Abstract
We report here the construction of Tubby-RFP balancers for the X, 2nd and 3rd chromosomes of Drosophila melanogaster. The insertion of a 2xTb-RFP transgene on the FM7c, CyO and TM3 balancer chromosomes introduces two easily scorable, dominant, developmental markers. The strong Tb phenotype is visible to the naked eye at the larval L2, L3 and pupal stages. The RFP associated with the cuticle is easily detected at all stages from late embryo to adult with the use of a fluorescence stereomicroscope. The FM7c Bar 2xTb-RFP, CyO Cy 2xTb-RFP and TM3 Sb 2xTb-RFP balancers will greatly facilitate the analysis of lethals and other developmental mutants in L2/L3 larvae and pupae, but also provide coverage of other stages beginning in late embryogenesis through to the adult.
Keywords: Tubby, DsRed, RFP, Tb, Tb1, Balancer, Drosophila
An extremely useful tool available to the Drosophila geneticist is the “balancer” chromosome. This marked chromosome allows recessive lethal mutants to be maintained as true-breeding stocks thanks to the very efficient suppression of recombination between homologues. Moreover, since the balancer chromosome can be easily identified through a visible, dominant trait, segregation of the recessive lethal chromosome can be readily followed in genetic crosses [Greenspan, 1997].
The origin of balancers goes back to the beginnings of Drosophila genetics. In 1918, Hermann Muller determined that a lethal mutation could be kept as a stock when another lethal mutation was located on the corresponding homologous chromosome. Muller referred to these as ‘balanced lethals’ [Muller, 1918]. Shortly after, Alfred Sturtevant proposed that inversions present on one of the homologous chromosomes suppressed recombination between the pair [Sturtevant, 1921]. Inversions in combination with lethals allowed balancing of larger chromosomal regions and created the foundation for the balancers in use today.
Today’s autosomal balancers are complex chromosomes, containing 1) multiple, overlapping inversions that effectively suppress recombination across much of the chromosome, 2) a lethal, recessive mutation that prevents survival of homozygous balancer flies, and 3) at least one visible, dominant marker that unequivocally identifies the balancer in the adult fly. X chromosome balancers are similarly constructed except for the lack of recessive lethal loci. However, most include a recessive female-sterile locus, thereby restricting fertility to the heterozygous females while ensuring the viability of the hemizygous males.
As mentioned above, all balancers carry a visible dominant adult marker, such as the 3rd chromosome traits stubble bristles (Sb) and serrated wings (Ser) found on TM3 balancers, or the curly wings (Cy) associated with all 2nd chromosome balancers, including CyO or the SM# series. Only the 3rd chromosome balancers TM6B and TM6C also carry a dominant visible larval/pupal marker, Tb1, in addition to the dominant adult bristles markers Humeral (Hu) and Sb, respectively. The Tubby phenotype due to the Tb1 mutant allele consist of a short and stout larva, and eventually pupa, clearly distinguishable from the slender body shape of the wt particularly at the L3 and pupal stages [Crymer 1980, 1984]. This larval marker makes the TM6B and TM6C balancers more versatile than any other and a much appreciated tool of the developmental geneticist.
First identified by Charlotte Auerbach in 1943, the Tb1 phenotype can always be distinguished from Tb+ in both homozygous and heterozygous larvae (L2, L3) or pupae [Craymer, 1980]. The phenotype is visible to the naked eye and thus allows one to quickly detect the segregation of mutant alleles balanced over TM6B or TM6C at the larval and pupal stages, thus facilitating the analysis of developmental mutants. Another particularly effective use of this feature is in large-scale screens for the identification of recessive lethal alleles on the 3rd chromosome. Scoring of the Tb marker at the pupal stage avoids the need to carefully score adult progeny, greatly improving the efficiency of such screens. Moreover, thanks to its normal fertility and viability, the use of the Tb marker does not adversely affect any crosses [Guan et al., 2006].
Over time, transgenic embryonic and larval markers have been added to several 1st, 2nd, and 3rd chromosome balancers for the study of developmental loci. GFP-expressing transgenes allow visual scoring of progeny at these early stages through the use of fluorescence microscopy [Reichhart and Ferrandon, 1998; Casso et al., 1999]. Nonetheless, the simplicity of the Tb1 marker, which can be detected visually without use of a stereomicroscope, remains unbeaten.
Recently, the Tb locus has been identified as a member of the Twdl gene family. Twdl type proteins are involved in cuticle formation and are characterized by 2 conserved motifs found in all members of this insect protein family (Guan et al., 2006). By sequence analysis, Guan and colleagues (2006) identified a 23 amino acid deletion within one of the evolutionarily conserved domains of TwdlA to be the cause of the dominant Tb1 phenotype. A similar deletion in TwdlD, TwdlD1, could also induce the short body type (Guan et al., 2006). The mutant phenotype was dose dependent and best visible at 1:1, or greater, ratios of mutant to wt alleles (Guan et al., 2006). A quantitative description of this trait is provided by the axial ratio (AR), defined as the ratio of the larval/pupal body length to the body width. This value has been shown to possess a constant mean that is characteristic of a fly strain and independent of the overall size of a pupa [Letsou et al., 1991]. Tb+ stocks have a pupal AR of ~3.0, whereas the AR of Tb1 pupae is around 2.0.
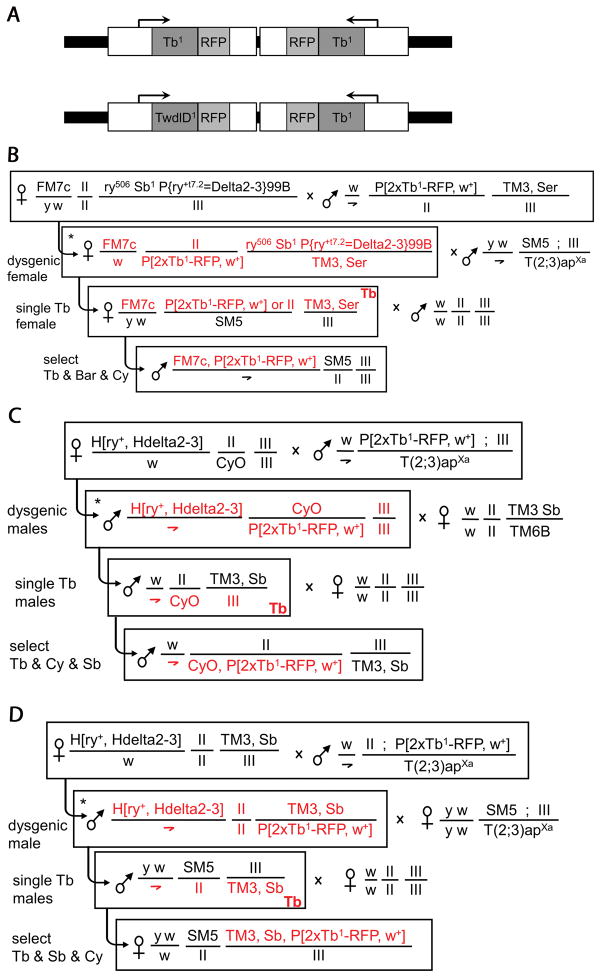
We report the construction of new Tb-RFP balancers for the X, II and III chromosomes of Drosophila. We first generated two types of ‘Tubby’ P-element constructs: one with two copies of the Tb1-RFP fusion and another with one copy of Tb1-RFP and one of TwdlD1-RFP (Guan et al., 2006) (Fig. 1A). Two Tb-inducing alleles were used in order to ensure at least a 1:1 ratio of mutant to wt alleles. Both constructs were then transformed into w1118 creating several Tb-looking fly lines. Transgenic lines containing the 2xTb1-RFP transgene displayed a more severe Tb phenotype than TwdlD1-RFP + Tb1-RFP lines and were used as donors for transposition of the P-elements onto balancers for the X (FM7c, Bar), the 2nd (CyO, Cy) and 3rd (TM3, Sb) chromosomes (Fig. 1B-D).
Figure 1. Constructs and genetic crosses.
A) Top: the P[2xTb1-RFP] construct contains two copies of the Tb1-RFP fusion gene previously described in Guan et al., 2006. Bottom: the P[TwdlD1-RFP, Tb1-RFP] construct contains one copy of the TwdlD1-RFP fusion gene and one copy of the Tb1-RFP previously described in Guan et al., 2006. Shaded boxes show coding regions, open boxes regulatory DNA and 5′/3′ UTRs (see Methods for details). B-D) Outline of genetic crosses for P-element transposition onto non-Tb balancer chromosomes, FM7c (B), CyO (C), TM3 (D). The asterisk denotes the dysgenic male or female; any of the chromosomes present in the dysgenic fly can acquire a new P-element insertion and are marked in red in the descendents; roman numerals indicate the corresponding unmarked chromosome.
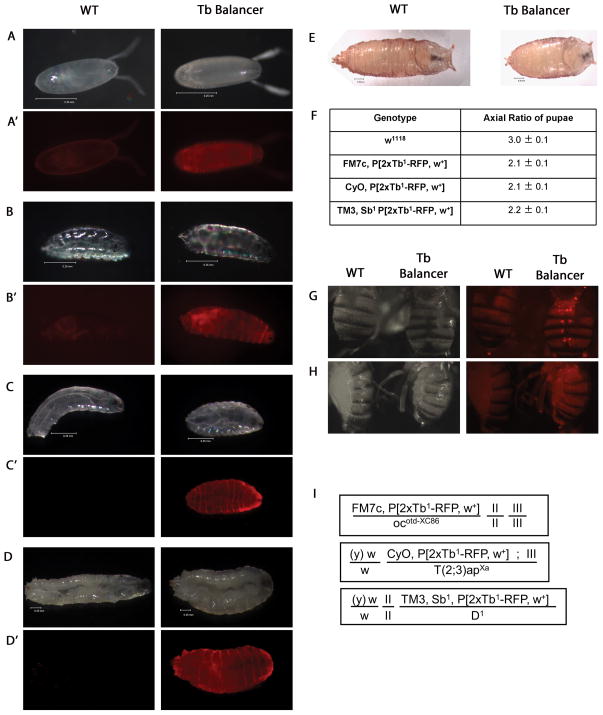
The Tb phenotype of the resulting new balancers is easily scored at the L2, L3 and pupal stages, and the presence of RFP extends this range to include late embryos, L1 larvae and adults (Fig. 2). In addition, since expression of the Tb1-RFP fusion protein is associated nearly exclusively with the cuticle, its presence does not interfere with the staining of internal tissues (e.g. larval imaginal discs) permitting triple-staining of balancer siblings as controls.
Figure 2. Tubby and RFP detection in embryos, larvae, pupae and adults.
A-E; G-H) Bright field and fluorescence images of w1118 in all “WT” panels and different 2xTb-RPF balancer lines in “Tb Balancer” panels. No significant differences were seen in the intensity of the fluorescence or the expressivity of the Tb trait between different balancers. Balancers shown are: A,A′) TM3, Sb1 P[2xTb1-RFP, w+] embryo; B,B′) CyO, P[2xTb1-RFP, w+] L1 larva; C,C′) TM3, Sb1 P[2xTb1-RFP, w+] L2 larva; D,D′) FM7c, P[2xTb1-RFP, w+] L3 larva; E) TM3, Sb1 P[2xTb1-RFP, w+] pupa; G) TM3, Sb1 P[2xTb1-RFP, w+] fly; H) TM3, Sb1 P[2xTb1-RFP, w+] fly. Images were taken on Zeiss SV11 and Leica MZ16F fluorescence stereomicroscopes and processed in Adobe Photoshop. F) AR of WT pupae (w1118; Tb+), N=25, or pupae carrying Tb-balancer chromosomes, N=40 for each new balancer. I) Genotypes of Tubby balancer stocks deposited at the Bloomington Drosophila stock center.
As expected for a 1:1 ratio of Tb to Tb+ loci, the mean AR of pupae from the 2xTb1-RFP balancers is ~2.0, significantly lower than the ~3.0 AR of w1118 pupae (Fig. 2E, 2F) or of the original balancer lines (see Fig. 2 legend). Tb1-RFP expression begins in late embryogenesis during larval cuticle deposition, and the fusion protein can be detected with a fluorescence stereomicroscope at this time (Fig. 2A′). In larvae, Tb1-RFP is present throughout the cuticle and is easily visible during movement when the cuticle is compressed permitting detection in live larvae even at the L1 stage (Fig. 2B′, 2C′, 2D′). Adult flies also fluorescence. In the abdominal region, two lines of fluorescent dots are clearly visible through the dorsal cuticle (Fig. 2G) and diffuse fluorescence is present on the ventral side (Fig. 2H). Whereas detection of the RFP will be most informative at the embryonic and L1 stages, it may also be useful in adult flies particularly if the adult visible trait associated with the balancer (Bar, Cy or Sb) is masked by, or similar it is to, phenotypes associated with other chromosomes present in the stock (e.g., the dominant Bar eye associated with FM7 cannot be scored in an genetic background that causes an adult eyeless phenotype).
In summary, the presence of the 2xTb1-RFP transgene on these balancers (FM7c, Bar 2xTb-RFP; CyO, Cy 2xTb-RFP; TM3, Sb 2xTb-RFP) greatly facilitates the analysis of lethals and other developmental mutants in L2/L3 larvae and pupae, but also extends this range from late embryos all the way to adults. At the time of this submission, the Gatti group reported the construction of Tb-tagged FM7a and CyO-Sbw balancers (Lattao et al., 2011). Our balancers differ in that they carry 2 copies of Tb1 and additionally the RFP-fusion marker. Moreover, FM7a and FM7c differ significantly in that FM7a is homozygous fertile whereas FM7c is sterile and better suited for the maintenance of X-linked lethals and weaker X chromosome. Thus, these new balancers significantly expand the options of available Tb balancers. The FM7c, Bar 2xTb-RFP, CyO, Cy 2xTb-RFP, and TM3, Sb 2xTb-RFP balancer stocks listed in Fig. 2I have been deposited at the Bloomington Drosophila Stock Center (http://flystocks.bio.indiana.edu).
METHODS
Cloning
The P[2xTb1-RFP] construct was generated by cloning a SpeI/XbaI Tb1-RFP fragment of pCaSpeR Tb1-RFP (Guan et al., 2006) [containing the Tb1 mutant genomic DNA from the 5′ regulatory region through the 3′UTR with the RFP coding region fused in frame with Tb1] into the SpeI sites of pCaSpeR4. A second SpeI/XbaI Tb1-RFP fragment was then inserted in the XbaI site of the newly generated P[1xTb1-RFP] plasmid resulting in the final P[2xTb1-RFP] construct. The P[TwdlD1-RFP, Tb1-RFP] construct was generated by cloning a EcoRI/KpnI TwdlD1-RFP fragment from pCaSpeR TwdlD1-RFP (Guan et al., 2006) [containing a TwdlD1 mutant genomic DNA from the 5′ regulatory region through the 3′UTR with the RFP coding region fused in frame with TwdlD1] into the EcoRI/KpnI sites of pCaSpeR4. The SpeI/XbaI Tb1-RFP isolated from pCaSpeR Tb1-RFP (Guan et al., 2006) was then inserted into the SpeI site of the P[TwdlD1-RFP] plasmid generating the final P[TwdlD1-RFP, Tb1-RFP] construct.
P-element transposition
An outline of the genetic crosses for P-element transposition is shown in Fig. 1B-D. Progeny from a dysgenic male or female containing the P[2xTb1-RFP] donor chromosome, a source of transposase and the target balancer were screened individually for co-segregation of the Tb trait and the balancer chromosome. Flies confirmed to have P-element insertions on the balancers were backcrossed to w1118 and a marked chromosome was introduced to create a balanced stock. Specific genotypes of the flies used for the cross schemes in Fig. 1 were as follows:
y H[ry+, Hdelta2-3] pn; so8/CyO
w; P[2xTb1-RFP, w+]; III/T(2,3)apXa
w; TM3, Sb1/ TM6B, Tb Hu
y w; SM5; III / T(2,3)apXa,
w; KrIf-1/CyO; D1/TM6B, Tb+
FM7c /y1 w, 2) ry506 Sb1 P{ry+t7.2=Delta2-3}99B/III
w; P[2xTb1-RFP, w+]/II; TM3, Ser1/III
w; II; SM5; III/T(2,3)apXa
ocotd-XC86/FM7a.
Roman numerals denote corresponding unmarked chromosomes. The H[ry+, Hdelta2-3] transposase was a gift of B. Calvi (Calvi et al., 1994). The P{ry+t7.2=Delta2-3}99B transposase and all other chromosomes were obtained from the Bloomington Drosophila Stock Center.
Acknowledgments
Many thanks to Dr. S. Wasserman for Tb-RFP DNA clones, the Bloomington stock center and Dr. B. Calvi for fly stocks. This work was supported by NEI grants R01EY013167 and R01EY017097 to FP and an RPB unrestricted grant to the Dept. of Ophthalmology, Upstate Medical University.
References
- Calvi BR, Gelbart WM. The basis for germline specificity of the hobo transposable element in Drosophila melanogaster. EMBO Journal. 1994;13:1636–44. doi: 10.1002/j.1460-2075.1994.tb06427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casso D, Ramirez-Weber FA, Kornberg TB. GFP-tagged balancer chromosomes for Drosophila melanogaster. Mech Dev. 1999;88:229–232. doi: 10.1016/s0925-4773(99)00174-4. erratum (2000) 91, 451–454. [DOI] [PubMed] [Google Scholar]
- Craymer L. New mutants report. DIS. 1980;55:197–200. [Google Scholar]
- Craymer L. New mutants report. DIS. 1984;60:234–236. [Google Scholar]
- Greenspan R. Fly Pushing: The Theory and Practice of Drosophila Genetics. Cold Spring Harbor (NY): Cold Spring harbor Laboratory Press; 1997. [Google Scholar]
- Guan X, Middlebrooks B, Alexander S, Wasserman S. Mutation of TweedleD, a member of an unconventional cuticle protein family, alters body shape in Drosophila. Proc Natl Acad Sci USA. 2006;103:16794–16799. doi: 10.1073/pnas.0607616103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges KE, Justice MJ. Checks and balancers: Balancer chromosomes to facilitate genome annotation. Trends Genet. 2004;20:252–259. doi: 10.1016/j.tig.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Lattao R, Bonaccorsi S, Guan X, Wasserman SA, Gatti M. Tubby-tagged balancers for the Drosophila X and second chromosomes. Fly. 2011 Oct-Nov;5 doi: 10.4161/fly.5.4.17283. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsou A, Alexander S, Orth K, Wasserman SA. Genetic and molecular characterization of tube, a Drosophila gene maternally required for embryonic dorsoventral polarity. Proc Natl Acad Sci USA. 1991;88:810–814. doi: 10.1073/pnas.88.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ. Genetic variability, twin hybrids, and constant hybrids, in a case of balanced lethal factors. Genetics. 1918;3:422–499. doi: 10.1093/genetics/3.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart ML, Ferrandon D. Green balancers. DIS. 1998;81:201–202. [Google Scholar]
- Sturtevant AH. A case of rearrangement of genes in Drosophila. Proc Natl Acad Sci USA. 1921;7:235–237. doi: 10.1073/pnas.7.8.235. [DOI] [PMC free article] [PubMed] [Google Scholar]