Abstract
For many years, aging was thought to be accompanied by significant decreases in total neuron number across multiple brain regions. However, this view was revised with the advent of modern quantification methods, and it is now widely accepted that the hippocampus and many regions of the cortex show substantially preserved numbers of neurons during normal aging. Nonetheless, age-related changes in neuron number do occur in focal regions of the primate prefrontal cortex (PFC), but the question of whether age-related neuron loss is an exclusive characteristic of the PFC in primates remains relatively unexplored. To investigate the loss of neurons with normal aging in rodents, we used unbiased stereological methods to quantify the number of principal neurons and interneurons in the PFC of young and aged rats. We observed a significant age-related decline in the number of principal neurons in the dorsal PFC. The number of interneurons positively stained with antibodies to glutamic acid decarboxylase 67 was also reduced in the dorsal PFC of aged rats. These observations indicate that the dorsal PFC is susceptible to neuron loss with aging in rodent brain and suggest some common basis for vulnerability in cortical circuits across species.
Indexing Terms: cingulate cortex, stereology, GAD67, interneuron
Age-related alterations in prefrontal cortical structure have been reported in humans, in terms of both regional volume (Tisserand et al., 2002) and neuronal morphology (de Brabander et al., 1998). Similar changes, including synaptic atrophy, reductions in dendritic spine density, and loss of neurons have been detected in non-human primates (Peters et al., 1998; Dumitriu et al., 2010; Smith et al., 2004). Neuronal loss occurs focally in area 8a, a component of the dorsolateral prefrontal cortex (PFC) with particular relevance for attention and working memory (Levy and Goldman-Rakic, 1999). The selective vulnerability of area 8a is supported by the maintenance of total neuron number across other components of the dorsolateral prefrontal cortex (dPFC), such as area 46 (Smith et al., 2004). However, the question of whether neuronal loss also occurs in the PFC of aging rodents has received comparatively less attention.
Although clear species differences exist in PFC anatomy in primates and rodents (Uylings et al., 2003), pre-frontal regions in the rat can be subdivided into a dorsal and ventral component based on cytoarchitectural and hodological criteria. The dorsal component, comprised of cingulate areas 1 and 2, is differentiated in a laminar pattern, whereas the ventral regions, which include the infralimbic and prelimbic areas, exhibit less clear lamination (Uylings et al., 2003). The connectivity also differs between the dPFC and the ventral prefrontal cortex (vPFC) in a manner consistent with primate connectional anatomy (Fuster, 2008). The dPFC receives and reciprocates projections from motor, somatosensory, visual, and retrosplenial cortices, whereas the vPFC is distinguishable by relatively greater connectivity with the hippocampus and associated cortical structures (Uylings et al., 2003). In this regard, the rat dPFC and vPFC share many characteristics with the primate, and may exhibit similar features of structural change with aging.
Here we evaluated age-related alterations in prefrontal cortical neuron number, by using a well-characterized rat model of cognitive decline (Gallagher et al., 1993). We observed that aging was associated with loss of neurons in the dPFC, with maintenance of neuron number in the vPFC. The number of cells stained with antibodies against glutamic acid decarboxylase 67 (GAD67) was also reduced in aged rats. The changes in prefrontal cortical neuron number were not correlated with behavioral impairment in the assessment of hippocampus-dependent spatial memory, consistent with the functional dissociation between hippocampal-mediated and prefrontally mediated memory systems reported in other studies (Barense et al., 2002).
Materials and Methods
Animals and tissue preparation
All animal procedures followed NIH guidelines and were approved by the Animal Care and Use Committee of Johns Hopkins University. Male Long-Evans rats (Charles River Laboratories, Wilmington, MA) were 6 (adult) or 24 (aged) months of age at the time of the studies. Aged rats were obtained at 8–9 months old and were housed in a Johns Hopkins University vivarium until they were 24 months old. For euthanasia, rats were anesthetized with isoflurane and perfused transcardially with sterile saline, followed by 4% paraformaldehyde in phosphate buffer (pH 7.4). After 24 hours of postfixation at 4°C, brains were moved into 10% glycerol in phosphate buffer for 24 hours at 4°C, followed by an additional 24 hours in 20% glycerol at 4°C. Brains were then sectioned on the coronal plane in a 1:10 series at 50 μm thickness by using a freezing microtome. Sections were stored in cryoprotectant at −80°C.
Behavioral categorization
The rats used in this study were behaviorally categorized by using the water maze task, as described (Stranahan et al., 2011). Behavioral testing took place during the light phase, with training over 8 days in sessions of three trials per day. At the start of each trial, rats were placed in the water at the perimeter of the pool, with starting locations varied across trials. Each trial lasted for 90 seconds or until the rat successfully located the platform, with a 60-second intertrial interval. Every sixth trial was a probe trial to assess the rat's spatial bias during its search. Rats were permitted to escape on probe trials when a retracted platform was made available after 30 seconds for completion of those trials. An index score, derived from the proximity of the rat to the escape platform location during the 30-second free swim on probe trials, was used to characterize performance of the rats in the maze for the purpose of neurobiological analyses. This index is the sum of the weighted proximity scores measured during the probe trials, with lower scores reflecting better spatial memory as indicated by shorter average distances from the platform location (Gallagher et al., 1993).
Histology and immunohistochemistry
For cresyl violet staining, a 1:5 series of sections was mounted onto coated slides, dried, and stained in 2.5% cresyl violet acetate (Sigma, St. Louis, MO). Stained slides were then dehydrated through increasing concentrations of ethanol, cleared with Citrisolv, and cover-slipped under Permount. For immunohistochemistry, we used a mouse monoclonal antibody against GAD67 (MAB5406, clone 1G10.2; lot #LV1721349; Millipore, Bedford, MA). This antibody was raised against a recombinant fusion protein containing the N-terminal regions (amino acids 4–101) of human GAD67. The N-terminal region is not shared by GAD65, and the antibody showed no cross-reactivity with the 65-kDa isoform of GAD in rat brain lysates (Millipore datasheet). The staining showed only the expected pattern of cytoplasmic labeling in neurons. Expected labeling pattern was based on comparison with immunolabeling in areas with a high density of γ-aminobutyric acid (GABA)ergic neurons, such as the basal ganglia (Gonzales et al., 1991). Western blot analysis of rat hippocampal protein extracts revealed a single band at 67 kDa.
Sections were washed in 0.1 M Tris-buffered saline (TBS; pH 7.6) to remove cryoprotectant, and endogenous peroxidases were quenched in 0.3% H2O2 in TBS. After additional TBS washes, sections were blocked in 5% normal horse serum in TBS with 0.3% Tween-20. After blocking, sections were incubated with primary antibody at 1:1,000 dilution in TBS containing 0.15% Tween-20 and 3% normal horse serum for 2 days at 4°C with shaking. Following primary antibody incubation, sections were washed in TBS, and reacted with biotinylated secondary antibody goat anti-mouse IgG (Vector, Burlingame, CA) diluted 1:500 in TBS with 0.15% Tween-20 and 5% normal horse serum for 1 hour. After additional washes in TBS, the bound secondary antibody was detected with avidin-biotin complex (ABC Elite; Vector) for 1 hour. Sections were then washed in TBS, and the avidin-biotin complex was visualized with nickel-enhanced diaminobenzadine (Vector). After another set of TBS rinses, tissue sections were mounted onto coated slides and dried. To detect regional boundaries, GAD67-stained sections were counterstained with 1% Neutral Red. After Neutral Red staining, sections were dehydrated, cleared, and cover-slipped as described above for cresyl violet staining.
Unbiased stereology
The rostrocaudal extent of the prefrontal cortex was defined according to cytoarchitectural criteria (Van Eden and Uylings, 1985), with reference to Paxinos and Watson (1996). Cingulate areas 1 and 2 (Cg1 and Cg2) were taken to represent the dPFC, and the infralimbic and prelimbic areas were grouped to represent the vPFC (Fig. 1). The border between the prelimbic and Cg1 areas is marked by a widening of layer V and sparser distribution of layer III cells in the prelimbic area, compared with the Cg1. The posterior border of the Cg1 and Cg2 is defined by an increase in the density of cells in layer II, and by the presence of a clearly distinguishable layer III. The mediolateral border of the Cg1 and the secondary motor cortex (M2, also known as the medial precentral area; Van Eden and Uylings, 1985) is identifiable by a transition between the orderly laminar hem separating layers I and II in the Cg1, and the broader, less regular appearance of layer II in the M2 (Fig. 1). All cytoarchitectural boundaries were assessed at 10× magnification before being outlined at 5× magnification with the aid of StereoInvestigator software (MBF Bioscience, Williston, VT).
Figure 1.
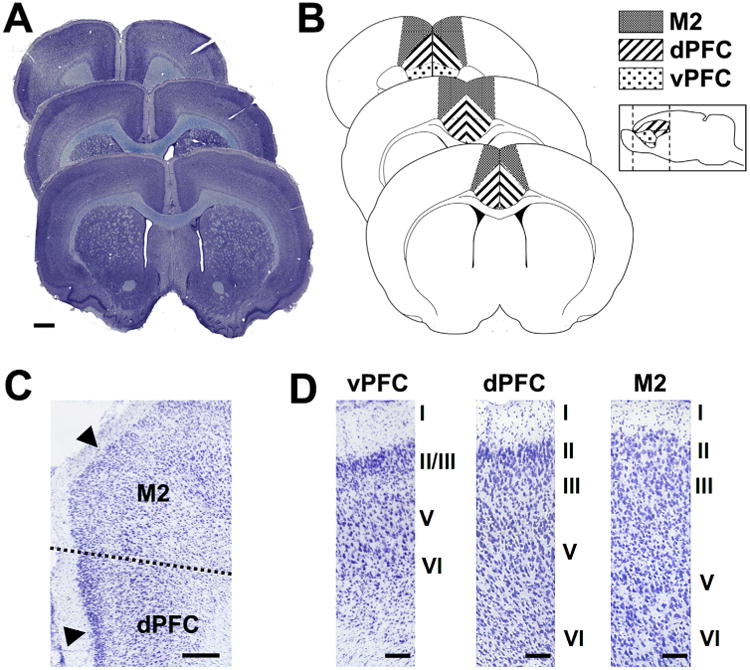
Anatomical characteristics of the rodent prefrontal cortex. A: Sagittal view of the rat forebrain showing the rostrocaudal extent of the dorsal (cingulate areas 1 and 2 [Cg1, Cg2]) and ventral (prelimbic and infralimbic areas [PreL, IL]) prefrontal cortex. B: Coronal schematic showing the position of the dorsal and ventral prefrontal regions, as well as secondary motor cortex (M2), which borders the dorsal prefrontal cortex (dPFC) mediolaterally. C: The mediolateral border between the dPFC and M2 is clearly distinguishable based on the clear laminar demarcation between layer I and II in dPFC relative to M2. D: Cytoarchitectural characteristics of the ventral prefrontal cortex (vPFC), the dPFC, and the M2 region. Scale bar = 1.0 mm in A; 250 μm in C,D.
Unbiased stereological analysis of total neuron numbers and GAD67-positive neuron numbers were carried out with the optical fractionator technique by using StereoInvestigator software. Stereological sampling parameters are given in Table 1. At each systematically selected site, cells were visualized by using a 100× oil immersion objective. Principal neurons were readily identifiable on cresyl violet-stained sections based on their pyramidal morphology with the apex pointed toward the pial surface, and a clearly defined nucleolus. Although soma size was not assessed systematically in this analysis, pyramidal cell diameters typically ranged between 10 and 20 lm, allowing them to be clearly distinguished from the smaller, darker glial cells, Section thickness was recorded at each site, and the average section thickness per animal was computed for stereological estimate calculations. StereoInvestigator software was used to calculate the total neuron number, the numbers of counted neurons, and the corresponding coefficient of error. For GAD67, we also measured antibody penetration by recording the average z-depth of labeled cells. Volume estimates for the dPFC and vPFC were computed via the Cavalieri method. Counting criteria for GAD67 were modified because the staining obscured the nucleus. Instead of using the top of the nucleus as the unique point for marker placement, the most superficial point at which the cell soma was visibly filled with the diaminobenzadine reaction product was identified and marked.
Table 1. Stereological Sampling Parameters for Analysis of Total Neuron Number and GAD67-Positive Cell Number in Young and Aged Rats1.
| Total neuron no. | GAD67+ cell no. | ||||
|---|---|---|---|---|---|
|
|
|
||||
| dPFC | vPFC | dPFC | vPFC | ||
| X step size (μm) | 128 | 127 | 150 | 140 | |
| Y step size (μm) | 138 | 118 | 150 | 140 | |
| Disector volume (μm3) | 1,392 | 1,392 | 2,500 | 2,500 | |
| No. of sampling sites | Young | 1,426 (192.6) | 652 (92.12) | 1,039 (159.3) | 503 (106.6) |
| Aged | 1,172 (235.2) | 596 (102.6) | 918 (93.7) | 570 (123.6) | |
| No. of sections | Young | 9.6 (1.3) | 7.0 (0.92) | 10.2 (1.1) | 6.4 (0.3) |
| Aged | 8.0 (2.4) | 7.6 (1.28) | 10.1 (1.4) | 7.7 (0.4) | |
| Section thickness (μm) | Young | 27.7 (1.4) | 26.6 (1.6) | 20.8 (2.3) | 17.2 (1.7) |
| Aged | 27.0 (3.4) | 25.5 (3.03) | 20.2 (1.7) | 15.9 (1.1) | |
| Coefficient of error | Young | 0.05 (0.004) | 0.08 (0.003) | 0.04 (0.006) | 0.07 (0.007) |
| Aged | 0.06 (0.008) | 0.08 (0.004) | 0.05 (0.008) | 0.06 (0.003) | |
Abbreviations: dPFC, dorsal prefrontal cortex; GAD67, glutamic acid decarboxylase 67; vPFC, ventral prefrontal cortex.
Values represent the group mean, with standard deviation in parenthesis.
Photomicrographs were acquired by using StereoInvestigator, stored as .tiff files, and adjusted for brightness and contrast by using Adobe Photoshop (Adobe Systems, San Jose, CA).
Statistical analysis
Total neuron numbers and GAD67-positive cell numbers were compared across young (n = 8) and aged (n = 16) rats by using bidirectional Student's t-tests in Graphpad Prism version 5.0 (La Jolla, CA). To evaluate whether water maze performance was associated with prefrontal cortical neuron number, aged rats were classified as aged-unimpaired (relative to young; Gallagher et al., 1993), or aged-impaired (n = 8 aged-unimpaired, n = 8 aged-impaired). The resulting three groups were analyzed by using one-way ANOVA with Tukey's post hoc test. For all statistical analyses, significance was set at P < 0.05.
Results
Aging reduces total neuron number in the dorsal prefrontal cortex
Aged rats had fewer neurons in the dorsal component of the prefrontal cortex (Figs. 2A, 3A, B; t23 = 3.78, P = 0.001; total neuron numbers, mean ± SEM, young = 694,624 ± 23,714, aged = 505,303 ± 34,887). Neuronal loss was not accompanied by global atrophy, as the volume of the dPFC was similar across young and aged rats (Fig. 2B; t23 = 0.39, P = 0.70; regional volume in mm3, mean ± SEM, young = 2.41 ± 0.11, aged = 2.34 ± 0.11). Section thickness, number of sampling sites, and corresponding coefficients of error derived from this analysis are shown in Table 1.
Figure 2.
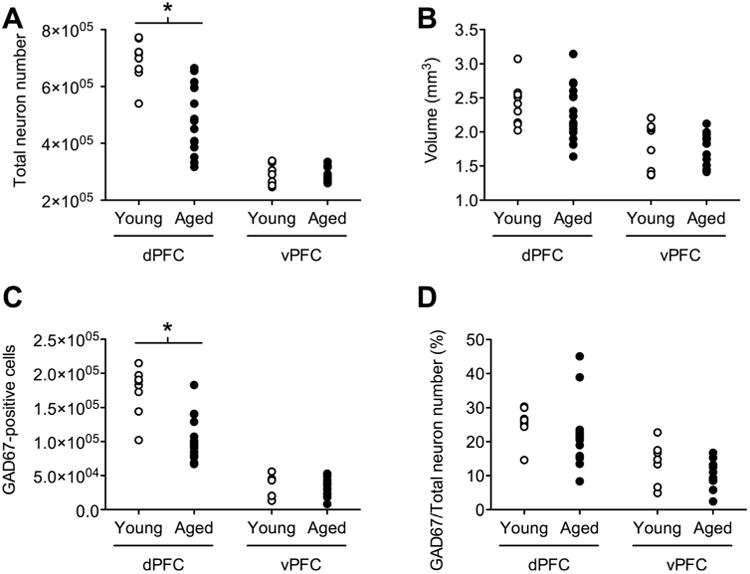
Aging reduced total neuron number and the number of GAD67-positive cells in the dorsal component of the rat prefrontal cortex. A: Unbiased stereological estimates of total neuron number reveal that aging is associated with neuronal loss in the dorsal prefrontal cortex (dPFC). There was no effect of aging on neuron number in the ventral prefrontal cortex (vPFC). B: Changes in total neuron number are not accompanied by gross regional atrophy, as indicated by volume estimates by using the method of Cavalieri. C: The number of GAD67-positive cells is reduced with aging in the dPFC. There was no change in the number of GAD67-positive cells in the vPFC. D: Because total neuron number is also reduced in the dorsal prefrontal cortex with aging, the ratio of GAD67-immunoreactive cells to principal neurons (expressed here as percent GAD67-positive cells relative to total pyramidal cell number on adjacent series of sections) is unchanged. *, Significant difference at P < 0.05 following Student's t-test.
Figure 3.
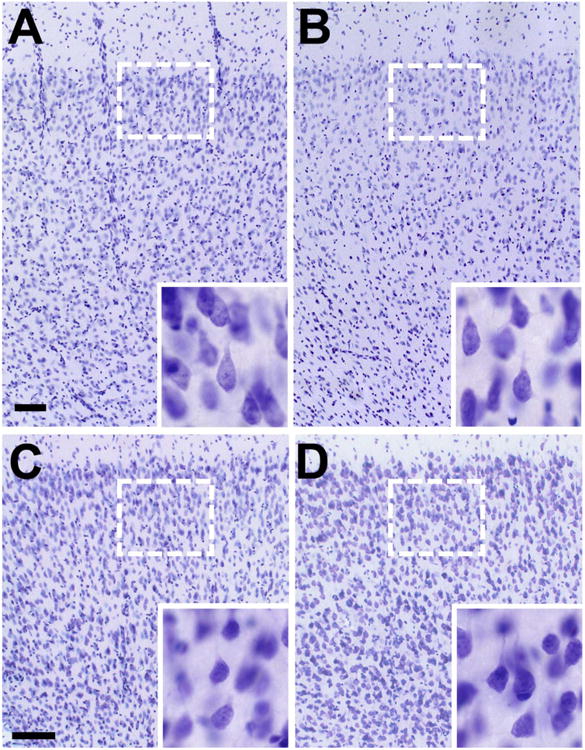
Histological evidence for neuronal loss in the dorsal component of the prefrontal cortex in aged rats. A,B: Principal neuron number is greater in the dorsal prefrontal cortex of young rats (A), relative to aged rats (B). C,D: The number of principal neurons is unchanged in the ventral prefrontal cortex of young (C) and aged (D) rats. Insets depict higher magnification images of Cresyl violet-stained cells in the dorsal (A,B) and ventral (C,D) prefrontal cortex. Scale bar = 100 lm in A (applies to A,B) and C (applies to C,D). Inset scale bar = 20 lm in A (applies to A,B) and C (applies to C,D).
The Cg1 and Cg2 (also known as dorsal and ventral cingulate cortices) were grouped together to represent the dPFC. This parcellation was determined on the basis of previously published work demonstrating connectional similarities between the two cingulate areas (Vertes et al., 2006). However, we also quantified total neuron number in the Cg1 and Cg2 separately. This analysis revealed that both the Cg1 and Cg2 exhibit neuron loss with aging (for Cg1, total neuron numbers, mean 6 SEM, young = 388,212 ± 16,709, aged = 286,959 ± 19,916, t23 = 3.43, P = 0.002; for Cg2, total neuron numbers, mean ± SEM, young = 306,411 ± 11,301, aged = 218,343 ± 16,529, t23 = 3.71, P = 0.001). Because similar trends were observed for the Cg1 and Cg2, these two regions were grouped together in subsequent analyses.
There was no change in total neuron number in the ventral prefrontal cortex (Figs. 2A, 3C,D; t17 = 0.36, P = 0.72; total neuron numbers, mean ± SEM, young = 286,614 ± 12,740, aged = 291,826 ± 7,772). Likewise, there was no change in the volume of the ventral prefrontal cortex (Fig. 2B; t17 = 0.17, P = 0.86; regional volume in mm3, mean ± SEM, young = 1.78 ± 0.12, aged = 1.75 ± 0.07). Section thickness, number of sampling sites, and coefficients of error in this analysis are shown in Table 1.
Aging reduces the number of GAD67-positive cells in the dorsal prefrontal cortex
In order to evaluate whether specific neuronal populations might be more susceptible to aging, we analyzed the number of cells that expressed GAD67. Again, aging reduced GAD67-positive cell number in the dorsal prefrontal cortex (Figs. 2C, 4A,B; t21 = 4.86, P = 0.001; total GAD67-positive cell numbers, mean ± SEM, young = 174,115 ± 12,543, aged = 103,813 ± 8,201). Section thickness, number of sampling sites, and corresponding coefficients of error are shown in Table 1. The relationship between principal neurons and GAD67-positive cell number in the dPFC was therefore unchanged (Fig. 2D; t21 = 1.08, P = 0.29; GAD67-positive cells as a percent of principal neuron number, mean 6 SEM, young = 25.4 ± 1.72, aged = 21.6 ± 2.41).
Figure 4.
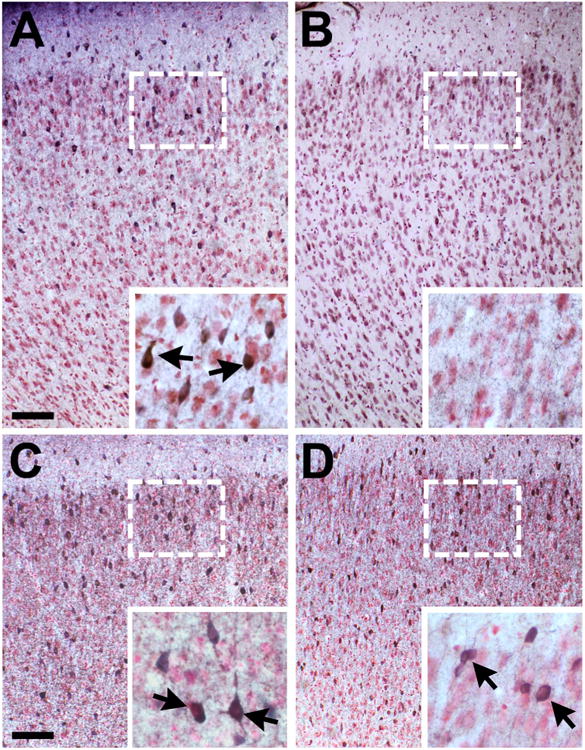
Reductions in GAD67-positive cell number with aging in the dorsal prefrontal cortex. A,B: Young rats (A) have more GAD67-positive cells than aged rats (B) in the dorsal prefrontal cortex. C,D: GA67-positive cell numbers are maintained in the ventral prefrontal cortex of young (C) and aged (D) rats. Higher magnification images (insets) with arrows indicating labeled cells. Scale bar = 100 μm in A (applies to A,B) and C (applies to C,D). Inset scale bar = 20 μm in A (applies to A,B) and C (applies to C,D)
There was no change in GAD67-positive cell number in the ventral prefrontal cortex (Figs. 2C, 4C,D; t21 = 0.46, P = 0.63; total GAD67-positive cell numbers, mean ± SEM, young = 34,923 6 5,847, aged = 32,202 ± 3,064). Section thickness, number of sampling sites, and corresponding coefficients of error are shown in Table 1. The percent of GAD67-positive cells relative to total neuron number was likewise unchanged (Fig. 2D; t16 = 1.47, P = 0.16; GAD67-positive cells as a percent of principal neuron number, mean ± SEM, young = 13.81 ± 2.35, aged = 10.25 ± 1.24). Antibody penetration, defined as the average z-depth of the counted cells, was similar across young and aged rats (data not shown).
Reductions in neuron number occur independently of spatial memory impairment
Rats were behaviorally categorized based on their performance in the hippocampus-dependent version of the water maze, as described (Stranahan et al., 2011). Aged rats that performed within the range of young, as measured by using an index score derived from proximity to the goal platform location during interpolated probe trials, were classified as aged-unimpaired (AU), whereas rats that performed outside of the range of young were designated aged-impaired (AI).
Neuronal loss in the dorsal prefrontal cortex occurred in both AU and AI rats (Fig. 5A; F2,22 = 8.84, P = 0.005; total neuron numbers, mean ± SEM, young = 694,624 ± 23,714, AU = 502,556 ± 56,310, AI = 508,050 ± 45,201). Neuron number was maintained in the ventral prefrontal cortex of aged rats, irrespective of spatial memory performance (F2,16 = 0.66, P = 0. 53; total neuron numbers, mean ± SEM, young = 286,614 ± 12,740, AU = 280,914 6 9,104, AI = 300,919 6 11,394). The number of GAD67-positive cells was reduced with aging, and this reduction was not associated with spatial memory performance (F2,22 = 12.87, P = 0.003; total GAD67-positive cell numbers, mean ± SEM, young = 174,115 ± 12,543, AU = 114,964 ± 14,757, AI = 94,056 ± 7,728).
Figure 5.
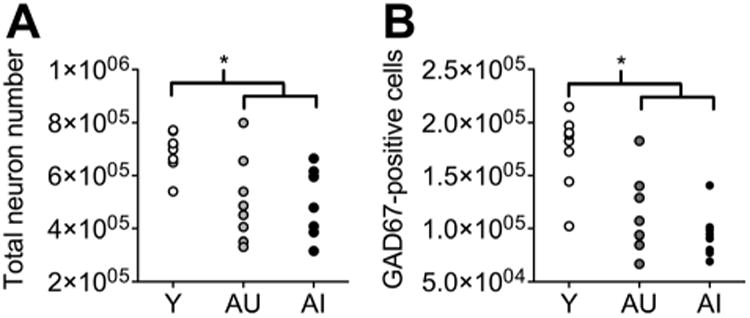
Hippocampus-dependent learning deficits do not correlate with loss of principal neurons or GAD67-positive cells in the dorsal prefrontal cortex. A: Total neuron numbers in the dorsal prefrontal cortex are comparably reduced in aged rats that exhibit spatial learning impairment (AI), and in aged rats that are unimpaired (AU) relative to young (Y) rats. B: The number of GAD67-positive cells is reduced in aged rats that differ in terms of spatial memory performance. *, Significance at P < 0.05 following one-way ANOVA with Tukey's post hoc test.
To gain greater insight into mechanisms that might be associated with spatial memory performance in aged rats, we analyzed the percent of GAD67-positive neurons relative to total neuron number. Age-related cognitive impairment was not associated with any difference in this relationship (F2,22 = 1.53, P = 0.24; ratio of total neurons to GAD67-positive cell number, mean ± SEM, young = 25.4 ± 1.73, AU = 24.6 ± 4.87; AI = 19.0 ± 1.34).
Discussion
In the current report, we observed neuronal loss in the dorsal component of the rat PFC. This loss occurred among principal neurons and GAD67-immunoreactive cells, such that a stable ratio of principal neurons to putative interneurons was maintained. Changes were confined to the dPFC, as no alterations in total neuron number or GAD67-positive cell number were observed in the vPFC, and age-related neuronal loss was not associated with spatial memory performance. We used behavioral characterization in a hippocampal-dependent task because individual differences in this model were previously shown to correlate with age-related reductions in both hippocampal and prefrontal cortical glucocorticoid receptor expression (Bizon et al., 2001). We believe that when these data are taken together with the current observation that both aged-impaired and aged-unimpaired rats exhibit decreased neuron number in the dorsal prefrontal region, to the extent that such neuron loss is detrimental in this behavioral model, some compensatory mechanisms might be recruited to maintain the performance of unimpaired rats.
In addition to interactions of the PFC with the medial temporal lobe, the PFC participates in the default mode network, defined as a set of anatomically connected cortical regions that exhibited correlated activation patterns at rest. Recent reports have demonstrated the existence of a default mode network in humans (Honey et al., 2009), non-human primates (Vincent et al., 2007), and rodents (Liang et al., 2011). Correlations between levels of activation across the default mode network and cognitive impairment with aging have also been demonstrated (Sperling et al., 2010), opening the possibility that neuron loss in one component of this system could disrupt the coordination of activity and information processing, with consequences for cognitive functions not limited to hippocampal-dependent memory.
Independent effects of aging across neurobehavioral systems have been reported and could be relevant to the current findings. Aged rats perform poorly during attentional set-shifting (Barense et al., 2002) in a task that has been demonstrated to depend on the integrity of the prefrontal cortex (Birrell and Brown, 2000). It is noteworthy that, in aged rats, individual differences in spatial memory performance are uncorrelated with set-shifting ability, suggesting that prefrontal cortical and medial temporal memory systems exhibit some dissociable effects of aging (Barense et al., 2002). The extent to which age-related reductions in prefrontal cortical neuron number and behavioral deficits in attentional set-shifting may be attributable to changes in activation of the default mode network remain to be determined.
Moreover, the question of whether neuron loss observed in the current study contributes to age-related deficits in attentional set-shifting has not yet been addressed. The behavioral significance of neuron loss detected in the cortex would be important to determine because cognitive deficits in hippocampal-dependent tasks are observed in the absence of altered neuron number in the medial temporal lobe but are correlated with functional alterations in largely intact circuits (Rapp and Gallagher, 1996; for review, see Wilson et al. 2006).
Our observation of neuronal loss in the dorsal region and preservation of neuron number in the ventral region differs from a previous report (Yates et al., 2008). Yates and colleagues (2008) reported an age-dependent reduction in the vPFC, but not the dPFC. Although the regional boundaries identified in the current study were reliably detected on the same sections by two different observers, the possibility that this disparity arises from differences in regional parcellation across the two studies cannot be ruled out. However, genetic divergence between the Long-Evans hooded rats used by Yates et al. (2008), derived from an in-house breeding colony, and the Long-Evans rats purchased from Charles River Laboratories for use in the current study is another possible reason for differences in the results. Because genetic differences in the effect of ventral prefrontal lesions on behavior have been demonstrated (Chang and Maren, 2010), it is possible that genetic drift could account for distinct trajectories of neuronal loss in subregions of the prefrontal cortex.
With respect to the current findings, the stereological data and prefrontal regional volumes derived from young rats in our study fall closely within the range of estimates from previous studies using unbiased stereological methods in frozen tissue (Cerqueira et al., 2005; Yates et al., 2008). Because using frozen sections could affect counts through the z-axis, the current observations might underestimate absolute neuron numbers. However, to our knowledge, no comparison values from tissue prepared by using Vibratome sectioning exists for these cortical regions, so it is difficult to assess what effect sectioning on a freezing microtome might have had on total neuron numbers. In that context, the numerical trends observed in the current study may also under-represent the absolute number of prefrontal GAD67-positive interneurons, and numbers of cells expressing GAD65 were not assessed.
Disinhibition of the PFC leads to deficits in cognitive flexibility (Gruber et al., 2010), in a manner reminiscent of the impaired reversal learning reported in a subset of aged rats (Schoenbaum et al., 2006). We quantified the number of GAD67-positive cells and expressed the ratio of GAD67-positive cells to principal neurons to estimate whether this population might be specifically vulnerable to aging in the prefrontal cortex. Because both the number of principal neurons and the number of GAD67-positive cells was reduced, the numerical relationship between excitatory and GAD67-expressing inhibitory neuron number was maintained. This suggests that neuronal loss in the aging dPFC is not limited to a specific neuronal phenotype. Perhaps most importantly, a focal age-related decline in prefrontal cortical neuron number appears to be conserved across rodents in the current study and in primate species (Smith et al., 2004), suggesting that a common basis for such vulnerability in cortical circuits may exist in the mammalian brain.
Acknowledgments
M.G. is the founder of AgeneBio Incorporated, a biotechnology company that is dedicated to commercializing therapies to treat cognitive impairment in aging, and she has a financial interest in the company.
Grant sponsor: National Institutes of Health National Research Service Award; Grant number: F32AG03481801; Grant sponsors: Ford Foundation/National Research Council (to A.M.S.); Provost's Undergraduate Research Award, Johns Hopkins University (to N.T.J.); program project grant, Johns Hopkins University; Grant number: P01AG009973-18 (to M.G).
Literature Cited
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Helm KA, Han JS, Chun HJ, Pucilowska J, Lund PK, Gallagher M. Hypothalamic-pituitary-adrenal axis function and corticosterone receptor expression in behav-iorally categorized young and aged Long-Evans rats. Eur J Neurosci. 2001;14:1739–1751. doi: 10.1046/j.0953-816x.2001.01781.x. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Pêgo JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Strain difference in the effect of infralimbic cortex lesions on fear extinction in rats. Behav Neurosci. 2010;124:391–397. doi: 10.1037/a0019479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brabander JM, Kramers RJ, Uylings HB. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci. 1998;10:1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J. The prefrontal cortex. Fourth. Academic Press; 2008. [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gonzales C, Kaufman DL, Tobin AJ, Chesselet MF. Distribution of glutamic acid decarboxylase (Mr 67,000) in the basal ganglia of the rat: an immunohistochemical study with a selective cDNA-generated polyclonal antibody. J Neurocytol. 1991;20:953–961. doi: 10.1007/BF01187913. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O'Donnell P. More is less: a disinhibited pre-frontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Association of storage and processing functions in the dorsolateral prefrontal cortex of the nonhuman primate. J Neurosci. 1999;19:5149–5158. doi: 10.1523/JNEUROSCI.19-12-05149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, King J, Zhang N. Uncovering intrinsic connec-tional architecture of functional networks in awake rat brain. J Neurosci. 2011;31:3776–3783. doi: 10.1523/JNEUROSCI.4557-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Stereotaxic Coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8:671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Deroche PS, Mao Y, Burwell RD. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb Cortex. 2002;12:1171–1179. doi: 10.1093/cercor/12.11.1171. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J Neurophysiol. 2006;95:1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of sub-cortical neurons. J Neurosci. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaVio-lette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Sel-koe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer's disease. Neuromol Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Haberman RP, Gallagher M. Cognitive decline is associated with reduced reelin expression in the entorhinal cortex of aged rats. Cereb Cortex. 2011;21:392–400. doi: 10.1093/cercor/bhq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based mor-phometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H. Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MA, Markham JA, Anderson SE, Morris JR, Juraska JM. Regional variability in age-related loss of neurons from the primary visual cortex and medial prefrontal cortex of male and female rats. Brain Res. 2008;1218:1–12. doi: 10.1016/j.brainres.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]


