Abstract
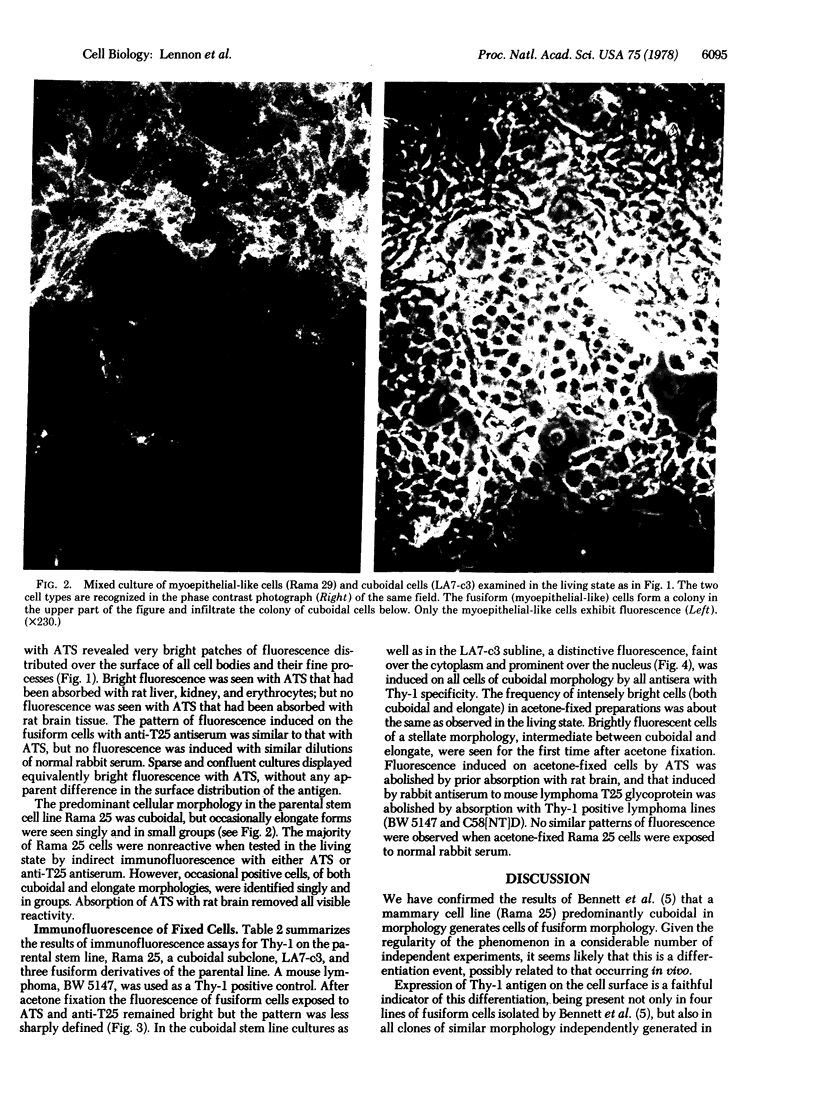
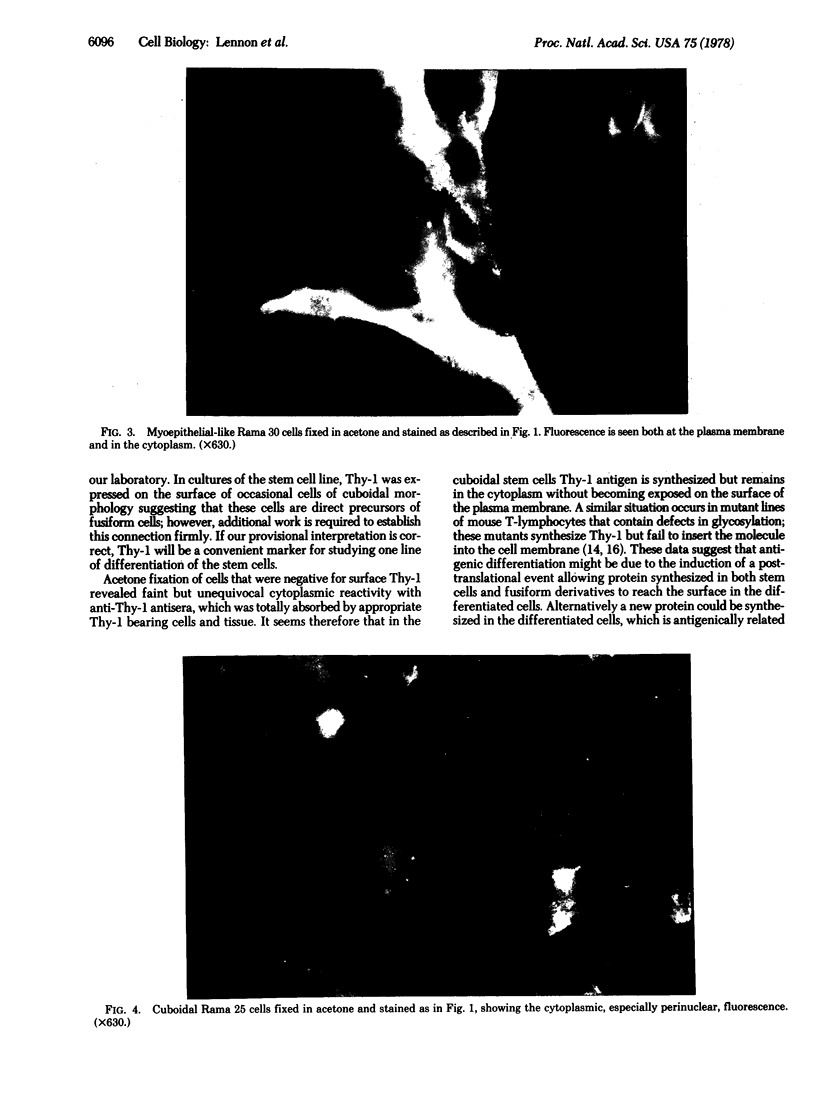
The rat mammary cell line Rama 25 [Bennett, D.C., Peachey, L.A., Durbin, H. & Rudland, P.S. (1978) Cell 15, 283--298] differentiates morphologically in vitro from a cuboidal form to a fusiform cell resembling myoepithelial cells. This differentiation occurs in all clonal isolates of the line. By using three different rabbit antisera specific for Thy-1, we have found that antigenic differentiation accompanies morphologic change to the fusiform state. Very few cuboidal cells had Thy-1 detectable on their surfaces in the living state; but after acetone fixation cytoplasmic Thy-1 was detected by immunofluorescence in all cells of the cuboidal type. Thy-1 specificity was established by the fact that immunofluorescence induced by rabbit anti-rat thymocyte serum was abolished by absorption with rat brain but not with erythrocytes, kidney, or liver; immunofluorescence induced by rabbit antiserum to purified Thy-1 glycoproteins from mouse lymphomas was absorbed by Thy-1 positive mouse and rat lymphomas. Surface Thy-1 provides a potentially valuable antigenic marker in the Rama 25 line for studying the differentiation of mammary myoepithelial cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Letarte-Muirhead M., Williams A. F., Faulkes R. A. Chemical characterisation of the Thy-1 glycoproteins from the membranes of rat thymocytes and brain. Nature. 1976 Oct 14;263(5578):563–567. doi: 10.1038/263563a0. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Letarte-Muirhead M., Williams A. F. Purification of the Thy-1 molecule from rat brain. Biochem J. 1975 Dec;151(3):699–706. doi: 10.1042/bj1510699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch R. S., Goldstein G. Induction of T-cell differentiation in vitro by thymin, a purified polypeptide hormone of the thymus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1474–1478. doi: 10.1073/pnas.71.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. C., Peachey L. A., Durbin H., Rudland P. S. A possible mammary stem cell line. Cell. 1978 Sep;15(1):283–298. doi: 10.1016/0092-8674(78)90104-6. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Hyman R., Trowbridge I., Singer S. J. Localization of T25 glycoprotein in wild-type and Thy 1- mutant cells by immunofluorescence and immunoelectron microscopy. J Exp Med. 1978 May 1;147(5):1348–1354. doi: 10.1084/jem.147.5.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R. W. Expression of Thy 1 antigen in normal and neoplastic mammary cells of mice. J Natl Cancer Inst. 1977 Jun;58(6):1629–1633. doi: 10.1093/jnci/58.6.1629. [DOI] [PubMed] [Google Scholar]
- Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974 Oct;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Haffke S. C., Seeds N. W. Neuroblastoma: the E. coli of neurobiology? Life Sci. 1975 Jun 1;16(11):1649–1657. doi: 10.1016/0024-3205(75)90048-x. [DOI] [PubMed] [Google Scholar]
- Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970;53:161–220. [PubMed] [Google Scholar]
- Hilgers J., Haverman J., Nusse R., van Blitterswijk W. J., Cleton F. J., Hageman P. C., van Nie P., Calafat J. Immunologic, virologic, and genetic aspects of mammary tumor virus-induced cell-surface antigens: presence of these antigens and the Thy 1.2 antigen on murine mammary gland and tumor cells. J Natl Cancer Inst. 1975 Jun;54(6):1323–1333. doi: 10.1093/jnci/54.6.1323. [DOI] [PubMed] [Google Scholar]
- Lesley J. F., Lennon V. A. Transitory expression of Thy-1 antigen in skeletal muscle development. Nature. 1977 Jul 14;268(5616):163–165. doi: 10.1038/268163a0. [DOI] [PubMed] [Google Scholar]
- Scheid M., Boyse E. A., Carswell E. A., Old L. J. Serologically demonstrable alloantigens of mouse epidermal cells. J Exp Med. 1972 Apr 1;135(4):938–955. doi: 10.1084/jem.135.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P. L. Theta alloantigen on mouse and rat fibroblasts. Nat New Biol. 1973 Nov 21;246(151):76–78. doi: 10.1038/newbio246076a0. [DOI] [PubMed] [Google Scholar]
- Stutman O. Correlation of in vitro and in vivo studies of antigens relevant to the control of murine breast cancer. Cancer Res. 1976 Feb;36(2 Pt 2):739–747. [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R., Mazauskas C. The synthesis and properties of T25 blycoprotein in Thy-1-negative mutant lymphoma cells. Cell. 1978 May;14(1):21–32. doi: 10.1016/0092-8674(78)90297-0. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]