Abstract
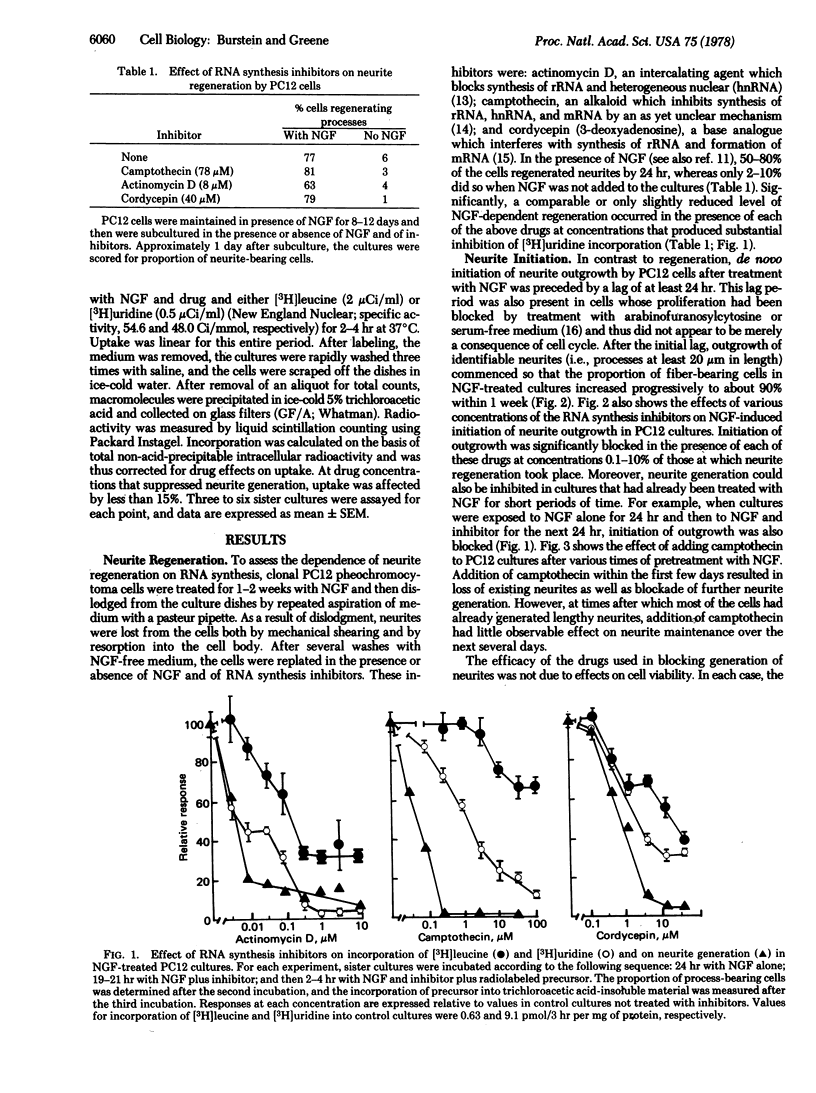
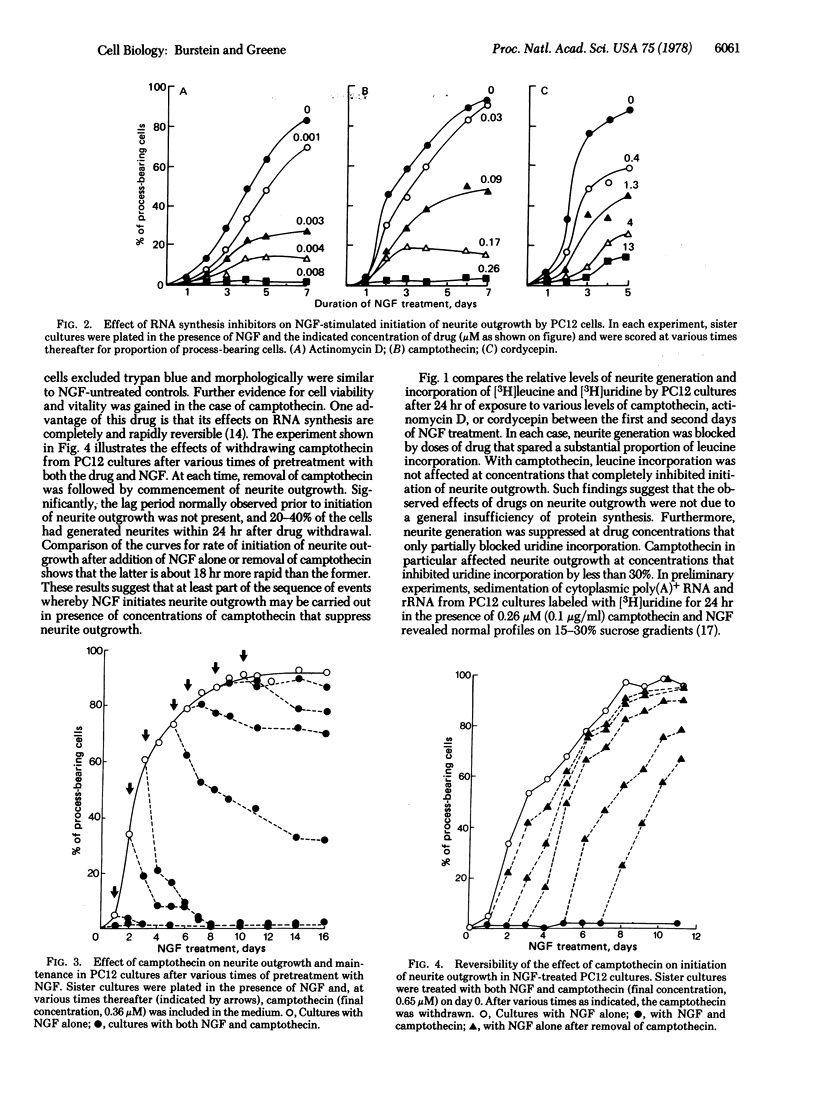
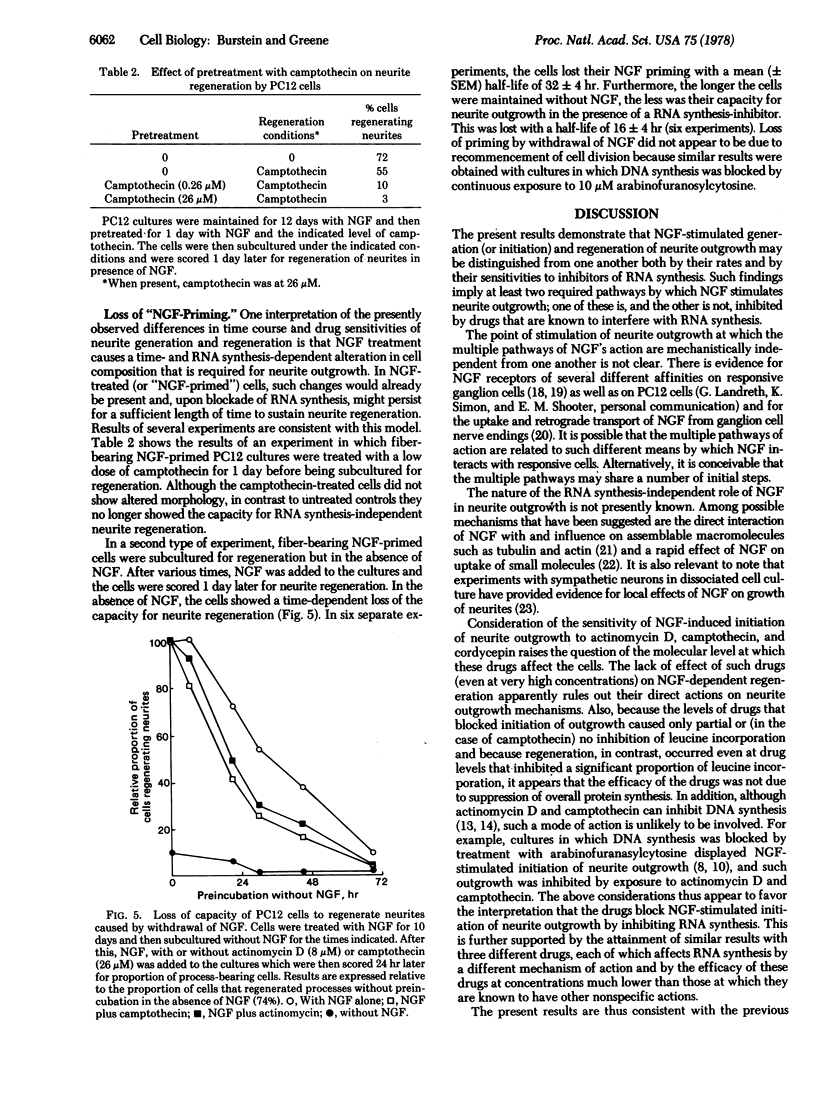
Studies on the mechanism of action of nerve growth factor (NGF) were carried out with PC12 rat pheochromocytoma cells. PC12 cells are uniquely useful for such studies because they respond to, but (unlike normal neurons) do not require, NGF and may undergo either generation or regeneration of neurites in response to NGF. Regeneration is defined here as NGF-dependent regrowth of neurites within 24 hr after subculture of NGF-treated PC12 cells. As in cultures of normal NGF-responsive neurons, neurite regeneration by PC12 cells occurs even in the presence of high concentrations of RNA synthesis inhibitors. Generation of neurites is defined as the de novo initiation of outgrowth when PC12 cells are exposed to NGF for the first time. In contrast to regeneration, neurite generation takes place with a lag of at least 24 hr and is blocked by low concentrations of RNA synthesis inhibitors. Such findings suggest that there are both RNA synthesis-dependent and -independent pathways in the mechanism whereby NGF stimulates neurite outgrowth. In addition, NGF-treated PC12 cells undergo a time-dependent loss of the capacity for neurite regeneration after pretreatment with RNA synthesis inhibitors or withdrawal of NGF. Such findings suggest that (i) initiation of neurite outgrowth requires NGF-stimulated, RNA synthesis-dependent accumulation of intracellular material(s), (ii) once such accumulation occurs, RNA synthesis-independent regeneration can occur (but only in the presence of NGF), and (iii) the turnover of such material(s) in the absence of their replacement leads to loss of the capacity for regeneration. A tentative sequence is presented for the events whereby NGF may stimulate neurite outgrowth.
Keywords: pheochromocytoma cells, differentiation, PC12 cell line
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGELETTI P. U., GANDINI-ATTARDI D., TOSCHI G., SALVI M. L., LEVI-MONTALCINI R. METABOLIC ASPECTS OF THE EFFECT OF NERVE GROWTH FACTOR ON SYMPATHETIC AND SENSORY GANGLIA: PROTEIN AND RIBONUCLEIC ACID SYNTHESIS. Biochim Biophys Acta. 1965 Jan 11;95:111–120. doi: 10.1016/0005-2787(65)90216-9. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham P. A., Varon S. Biosynthetic activities of dorsal root ganglia in vitro and the influence of nerve growth factor. Neurobiology. 1974;4(2):57–70. [PubMed] [Google Scholar]
- Calissano P., Monaco G., Castellani L., Mercanti D., Levi A. Nerve growth factor potentiates actomyosin adenosinetriphosphatase. Proc Natl Acad Sci U S A. 1978 May;75(5):2210–2214. doi: 10.1073/pnas.75.5.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot R. B. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A., Tischler A. S., Greene L. A. Nerve growth factor-induced increase in electrical excitability and acetylcholine sensitivity of a rat pheochromocytoma cell line. Nature. 1977 Aug 11;268(5620):501–504. doi: 10.1038/268501a0. [DOI] [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Bradshaw R. A. Properties of the specific binding of 125I-nerve growth factor to responsive peripheral neurons. J Biol Chem. 1974 Sep 10;249(17):5513–5519. [PubMed] [Google Scholar]
- Green L. A. A quantitative bioassay for nerve growth factor (NGF) activity employing a clonal pheochromocytoma cell line. Brain Res. 1977 Sep 16;133(2):350–353. doi: 10.1016/0006-8993(77)90770-3. [DOI] [PubMed] [Google Scholar]
- Greene L. A. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978 Sep;78(3):747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Rein G. Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheo-chromocytoma cells. Brain Res. 1977 Jul 1;129(2):247–263. doi: 10.1016/0006-8993(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Rein G. Synthesis, storage and release of acetylcholine by a noradrenergic pheochromocytoma cell line. Nature. 1977 Jul 28;268(5618):349–351. doi: 10.1038/268349a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S. R., Sander G., Kirschner M. W. Evidence for a functional role of RNA in centrioles. Cell. 1977 Mar;10(3):337–350. doi: 10.1016/0092-8674(77)90021-6. [DOI] [PubMed] [Google Scholar]
- Hendry I. A., Stöckel K., Thoenen H., Iversen L. L. The retrograde axonal transport of nerve growth factor. Brain Res. 1974 Mar 15;68(1):103–121. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- Horii Z. I., Varon S. Nerve growth factor action on membrane permeation to exogenous substrates in dorsal root ganglionic dissociates from the chick embryo. Brain Res. 1977 Mar 18;124(1):121–133. doi: 10.1016/0006-8993(77)90868-x. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., ANGELETTI P. U. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol. 1963 Mar;6:653–659. doi: 10.1016/0012-1606(63)90149-0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- McGuire J. C., Greene L. A., Furano A. V. NGF stimulates incorporation of fucose or glucosamine into an external glycoprotein in cultured rat PC12 pheochromocytoma cells. Cell. 1978 Oct;15(2):357–365. doi: 10.1016/0092-8674(78)90004-1. [DOI] [PubMed] [Google Scholar]
- Partlow L. M., Larrabee M. G. Effects of a nerve-growth factor, embryo age and metabolic inhibitors on growth of fibres and on synthesis of ribonucleic acid and protein in embryonic sympathetic ganglia. J Neurochem. 1971 Nov;18(11):2101–2118. doi: 10.1111/j.1471-4159.1971.tb05069.x. [DOI] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Dichter M. A., Biales B., Greene L. A. Neuroendocrine neoplasms and their cells of origin. N Engl J Med. 1977 Apr 21;296(16):919–925. doi: 10.1056/NEJM197704212961608. [DOI] [PubMed] [Google Scholar]



