Abstract
Context:
Noise acts as an environmental stressor as has been demonstrated by an increased brain acetyl cholinesterase activity as well as elevated plasma corticosterone and adrenocorticotropic hormone levels. Noise can lead to neurodegenerative changes in the brain and in the ear.
Aim:
This study was undertaken to investigate the effect of chronic noise on growth and development during the sensitive period of embryonic life.
Materials and Methods:
In this study, we analyzed the body weight, brain weight and brain size following prenatal chronic noise exposure. Fertilized eggs of domestic chicks were exposed to chronic excessive acoustic stimulation with frequency of the sound ranging from 30 to 3000 Hz with a peak at 2700 Hz was given at 110 dB sound pressure level from embryonic day (E) 10 until hatching.
Results:
An appreciable decrease in body weight, brain weight and brain size was evident in the experimental group exposed to noise. A generalized decrease in the neuronal nuclear size and increase in the density of neurons was also observed.
Conclusion:
These observations could be an indicator of growth and developmental retardation following exposure to noise.
Keywords: Development, environmental stressor, growth, noise
INTRODUCTION
In humans hearing is established in utero by the third trimester as indicated by fetal response to acoustic stimuli between 36 and 40 weeks gestational age.[1]
The hearing begins in the birds at an early stage. Though the response to sound and frequency selectivity emerges on embryonic day 15. Thereafter, rapid maturation of frequency selectivity occurs from embryonic day 16 to 18. However, in the period between embryonic days 12 and 16, the birds responded to direct columella footplate stimulation of the cochlea. Thus, two periods of ontogeny have been proposed. First is a pre-hearing period (E12-E16) of endogenous cochlear signaling that provides neurotrophic support and guides normal developmental refinements in central binaural processing pathways followed by a period (E16-E19) wherein, the cochlea begins to detect and encode airborne sound.[2]
Studies show that the prenatal exposure to sound can mould the learning process. Exposure to ambient environmental sounds like voice of mother and soothing music lead to increased weight gain/day for both males and females and thus, has a positive effect on growth and development in premature human neonates.[3] Prenatal exposure to music induced a rapid advance in motor ability such as sitting and standing in human neonates.[4]
There is a growing concern over the hazardous effects of noise pollution in the modern society and both physiological as well as morphological studies have been conducted to study the effect of acoustic trauma. Exposure to loud noise leads to an acoustic traumatization with a temporary threshold shift initially and with increasing exposure, intensity and duration, a permanent hearing loss. Chronic traffic noise (cars, trains, air planes) is usually not threatening to the ear, but it may represent a considerable subjective annoyance and a stress factor, leading to psychosomatic disturbances, neuro-vegetative symptoms and sleeping disorders.[5]
Most of the studies on noise exposure have been conducted on adult and neonatal animals. Few studies are there to study the effect of noise on prenatal growth and development.
MATERIALS AND METHODS
The number of animals is used and procedures to minimize the suffering of the animals are in accordance with the Ethics Committee on animal experiments of all India Institute of Medical Sciences, Delhi.
Incubation conditions
Fertilized eggs of White Leghorn chickens (Gallus domesticus), weighing between 50 and 60 g, were obtained from a registered poultry farm.
The eggs were incubated in a specially designed, double-walled, insulated, sound proof incubator (Widson Scientific Works Ltd.). Incubation conditions were maintained at 70-80% humidity and temperature of 37°C (36-38°C). The eggs were tilted 4 times a day and exposed to a photoperiod of 12-h light and 12-h dark cycle, controlled by automatic timer devices. Aeration was provided with a force draft of air.
A background sound of 40 dB, emanating from the motor 2 to 3 times in an hour, will be audible to all the groups and cannot be eliminated.
Experimental groups
Control (Group I)
The incubating eggs, which served as control received no additional auditory stimulus.
Experimental (Group II)
The incubating eggs were exposed to chronic excessive acoustic stimulation (unpatterned sound as noise) from E10 until the day of hatching.
Auditory stimulation protocol
The auditory stimuli were given for 15 min/h, over the period of 24 h, beginning from day 10 and continued till the day of hatching. This was achieved through two built in speakers connected to a stereo sound system provided with automatic setting with an electronic timer device.
To ensure that the embryos receive the auditory stimuli, a portion of the shell of approximately 3 mm size over the air sac will be removed on day 9.5 of incubation, maintaining the membranes intact.
Auditory stimuli characteristics
The recorded cassettes were pre-screened with sound analyzer at the National Physical Laboratories (Council of Scientific and Industrial Research, New Delhi) to determine the frequency range and modulation. An AD-3521 fast Fourier transform analyzer was used to visualize sound wave pattern and measure the frequency at every point of the wave pattern with the aid of a stylus. The cumulative frequency range of the recorded sound was plotted. The frequency of the sound ranging from 30 to 3000 Hz with a peak at 2700 Hz was given at 110 dB sound pressure level.
Tissue collection
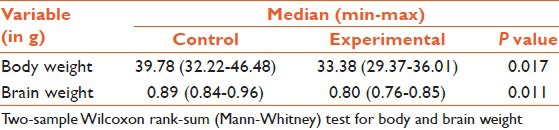
Chicks from the control and the experimental group were collected on the day of hatching (referred to as post-hatch day 1). The chicks after ether anesthesia were weighed. They were decapitated and the brain along with the brainstem was removed from the skull by severing all the cranial nerves and vessels at the base. The whole brain was weighed [Table 1].
Table 1.
Body and brain weight of chicks
Tissue processing
Immediately after the tissue was obtained, it was immersion fixed in 4% of paraformaldehyde at 4°C for 2 weeks. The forebrains were dehydrated, infiltrated and the blocks were prepared by embedding in paraplast. Serial coronal sections of 7 μm thickness were cut with a rotary microtome. The sections were mounted on egg albumin coated glass slides and subsequently stained for Nissl substance with 1% buffered thionin. A comparison of size of sections of brain of experimental and control groups at a distance of 2 mm from the rostral end of the brain was done.
Quantification
The measurement of neuronal nuclear area was determined using an image analyzing system Leica Q500MC. The measurements were made under a × 100 objective lens such that pixel size was 0.51 μm.
Data analysis
Wilcoxon rank-sum (Mann-Whitney) test was applied to compare the body and brain weight of control with experimental chicks. To test the statistical significance of change in the neuronal nuclear area two-tailed paired t-test was used. Further statistical analysis on the pooled data to assess the difference, if any, in the two groups studied-normal and experimental was done using t-test. Frequency distribution was analyzed using Fishers exact test.
RESULTS
Egg weight, embryo weight, brain weight
The weight of the eggs was almost the same for control and experimental groups ranging between 50 and 60 g. The body weight and brain weight of 50 experimental animals was compared with 50 animals used as controls. The body weight and the brain weight was significantly less in the experimental group on the post-hatch day 1 compared with the control group [Table 1]. The size of the brain was also reduced in the experimental group [Figure 1].
Figure 1.
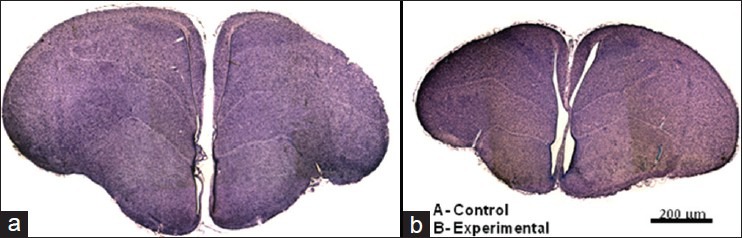
(a) Coronal section at 2 mm from the rostral end of the brain of chick on post-hatch day 1 of control group. (b) experimental group
Area and density of neurons
In the experimental group exposed to chronic excessive acoustic stimulation, an increase in the density of neurons and a significant decrease in the size of neuronal nuclear area were observed in the comparable areas [Figure 2].
Figure 2.
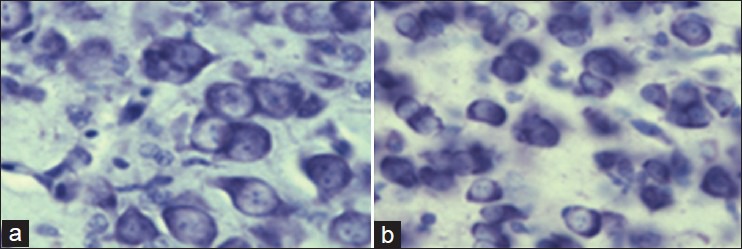
(a) Neurons in mediorostral neostriatum and hyperstriatum ventrale region of chick. Compare the diameter and density of the neurons in the control group. (b) and experimental group
DISCUSSION
The present study was undertaken to investigate the effects of prenatal chronic noise exposure on the growth and development of chicks.
The auditory stimuli in the present study were given at 110 dB sound pressure level with the frequency of the sound ranging from 30 to 3000 Hz with a peak at 2700 Hz. This is the level of sound encountered in many industrial work places, busy traffic intersection and discos. These unpatterned sounds are considered as noise. Hence the sound delivered to the chick embryos in the present study amounted to noise.
The noise stimulus in the present study was given for 15 min/h, over the period of 24 h, beginning from day 10 and continued till the day of hatching. This auditory stimulation protocol was based on the knowledge that in the basilar cochlear papillae of chick (G. domesticus), afferent synapses appear on the hair cells by about embryonic day E8.[6] It is also known that the cochlear nucleus of the chick responds to electrical stimulation from day E11 of incubation.[7] Thus the auditory apparatus and the pathway are functional early in development and hence the initiation of the present protocol by E10.
In the present study, the chronic excessive sound stimulation at 110 dB and high-frequency of 2700 Hz (30-3000 Hz) was given throughout the period of embryonic development. It has been demonstrated that during normal development the auditory evoked responses in the chick mature in a systematic pattern, by responding first to low frequency sounds prior to hatching and high frequency sounds after hatching.[7] Thus, in the present study, as opposed to developmental norms, the embryos have been subjected to high-frequency sound throughout their embryonic period.
Following prenatal exposure to chronic excessive sound, the post-hatch day 1 chicks showed a decrease in the body and brain weight and a reduced brain size. A similar growth retardation in pups indicated by decreased body weight was observed following prenatal exposure to noise of 95 dB from supersonic sound machine for 1 h once a day starting from 15th day of pregnancy in rats until the delivery. In this study on the rats, decreased neurogenesis in the CA1 region of hippocampus, as well as impaired spatial learning ability in the pups assessed by radial arm maze test have also been demonstrated.[8]
In additional to the observation of decrease in brain size and weight in the present study, we have interestingly noted an apparent reduction in the size of neuronal nuclear area and an increase in the neuronal density in the comparable regions of the brain.
The reduction in cell size could also be attributed to developmental retardation caused by stress due to chronic excessive sound exposure during the sensitive and critical phase of foetal development.
In the adult brain, stress related neuronal changes such as suppressed neurogenesis of the dentate gyrus granule neurons and atrophy of dendrites in the CA3 region of hippocampus[9] as well as spine synapse loss in the neurons in medial prefrontal cortex[10] have been demonstrated. Prenatal stress administered by restraining pregnant rats in a small cage for 240 min daily for 3 days (gestational day 15-17) results in enhanced corticotropin-releasing factor messenger ribonucleic acid (mRNA) expression and a significant decrease in the size of neuronal processes of the hypothalamic paraventricular nucleus in the 18-day-old rat fetus.[11] Thus, stress as indicated by the increase in mRNA and plasma levels of stress hormones can cause detrimental changes in the central nervous system.
Noise too acts as an environmental stressor as has been demonstrated by increased brain acetyl cholinesterase activity as well as elevated plasma corticosterone and adrenocorticotropic hormone levels in healthy adult rats, following acute and chronic exposure to noise of 100 dB sound pressure level.[12,13,14]
The functional development in normal brain causes neuronal number to decrease and size of neurons to increase. Nevertheless, in the present study a reduction in the size of neurons and increase in neuronal density, could be an indicator of developmental retardation attributed to stress. Further, a significant decrease in brain weight, body weight and brain size was observed.
Thus, the noise related stress can retard the growth and development of the body as well as the nervous system.
Footnotes
Source of Support: Nill
Conflict of Interest: None declared.
REFERENCES
- 1.Porcaro C, Zappasodi F, Barbati G, Salustri C, Pizzella V, Rossini PM, et al. Fetal auditory responses to external sounds and mother's heart beat: Detection improved by Independent Component Analysis. Brain Res. 2006;1101:51–8. doi: 10.1016/j.brainres.2006.04.134. [DOI] [PubMed] [Google Scholar]
- 2.Jones TA, Jones SM, Paggett KC. Emergence of hearing in the chicken embryo. J Neurophysiol. 2006;96:128–41. doi: 10.1152/jn.00599.2005. [DOI] [PubMed] [Google Scholar]
- 3.Standley JM. The effect of music and multimodal stimulation on responses of premature infants in neonatal intensive care. Pediatr Nurs. 1998;24:532–8. [PubMed] [Google Scholar]
- 4.Chen DG, Huang YF, Zhang JY, Qi GP. Influence of prenatal music and touch enrichment on the IQ, motor development and behaviour of infants. Chin J Psychol. 1994;8:148–51. [Google Scholar]
- 5.Häusler R. The effects of acoustic overstimulation. Ther Umsch. 2004;61:21–9. doi: 10.1024/0040-5930.61.1.21. [DOI] [PubMed] [Google Scholar]
- 6.Cohen GM, Fermin CD. The development of hair cells in the embryonic chick's basilar papilla. Acta Otolaryngol. 1978;86:342–58. doi: 10.3109/00016487809107513. [DOI] [PubMed] [Google Scholar]
- 7.Saunders JC, Coles RB, Gates GR. The development of auditory evoked responses in the cochlea and cochlear nuclei of the chick. Brain Res. 1973;63:59–74. doi: 10.1016/0006-8993(73)90076-0. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Lee MH, Chang HK, Lee TH, Lee HH, Shin MC, et al. Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain Dev. 2006;28:109–14. doi: 10.1016/j.braindev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS, Magarinos AM. Stress and hippocampal plasticity: Implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 10.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–48. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 11.Fujioka T, Sakata Y, Yamaguchi K, Shibasaki T, Kato H, Nakamura S. The effects of prenatal stress on the development of hypothalamic paraventricular neurons in fetal rats. Neuroscience. 1999;92:1079–88. doi: 10.1016/s0306-4522(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 12.Windle RJ, Wood S, Shanks N, Perks P, Conde GL, da Costa AP, et al. Endocrine and behavioural responses to noise stress: Comparison of virgin and lactating female rats during non-disrupted maternal activity. J Neuroendocrinol. 1997;9:407–14. doi: 10.1046/j.1365-2826.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- 13.Sembulingam K, Sembulingam P, Namasivayam A. Effect of acute noise stress on acetylcholinesterase activity in discrete areas of rat brain. Indian J Med Sci. 2003;57:487–92. [PubMed] [Google Scholar]
- 14.Manikandan S, Padma MK, Srikumar R, Jeya Parthasarathy N, Muthuvel A, Sheela Devi R. Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neurosci Lett. 2006;399:17–22. doi: 10.1016/j.neulet.2006.01.037. [DOI] [PubMed] [Google Scholar]


