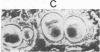
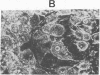
Abstract
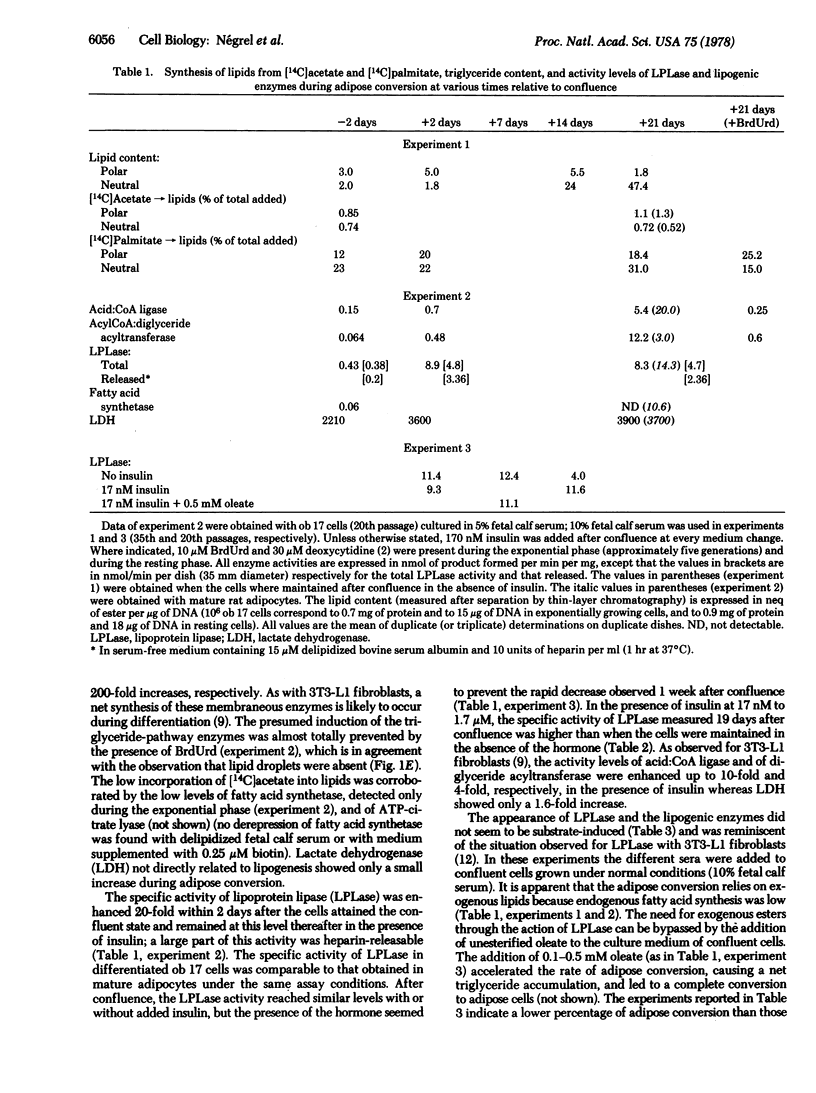
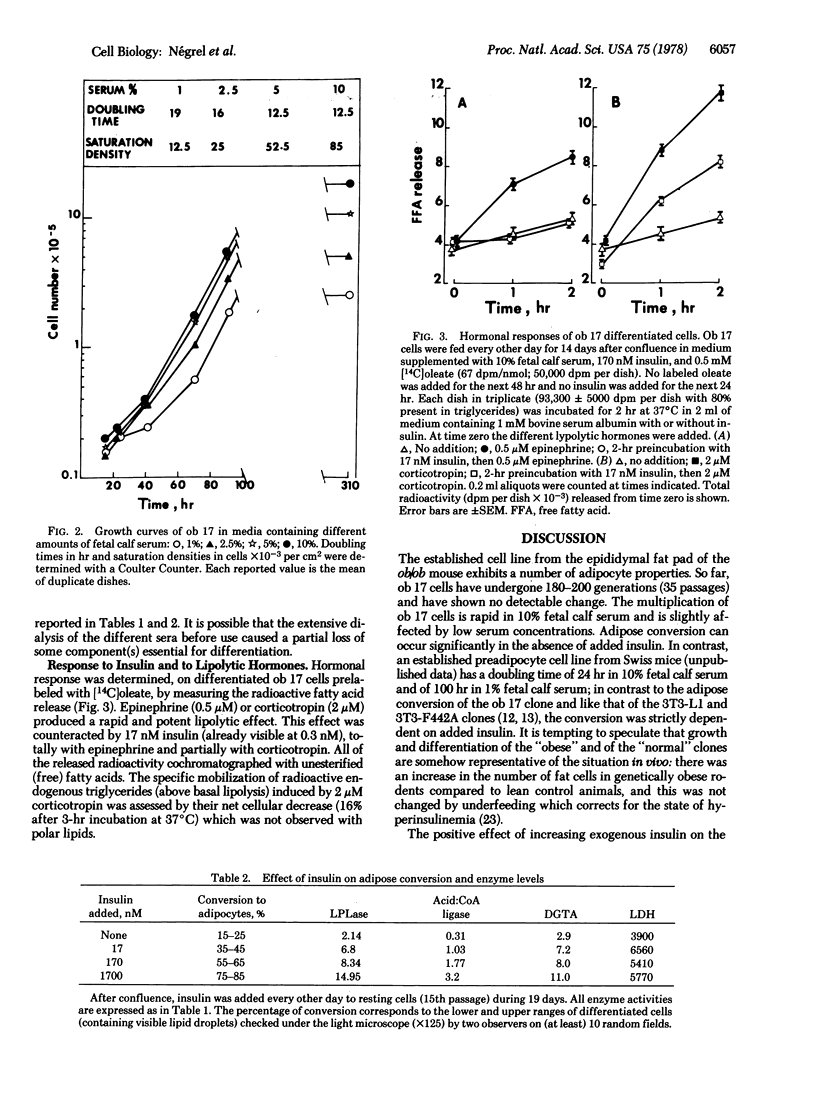
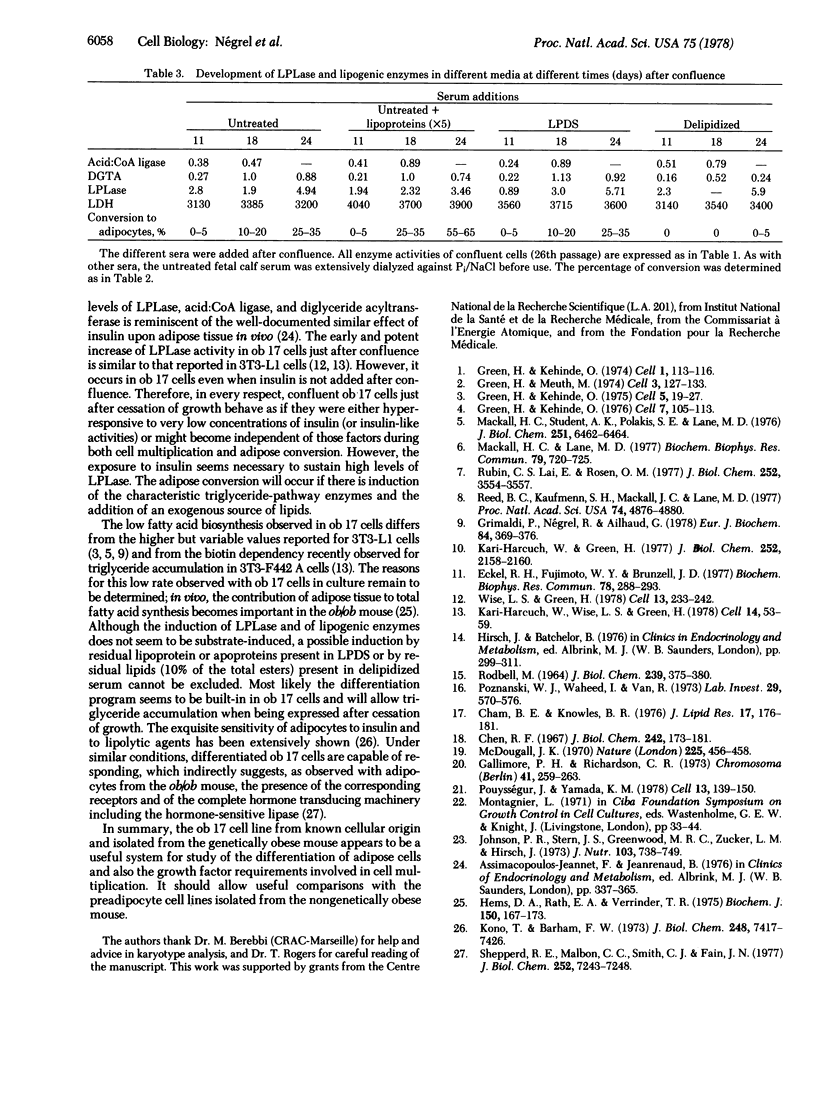
A clonal cell line that responds to insulin and to lipolytic hormones has been established from the epididymal fat pad of the C57BL/6J ob/ob mouse. This line, designated ob 17, has a doubling time of 12.5 or 19 hr in 10% or 1% fetal calf serum, respectively. It presents a heterogeneous chromosome number with 40% of the cells containing 35-44 chromosomes and expresses the characteristic H2-LA antigen. After cessation of growth, ob 17 cells accumulate droplets of triglycerides; this accumulation occurs to a significant extent even in the absence of insulin normally added after confluence. Lipoprotein lipase activity is negligible in exponentially growing cells but appears at its maximal level just after confluence with or without insulin. Acid:CoA ligase and acylCoA:diglyceride acyltransferase develop later than lipoprotein lipase. The appearance of lipolytic and lipogenic enzymes, but not of triglycerides, seems to be independent of the presence of lipoproteins or of unesterified fatty acids in the culture medium. Therefore, the differentiation program becomes operative when growth is arrested, and differentiation occurs, providing a source of exogenous lipids. Differentiated ob 17 cells in which endogenous triglycerides have been prelabeled on the fatty acid moiety do respond to epinephrine and corticotropin by release of radioactive fatty acid. This lipolytic response is counteracted by prior addition of insulin. The ob 17 cell line appears to be a useful model for study of growth and differentiation of adipose cells as compared to preadipocyte cell lines from the nongenetically obese mouse.
Keywords: adipose conversion, lipoprotein lipase, lipogenic enzymes
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cham B. E., Knowles B. R. A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res. 1976 Mar;17(2):176–181. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Eckel R. H., Fujimoto W. Y., Brunzell J. D. Development of lipoprotein lipase in cultured 3T3-L1 cells. Biochem Biophys Res Commun. 1977 Sep 9;78(1):288–293. doi: 10.1016/0006-291x(77)91252-9. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., Richardson C. R. An improved banding technique exemplified in the karyotype analysis of two strains of rat. Chromosoma. 1973;41(3):259–263. doi: 10.1007/BF00344020. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974 Oct;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Grimaldi P., Négrel R., Ailhaud G. Induction of the triglyceride pathway enzymes and of lipolytic enzymes during differentiation in a 'preadipocyte' cell line. Eur J Biochem. 1978 Mar 15;84(2):369–376. doi: 10.1111/j.1432-1033.1978.tb12177.x. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Rath E. A., Verrinder T. R. Fatty acid synthesis in liver and adipose tissue of normal and genetically obese (ob/ob) mice during the 24-hour cycle. Biochem J. 1975 Aug;150(2):167–173. doi: 10.1042/bj1500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Stern J. S., Greenwood M. R., Zucker L. M., Hirsch J. Effect of early nutrition on adipose cellularity and pancreatic insulin release in the Zucker rat. J Nutr. 1973 May;103(5):738–743. doi: 10.1093/jn/103.5.738. [DOI] [PubMed] [Google Scholar]
- Kono T., Barham F. W. Effects of insulin on the levels of adenosine 3':5'-monophosphate and lipolysis in isolated rat epididymal fat cells. J Biol Chem. 1973 Nov 10;248(21):7417–7426. [PubMed] [Google Scholar]
- Kuri-Harcuch W., Green H. Increasing activity of enzymes on pathway of triacylglycerol synthesis during adipose conversion of 3T3 cells. J Biol Chem. 1977 Mar 25;252(6):2158–2160. [PubMed] [Google Scholar]
- Kuri-Harcuch W., Wise L. S., Green H. Interruption of the adipose conversion of 3T3 cells by biotin deficiency: differentiation without triglyceride accumulation. Cell. 1978 May;14(1):53–59. doi: 10.1016/0092-8674(78)90300-8. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Role of pyruvate carboxylase in fatty acid synthesis: alterations during preadipocyte differentiation. Biochem Biophys Res Commun. 1977 Dec 7;79(3):720–725. doi: 10.1016/0006-291x(77)91171-8. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- McDougall J. K. Effects of adenoviruses on the chromosomes of normal human cells and cells trisomic for an E chromosome. Nature. 1970 Jan 31;225(5231):456–458. doi: 10.1038/225456a0. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Yamada K. M. Isolation and immunological characterization of a glucose-regulated fibroblast cell surface glycoprotein and its nonglycosylated precursor. Cell. 1978 Jan;13(1):139–140. doi: 10.1016/0092-8674(78)90145-9. [DOI] [PubMed] [Google Scholar]
- Poznanski W. J., Waheed I., Van R. Human fat cell precursors. Morphologic and metabolic differentiation in culture. Lab Invest. 1973 Nov;29(5):570–576. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reed B. C., Kaufmann S. H., Mackall J. C., Student A. K., Lane M. D. Alterations in insulin binding accompanying differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4876–4880. doi: 10.1073/pnas.74.11.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Lai E., Rosen O. M. Acquisition of increased hormone sensitivity during in vitro adipocyte development. J Biol Chem. 1977 May 25;252(10):3554–3557. [PubMed] [Google Scholar]
- Shepherd R. E., Malbon C. C., Smith C. J., Fain J. N. Lipolysis and adenosine 3':5'-monophosphate metabolism in isolated white fat cells from genetically obese-hyperglycemic mice (ob/ob). J Biol Chem. 1977 Oct 25;252(20):7243–7248. [PubMed] [Google Scholar]
- Wise L. S., Green H. Studies of lipoprotein lipase during the adipose conversion of 3T3 cells. Cell. 1978 Feb;13(2):233–242. doi: 10.1016/0092-8674(78)90192-7. [DOI] [PubMed] [Google Scholar]