, Rhie JW, Kwon H, Jun YJ, Seo JW, Yoo G, et al. In vivo cartilage formation using chondrogenic-differentiated human adipose-derived mesenchymal stem cells mixed with fibrin glue. J Craniofac Surg. 2010;21:468–72. doi: 10.1097/SCS.0b013e3181cfea50. [DOI] [PubMed] [Google Scholar]
- 11.Fanburg-Smith JC, Auerbach A, Marwaha JS, Wang Z, Rushing EJ. Reappraisal of mesenchymal chondrosarcoma: Novel morphologic observations of the hyaline cartilage and endochondral ossification and beta-catenin, Sox9 and osteocalcin immunostaining of 22 cases. Hum Pathol. 2010;41:653–62. doi: 10.1016/j.humpath.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Xu Q, Jiang X, Ke Y, Zhang S, Xu R, Zeng Y. Gene therapy in hemiparkinsonian rhesus monkeys: Long term survival and behavioral recovery by transplantation of autologous human tyrosine hydroxylase-expressing neural stem cells. Cytotherapy. 2010;12:226–37. doi: 10.3109/14653240903490371. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Feng Y, Fu XL, Cai XW. Effects of gene therapy with replication- defective adenovirus ericlosing Egr-1 promoter and Smad7 cDNA on irradiation-induced pulmonary fibrosis: Experiment with mice. Zhonghua Yi Xue Za Zhi. 2006;86:2847–52. [PubMed] [Google Scholar]
- 14.Campbell SE, Bennett D, Nasir L, Gault EA, Argyle DJ. Disease- and cell-type-specific transcriptional targeting of vectors for osteoarthritis gene therapy: Further development of a clinical canine model. Rheumatology (Oxford) 2005;44:735–43. doi: 10.1093/rheumatology/keh590. [DOI] [PubMed] [Google Scholar]
- 15.Alderuccio F, Chan J, Scott DW, Toh BH. Gene therapy and bone marrow stem-cell transfer to treat autoimmune disease. Trends Mol Med. 2009;15:344–51. doi: 10.1016/j.molmed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Cooper GM, Usas A, Olshanski A, Mooney MP, Losee JE, Huard J. Ex vivo Noggin gene therapy inhibits bone formation in a mouse model of postoperative resynostosis. Plast Reconstr Surg. 2009;123(2 Suppl):94S–103S. doi: 10.1097/PRS.0b013e318191c05b. [DOI] [PubMed] [Google Scholar]
- 17.Nakase J, Kitaoka K, Matsumoto K, Tomita K. Facilitated tendon-bone healing by local delivery of recombinant hepatocyte growth factor in rabbits. Arthroscopy. 2010;26:84–90. doi: 10.1016/j.arthro.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Qin L, Cheung W. Low-intensity pulsed ultrasound accelerated bone-tendon junction healing through regulation of vascular endothelial growth factor expression and cartilage formation. Ultrasound Med Biol. 2008;34:1248–60. doi: 10.1016/j.ultrasmedbio.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Saltzman CL, Goulet JA, McClellan RT, Schneider LA, Matthews LS, Michgan AA. Results of treatment of displaced patellar fracture by partial patellectomy. J Bone Joint Surg. 1990;72:1279–85. [PubMed] [Google Scholar]
- 20.Hung LK, Lee SY, Leung KS, Chan KM, Nicholl LA. Partial patellectomy for patellar fracture: Tension and band wiring and early mobilization. J Orthop Trauma. 1993;7:252–60. doi: 10.1097/00005131-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Wong MW, Qin L, Lee KM, Leung KS. Articular Cartilage Increases Transition Zone Regeneration in Bone-tendon Junction Healing. Clin Orthop Relat Res. 2009;467:1092–100. doi: 10.1007/s11999-008-0606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays PL, Kawamura S, Deng XH, Dagher E, Mithoefer K, Ying L, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90:565–79. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin M, Ralphs JR. Fibrocartilage in tendon and ligaments: An adaptation to compressive load. J Anat. 1998;193:481–94. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10:64–70. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J, Zhang P, Qin L, Pan XH. Hypoxia is essential for bone-tendon junction healing: The molecular biological evidence. Int Orthop. 2011;35:925–8. doi: 10.1007/s00264-010-1157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


