Abstract
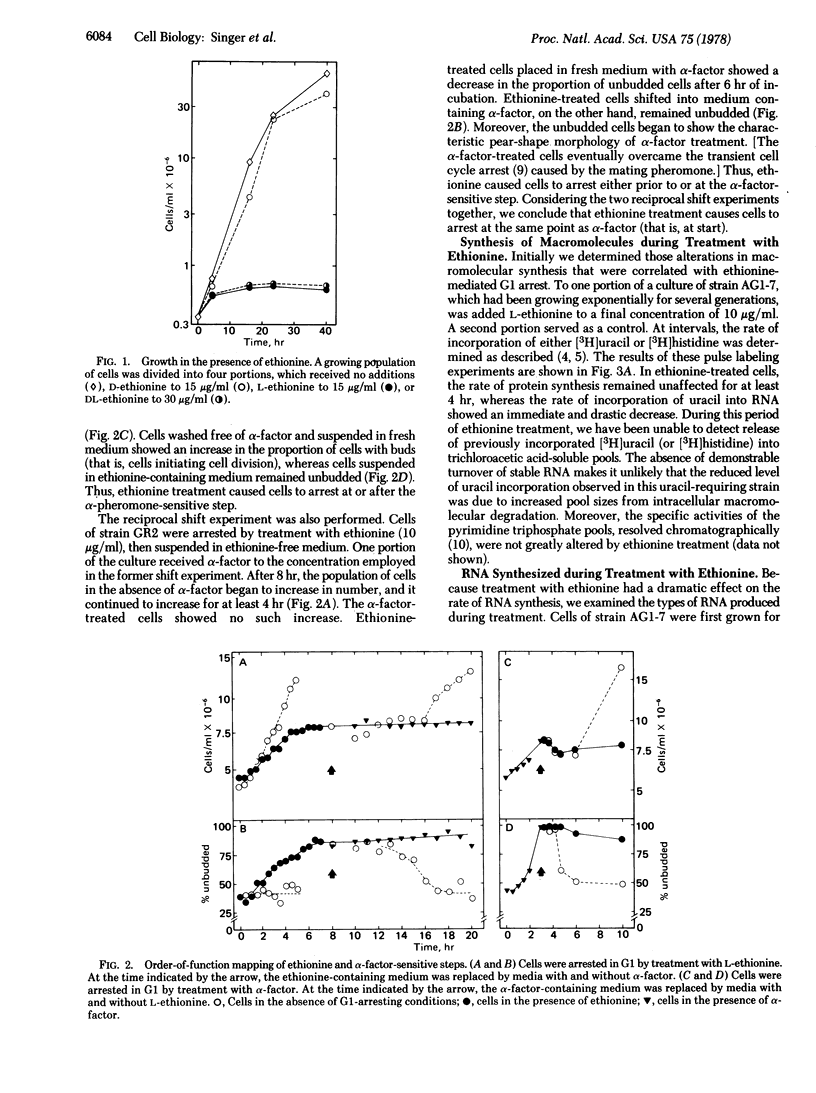
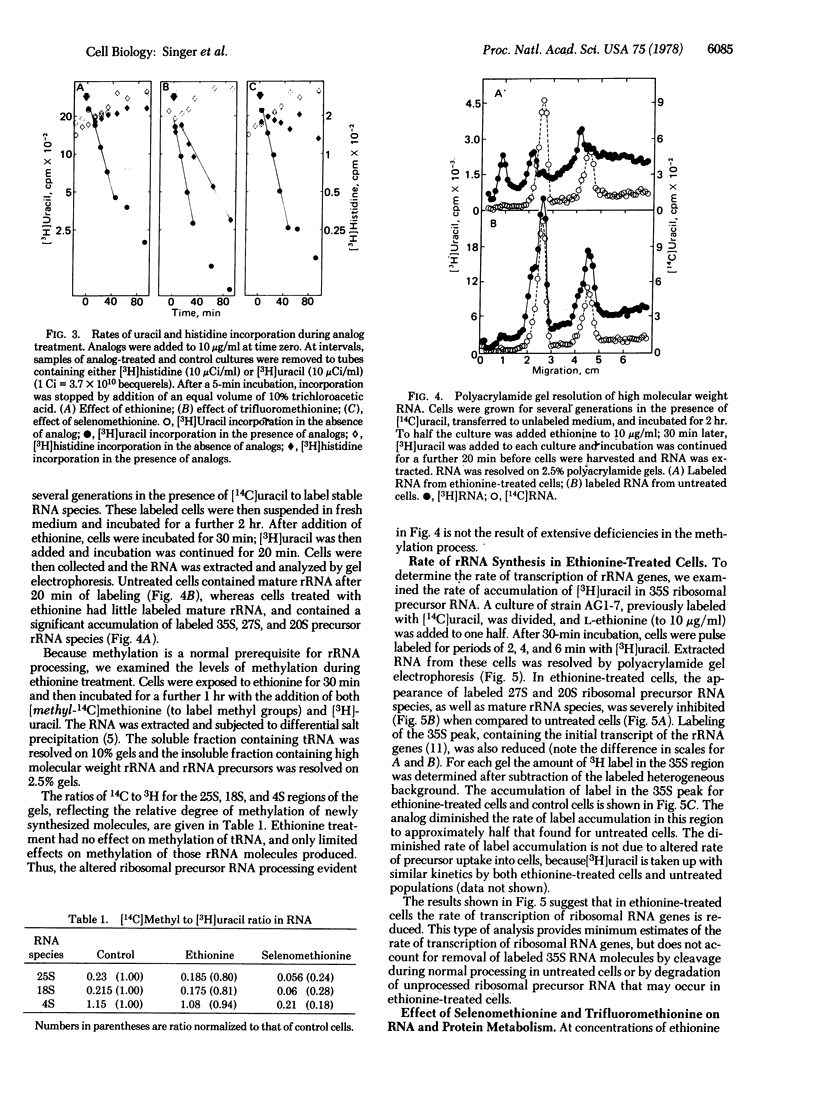
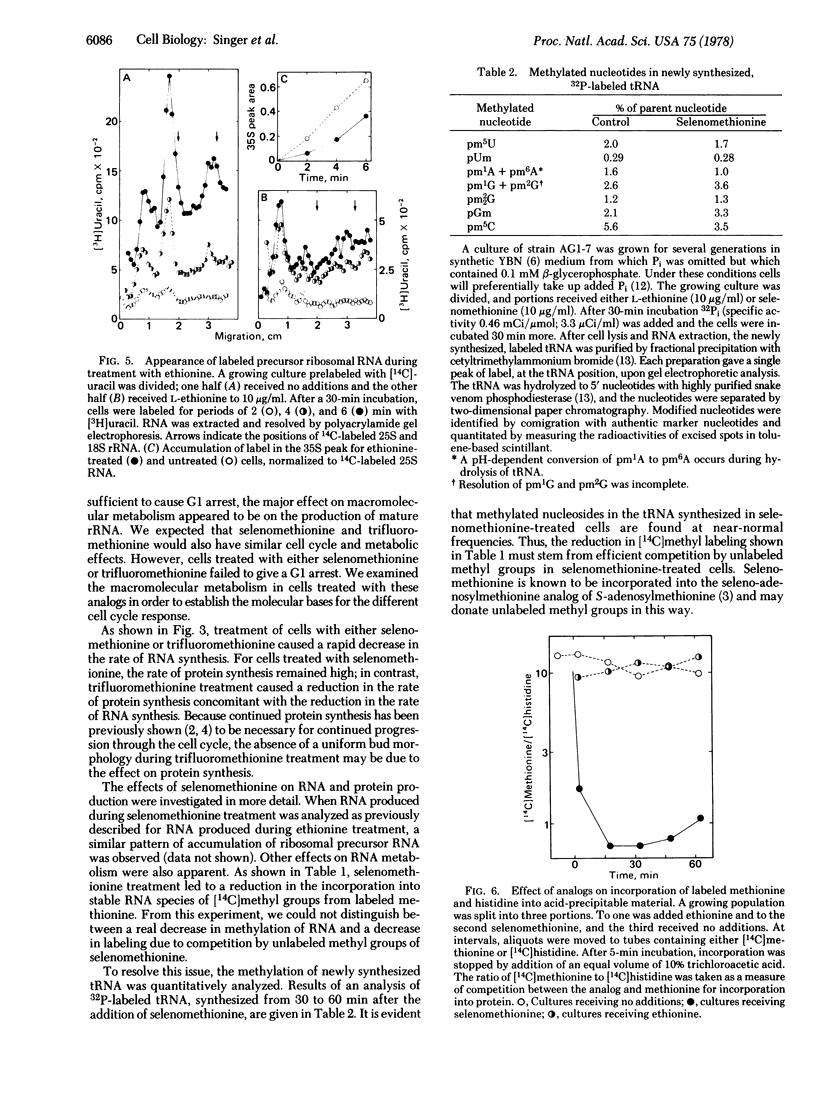
Methionine analogs such as ethionine, selenomethionine, and trifluoromethionine all arrest growth and division of the yeast Saccharomyces cerevisiae. One analog, ethionine, caused cells of the yeast to arrest specifically within G1; reciprocal shift experiments showed that ethionine and alpha-factor arrested cells at the same step ("start"). The major effect of ethionine on synthesis of macromolecules was to reduce both the rate of appearance of 35S ribosomal precursor RNA and the rate of production of mature rRNA. Synthesis of protein was relatively unaffected by ethionine. Selenomethionine and trifluoromethionine caused cells to arrest randomly in the cell division cycle. Although treatment of cells with either selenomethionine or trifluoromethionine also reduced the rate of total RNA synthesis, each of these analogs had other effects that presumably prohibited completion of the cell cycle. We propose that the rate of rRNA production is an important regulatory event in the cell cycle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bücking-Throm E., Duntze W., Hartwell L. H., Manney T. R. Reversible arrest of haploid yeast cells in the initiation of DNA synthesis by a diffusible sex factor. Exp Cell Res. 1973 Jan;76(1):99–110. doi: 10.1016/0014-4827(73)90424-2. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Chan R. K. Recovery of Saccharomyces cerevisiae mating-type a cells from G1 arrest by alpha factor. J Bacteriol. 1977 May;130(2):766–774. doi: 10.1128/jb.130.2.766-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y., Antoniewski J., de Robichon-Szulmajster H. Effects of regulatory mutations upon methionine biosynthesis in Saccharomyces cerevisiae: loci eth2-eth3-eth10. J Bacteriol. 1973 Sep;115(3):1084–1093. doi: 10.1128/jb.115.3.1084-1093.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani F., Cherest H., de Robichon-Szulmajster H. Biochemical and regulatory effects of methionine analogues in Saccharomyces cerevisiae. J Bacteriol. 1975 May;122(2):375–384. doi: 10.1128/jb.122.2.375-384.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Periodic density fluctuation during the yeast cell cycle and the selection of synchronous cultures. J Bacteriol. 1970 Dec;104(3):1280–1285. doi: 10.1128/jb.104.3.1280-1285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A., McFarlane S. Growth and cell division during nitrogen starvation of the yeast Saccharomyces cerevisiae. J Bacteriol. 1977 Nov;132(2):723–730. doi: 10.1128/jb.132.2.723-730.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. RNA synthesis and control of cell division in the yeast S. cerevisiae. Cell. 1978 Aug;14(4):951–958. doi: 10.1016/0092-8674(78)90349-5. [DOI] [PubMed] [Google Scholar]
- Maw G. A. Incorporation and distribution of ethionine-sulfur in the protein of ethionine-sensitive and ethionine-resistant yeasts. Arch Biochem Biophys. 1966 Aug;115(2):291–301. doi: 10.1016/0003-9861(66)90277-3. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. Three forms of the 5.8-S ribosomal RNA species in Saccharomyces cerevisiae. Eur J Biochem. 1974 Jan 3;41(1):197–202. doi: 10.1111/j.1432-1033.1974.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Shulman R. W., Sripati C. E., Warner J. R. Noncoordinated transcription in the absence of protein synthesis in yeast. J Biol Chem. 1977 Feb 25;252(4):1344–1349. [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. Ribosomal RNA synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):227–242. doi: 10.1016/0022-2836(72)90279-3. [DOI] [PubMed] [Google Scholar]
- Unger M. W., Hartwell L. H. Control of cell division in Saccharomyces cerevisiae by methionyl-tRNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1664–1668. doi: 10.1073/pnas.73.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Gorenstein C. The synthesis of eucaryotic ribosomal proteins in vitro. Cell. 1977 May;11(1):201–212. doi: 10.1016/0092-8674(77)90331-2. [DOI] [PubMed] [Google Scholar]


