Abstract
Ancestral homologues of the major eukaryotic cytoskeletal families, tubulin and actin, play critical roles in cytokinesis of bacterial cells. FtsZ is the ancestral homologue of tubulin and assembles into the Z ring that determines the division plane. FtsA is an ancestral homologue of actin and is involved in coordinating cell wall synthesis during cytokinesis. FtsA assists in the formation of the Z ring and also has a critical role in recruiting downstream division proteins to the Z ring to generate the divisome that divides the cell. Spatial regulation of cytokinesis occurs at the stage of Z ring assembly and regulation of cell size occurs at this stage or during Z ring maturation.
Introduction
Bacterial cytokinesis has been studied primarily in E. coli and B. subtilis, two bacteria with a similar rod shape. A comparison between these two organisms, which are separated in evolutionary time longer than yeast and humans, has revealed the basic components of the cytokinetic machinery (Errington et al., 2003). Furthermore, defining the machinery in these two bacteria has demonstrated a core of essential components that are used by many bacteria. These investigations have facilitated the study of cytokinesis in other bacteria, by allowing investigators to hone in on differences. Chloroplasts and many Archaea use FtsZ for division but this will not be discussed here (Miyagishima, 2011; Wang and Lutkenhaus, 1996). In constrast, some Archaea and most mictochondria use Escrt or dynamin based cell division machineries respectively (Bernander and Ettema, 2010; Osteryoung, 2001).
Cytokinesis in bacteria can be divided into at least 3 steps (de Boer, 2010). First, the Z ring is assembled on the cytoplasmic membrane with the aid of membrane tethering proteins (Pichoff and Lutkenhaus, 2002). This step is under spatial and temporal control to ensure that the Z ring is assembled between segregated chromosomes (Lutkenhaus, 2007). In the second step, which usually occurs after a considerable lag, the remaining cell division proteins are added to the Z ring to form the complete divisome (Aarsman et al., 2005; Gamba et al., 2009). Formation of this complex adds 7 essential proteins and an increasing number of nonessential proteins that have partially overlapping functions (de Boer, 2010; Goehring and Beckwith, 2005; Vicente and Rico, 2006). Third, the divisome is activated to synthesize septal peptidoglycan which has to be split so that the progeny cells can separate (Gerding et al., 2009). This third step is under complex control so that cell wall degrading enzymes are only activated at the correct place after septal cell wall synthesis has initiated (Uehara and Bernhardt, 2011).
FtsZ and assembly of the Z ring
FtsZ
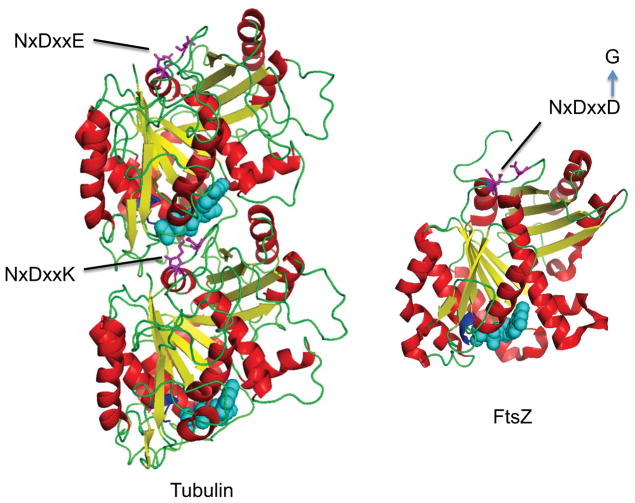
FtsZ is considered the ancestral homologue of eukaryotic tubulins (Nogales et al., 1998). Overall the amino acid identity is only on the order of 10% with the highest degree of conservation involving residues required for GTP binding and hydrolysis (Erickson, 2007) (Fig. 1). GTP is bound on one end of an FtsZ/tubulin (the + end) subunit and this involves the signature FtsZ/tubulin loop (GGGTG[S/T]G) binding to the phosphates. The GTPase catalytic site is formed during filament assembly when the synergy loop (NxDxx[D/E]) from the incoming subunit is added. Despite this limited sequence identity, however, the similar structures of the monomers and filaments, as well a similar mechanism of the GTPase, argue that these two proteins are evolutionary related filament forming proteins (Michie and Lowe, 2006). Furthermore, the recent isolation of an inhibitor of FtsZ that stabilizes FtsZ filaments and the realization that its binding site is analogous to the taxol binding site in tubulin that stabilizes microtubules further highlights the similarity (Andreu et al., 2010; Haydon et al., 2008).
Fig. 1.
Structures of FtsZ and tubulin. The residues that are most conserved in FtsZ (PDB 2VAW) and tubulin (PDB 1TUB) are involved in the binding and hydrolyzing of GTP and include the synergy loop (NxDxx[D/E] (residues in caps and colored magenta) and the signature loop (GGGTG[T/S]G, colored blue) that binds the phosphates. The synergy loop in β-tubulin does not induce GTP hydrolysis due to a positive charged residue (K) substituted for an acidic residue (E) in the loop. A substitution of the acidic residue in FtsZ with G (FtsZ2) results in loss of GTPase activity.
FtsZ forms dynamic filaments in the presence of GTP that are structurally similar to a protofilament present in a microtubule (Lowe and Amos, 1999; Mukherjee and Lutkenhaus, 1998). The filaments readily bundle depending upon the in vitro conditions, however, the basic unit of assembly is a filament that is a single subunit thick (Chen et al., 2005; Mukherjee and Lutkenhaus, 1994). Under conditions that favor maximal GTPase activity the average filament contains about 30 subunits, however, anything that slows down the GTPase leads to lateral bundling and longer filaments (Chen and Erickson, 2009). Similarly, anything that promotes bundling, including a higher concentration of FtsZ or molecular crowding, slows down the GTPase activity. However, close inspection of bundled filaments did not reveal an orderly arrangement and it was argued that lateral bonds do not exist (Erickson et al., 2010).
Although there was some concern whether assembly of such a filament (single molecule thick) would undergo nucleated assembly and display a critical concentration (Romberg et al., 2001), it is now clear, that FtsZ displays a critical concentration around 1 μM, remarkably similar to tubulin (Chen et al., 2005; Mukherjee and Lutkenhaus, 1998). The nucleated assembly of a linear filament is accounted for by having an inactive monomer undergo an izomerization reaction to an active form before association with the next monomer (Dajkovic et al., 2008; Huecas et al., 2008; Miraldi et al., 2008).
FtsZ filaments display different degrees of curvature depending upon the nucleotide. Initially, straight filaments were associated with bound GTP and highly curved filaments with GDP (Lu et al., 2000). However, bound GTP has also been associated with a gently curved form by both AFM and electron microscopy and the reader is referred to a comprehensive review on the in vitro properties and behavior of FtsZ (Erickson et al., 2010). The possibility that GTP containing filaments adopt a fixed curvature, which can deform membranes in vitro, has been used to as a basis for a model for FtsZ to provide the force for constriction.
Z ring
The Z ring was first visualized as an entity by immunoelectron microscopy (Bi and Lutkenhaus, 1991), then by immunofluorescence microscopy (Addinall et al., 1996; Levin and Losick, 1996), but it is now readily visualized in live cells by tagging FtsZ or any of a number of components that are recruited to the Z ring with GFP (Ma et al., 1996). So far all studies have been done with GFP tagged FtsZ, which can not substitute for FtsZ and is toxic. However, the expression of GFP-FtsZ does not appear to interfere with division if the expression level is less than 25% of the native FtsZ. The use of GFP-FtsZ revealed that the Z ring is assembled in two steps with FtsZ being initially loosely organized at midcell before eventually coalescing into a ring. This transition correlates with the segregation of the nucleoids (Inoue et al., 2009) and is promoted by various nonessential Z interacting proteins, which have the ability to bundle FtsZ filaments in vitro (Dajkovic et al., 2010; Gueiros-Filho and Losick, 2002; Monahan et al., 2009), suggesting that some degree of bundling of FtsZ filaments occurs in the Z ring.
Efforts to visualize the Z ring by cryoelectron microscopy in Caulobacter, which is amenable to this technique due to its small diameter, yielded only a few scattered short filaments in the septal region perpendicular to the long axis of the cell (Li et al., 2007). However, quantitative fluorescence measurements have estimated that in E. coli and B. subtilis about 30–40% of the FtsZ in the cell is in the Z ring, which is enough FtsZ to form two to three filaments completely encircling the septum (Anderson et al., 2004{Geissler, 2007 #569)}(Geissler et al., 2007). This suggests not all FtsZ filaments are captured by cryoelectron microscopy. FRAP studies have shown that the subunits in the Z ring are rapidly turning over (T1/2 of 8–10 seconds) (Erickson et al., 2010; Stricker et al., 2002). To account for the amount of FtsZ in the Z ring and the rapid turnover, it was proposed that the Z ring consists of overlapping short filaments. One of the major questions in the field is the substructure of the Z ring. Overproduction of FtsZ or removal of FtsA and Min leads to multiple Z ring at midcell and the poles of the cell, or between nucleoids in long cells rather than a thickening of the existing ring suggesting that Z rings have a defined structure (Bi and Lutkenhaus, 1990; Yu and Margolin, 1999).
The first known step in bacterial cytokinesis is the assembly of the Z ring at the future division site (Bi and Lutkenhaus, 1991). In E. coli formation of the Z ring requires the presence of one of two proteins (ZipA or FtsA) that can tether FtsZ to the membrane (Pichoff and Lutkenhaus, 2002) (Fig. 2). Other nonessential FtsZ interacting proteins (ZapA–D), at least two of which are highly conserved (ZapA and ZapD), are also present and have a partially overlapping function in promoting the integrity of the Z ring (de Boer, 2010; Durand-Heredia et al., 2012; Gueiros-Filho and Losick, 2002; Hale et al., 2011), ZipA has a transmembrane domain attached by a long flexible linker to the FtsZ binding domain and FtsA binds the membrane through a C-terminal amphipathic helix that is attached to the main body of FtsA by a long flexible linker (Hale and de Boer, 1997; Pichoff and Lutkenhaus, 2005). Both proteins bind to a short highly conserved tail of FtsZ (the C-terminal 12 amino acids) that is connected to the main body of FtsZ by a flexible linker (Ma and Margolin, 1999). Even though these conserved residues are important for binding to both FtsA and ZipA, crystal structures of ZipA and FtsA complexed with the conserved tail reveal that the tail is in different conformations in the two complexes and the respective binding sites in ZipA and FtsA have no similarity (Mosyak et al., 2000; Szwedziak et al., 2012) (Fig. 2). This tail of FtsZ is extremely conserved in evolution even in bacteria that lack ZipA and FtsA. In such bacteria the tail probably interacts with other proteins. In B. subtilis the interaction between FtsZ and both SepF (Singh et al., 2008) and EzrA (Singh et al., 2007) [and presumably FtsA] depends upon the FtsZ tail sequence). The FtsZ tail also binds to at least one antagonist of FtsZ assembly, MinC/MinD and to one of the Z interacting proteins, ZapD (Durand-Heredia et al., 2012; Shen and Lutkenhaus, 2009). Perhaps it also binds to others.
Fig. 2.
FtsZ filaments are tethered to the membrane by FtsA and ZipA. ZipA and FtsA bind to the highly conserved C-tail of FtsZ, which is connected to main body of FtsZ by a flexible linker. Both ZipA and FtsA are tethered to the membrane by a flexible linker that connects the membrane binding domains of these proteins to the main body of the protein.
The fact that either of two unrelated proteins can promote Z ring formation suggests that the self organization leading to Z ring formation is largely a property of FtsZ assembly and membrane attachment. This concept was reinforced by the experiments from the Erickson lab that demonstrated that the addition of a membrane targeting sequence (MTS) to FtsZ (also tagged with GFP for visualization) leads to dynamic formation of Z rings inside artificial phospholipid tubules that coalesce and partially constrict the tubule (Osawa et al., 2008). Placing the MTS at the other end of FtsZ (so it would be on the other side of the filament) leads to formation of Z rings on the outside of the lipid tubule that coalesce to cause a constriction (Osawa and Erickson, 2011). This assembly on the inside versus outside based on the sidedness of the membrane tether argues for the importance of intrinsic filament curvature. In the absence of GTP hydrolysis Z rings assemble and constriction occurs although not as deep. This suggests that the intrinsic curvature of GTP bound FtsZ filaments along with their lateral association can deform a vesicle surface. It was suggested that the remodeling of the filaments driven by GTP hydrolysis is required to get deeper constrictions.
Mutations in ftsZ that prevent GTP hydrolysis are lethal, however, at least one hydrolysis deficient mutant can support growth in the presence of an unknown suppressor mutation (Bi and Lutkenhaus, 1992; Dajkovic and Lutkenhaus, 2006) (Mukherjee et al., 2001; Osawa and Erickson, 2006). This mutant, D212G, has a substitution in the synergy loop required for GTP hydrolysis (Fig. 1). The most notable phenotype of this mutant is a large fraction of cells displaying a contorted septum suggesting that GTP hydrolysis by FtsZ is not essential for cytokinesis but remodeling of FtsZ at the Z ring is required for symmetrical invagination of the septum (Addinall and Lutkenhaus, 1996). One possibility is that constant remodeling of FtsZ at the leading edge of the septum (and adapting to the constriction of the cell) acts as a guide to septal peptidoglycan synthesis (which would be the primary motor for invagination). This possibility is suggested by mutants of FtsZ that form spiral shaped structures and spiraled shaped septa and also by the fact that cells inhibited for septal peptidoglycan synthesis do not invaginate the inner membrane (Addinall and Lutkenhaus, 1996).
Spatial regulation of the Z ring
The spatial regulation of Z ring assembly has been extensively studied in 3 widely divergent rod shaped bacteria, E. coli, B. subtilis and Caulobacter crescentus, but is beginning to be studied in others (Lutkenhaus, 2012). The theme that emerges from these studies is that antagonists of FtsZ assembly are positioned away from midcell to prevent Z ring formation in their vicinity (Lutkenhaus, 2007). For at least three of these negative regulators that have been studied in vitro, MinC, SlmA and MipZ, the mechanism appears similar, when activated, they break FtsZ filaments (Cho et al., 2011; Hu et al., 1999; Thanbichler and Shapiro, 2006; Tonthat et al., 2011). By doing so in vivo they would prevent FtsZ filaments from obtaining the necessary length to coalesce into a Z ring.
The filament breaking mechanism used by spatial regulators is in contrast to the best studied FtsZ inhibitor, SulA, which prevents assembly by sequestering FtsZ monomers (Chen et al., 2012; Cordell et al., 2003; Dajkovic et al., 2008). This inhibitor is produced in E. coli as part of the SOS (DNA damage) response (Huisman and D’Ari, 1981). When produced it also leads to the rapid dissolution of dynamic Z rings as monomers released by the dynamic turnover are sequestered (Dajkovic et al., 2008).
E. coli and B. subtilis employ two negative regulatory systems, Min (minicell) and NO (nucleoid occlusion), named for their effect on positioning of the septum (Z ring). Eliminating the Min system leads to Z ring assembly at the poles and minicell formation. In contrast, eliminating the NO system has little phenotype in exponential cells but leads to Z ring assembly occurring over the nucleoid under conditions that delay replication or segregation (Bernhardt and de Boer, 2005; Wu and Errington, 2004). Elimination of both systems leads to filamentous cell death as cells are unable to divide, presumably due to FtsZ being scattered among many immature FtsZ assemblies throughout the cell (Bernhardt and de Boer, 2005). By preventing these spurious assemblies Min and NO ensure that there is sufficient FtsZ available to construct a complete Z ring at midcell. Consistent with this, overproduction of FtsZ is able to rescue a double mutant lacking both systems.
Min system
The Min system prevents Z ring assembly near the poles of the cell through the spatial regulation of the FtsZ antagonist MinC (Lutkenhaus, 2007) (Fig. 3). MinC is tethered to the membrane by binding MinD, which not only concentrates MinC at the membrane but also enhances MinC’s ability to bind to the conserved tail of FtsZ (Johnson et al., 2002; Shen and Lutkenhaus, 2009). This binding to the tail of FtsZ, which requires the C-terminal domain of MinC, positions the N-terminus of MinC near the FtsZ filament. Genetic and biochemical evidence indicates the N-terminal domain attacks FtsZ filaments at the junction of two subunits following GTP hydrolysis (Shen and Lutkenhaus, 2010).
Fig. 3.
Antagonists of FtsZ assembly are positioned in the cell to spatially regulate tZ ring assembly. Nucleoid occlusion (NO) is mediated by SlmA in E. coli (Noc in B. subtilis) and prevents Z ring formation over the nucleoid. SlmA and Noc are localized by binding to sequences in the origin proximal region of the chromosome. The Min system prevents Z ring assembly near the poles. MinC is activated by being recruited to the membrane by MinD and oscillates between the poles of the cell under the control of MinD and MinE. In B. subtilis MinC/MinD are recruited to incipient septa by MinJ (not shown) and DivIVA. In Caulobacter crescentus MipZ forms a gradient on the nucleoid. The gradient emanates from its partner ParB, which is bound near the origin and anchored to the pole by interaction with PopZ. Following initiation of replication one origin segregates to the opposite pole where it dislodges FtsZ left over from the previous division. This FtsZ, along with newly synthesized FtsZ, assembles at the lowpoint of the bipolar MipZ gradient.
MinC and MinD are positioned by one of two regulators (Rothfield et al., 2005). In E. coli, and most Gram negative bacteria, the regulator is MinE, which undergoes a coupled oscillation along with MinC/MinD between the poles of the cell (Fu et al., 2001; Hale et al., 2001; Hu and Lutkenhaus, 1999; Raskin and de Boer, 1999) (Fig. 3). MinD, an ATPase that dimerizes and binds the membrane in the presence of ATP, and MinE, an activator of the MinD ATPase, constitute the oscillator whereas MinC is a passenger. Many of the details of this oscillatory mechanism have been elaborated and it has been modeled extensively (Howard and Kruse, 2005; Lutkenhaus, 2007). Importantly, only MinD and MinE are needed for the oscillation. Consistent with this, MinD and MinE are able to dynamically self organize in vitro (Ivanov and Mizuuchi, 2010; Loose et al., 2008). They form traveling waves on a lipid bilayer fueled by the ATP hydrolysis of MinD, which is stimulated by MinE. The in vitro waves have characteristics that mimic the in vivo oscillation, most notably, a maximum concentration of MinE at the trailing edge of the wave, which is estimated to be in a 1:1 ratio with MinD (Loose et al., 2011). MinD promotes conformational changes in MinE resulting in an active form that binds MinD and the membrane which allows MinE to swing from one membrane bound MinD to the next despite knocking MinD off the membrane (Park et al., 2011).
In B. subtilis, and most Gram positive bacteria, the regulator of MinC/MinD is a coiled-coil protein designated DivIVA (Marston et al., 1998). This protein is recruited to the site of membrane curvature generated by cytokinesis (Eswaramoorthy et al., 2011) (Fig. 3). DivIVA recruits MinC and MinD through an intermediary designated MinJ (Bramkamp et al., 2008; Patrick and Kearns, 2008). Thus, a DivIVA ring, decorated with the Min proteins, is formed on either side of the incipient septum and prevents FtsZ released from the ongoing cytokinesis from reforming a ring at the newly forming poles (Gregory et al., 2008).
NO system
Remarkably, the NO systems of B. subtilis and E. coli employ two unrelated DNA binding proteins, Noc and SlmA respectively, to perform the same task (Bernhardt and de Boer, 2005; Wu and Errington, 2004). Both proteins bind to their specific DNA binding sites, which are scattered in the origin proximal 2/3 of the circular chromosome (Wu et al., 2009). For SlmA, it has been shown that it is activated to attack FtsZ filaments upon binding to its cognate sequence (Cho et al., 2011; Tonthat et al., 2011). Therefore, as the duplicating chromosomes segregate the tethered regulators are moved away from the cell center making it permissive for Z ring assembly (Fig. 3). Thus, the NO systems seem ideally suited to couple DNA segregation to formation of the Z ring, but as pointed out above, elimination of NO has little phenotype in exponentially growing cells, suggesting its primary role is to prevent guillotining of the chromosome under conditions of replication/segregation stress.
B. subtilis offers an advantage in the study of cytokinesis since examination of the Z ring in germinating spores offers a chance to look at formation of a Z ring in the absence of influences of the previous division (Migocki et al., 2002). Spores of a mutant lacking Min and NO (generated under permissive growth conditions for the double mutant) were germinated and followed for 1–2 generations of growth. Z ring formation, although delayed, still occurred primarily at midcell (Rodrigues and Harry, 2012). This result indicates that an additional factor, other than Min or NO, positions the Z ring and remains to be identified. Also, it is clear that the proteins identified to mediate NO, SlmA and Noc, represent only part of NO and that additional factor (s) are involved.
Together the results favor a model in which there is a limited amount of FtsZ available in the cell but FtsZ assembly is promiscuous and is actively countered by localized antagonists, Min and NO. By limiting this promiscuous behavior away from midcell, they ensure there is sufficient FtsZ available to assemble a complete Z ring at the desired location. In their absence FtsZ assembly between nucleoids is favored, however, the free FtsZ is insufficient to form a complete ring due to too much FtsZ tied up in spurious assemblies. Consistent with this, the double mutant can be suppressed by increasing FtsZ (also by a slower growth rate). Also, growth of E. coli in chambers smaller than the diameter of the cell selects for flattened cells. In these deformed cells a Z ring forms between segregated nucleoids even in the absence of SlmA and Min suggesting that NO is the primary determinant of Z ring placement and that SlmA is not the major component of the NO system (Mannik et al., 2012).
Another spatial regulator
Although Min and Noc are widely distributed, especially among rod shaped bacteria, they are not present in all bacteria. Caulobacter lacks Min and both NO homologues and spatial regulation is due to MipZ, a protein related to MinD (Thanbichler and Shapiro, 2006). Monomers of MipZ are recruited to the pole of the cell by interaction with ParB, which is bound near the origin of replication, and along with ParA, is involved in chromosome segregation (Fig. 3). ParB promotes MipZ dimerization, the form that antagonizes FtsZ assembly, and the dimers diffuse away and bind nonspecifically to the chromosome (Kiekebusch et al., 2012). This binding is transient as the intrinsic ATPase of MipZ causes release from the DNA. Repeated cycling of MipZ between the DNA and ParB leads to a gradient of MipZ on the chromosome that is highest near ParB. When the origin duplicates, one segregates to the other pole, resulting in a bipolar gradient of MipZ with the low point near midcell.
Z ring assembly during sporulation
Although spatial regulation of Z ring assembly is focused mostly on exponentially growing cells, sporulation offers unique opportunities to examine spatial regulation. In sporulating cells of B. subtilis the Z ring formed at midcell spirals away to form a Z ring at each of the cell’s poles, one of which leads to asymmetric septation and the other is dismantled (Ben-Yehuda and Losick, 2002). Although the switch involves an increase in FtsZ expression and the production of a membrane protein that binds FtsZ (SpoIIE) a detailed mechanism is lacking. Streptomyces species also sporulate and have a life style similar to filamentous fungi with a mycelial growth containinng few septa and the formation of aerial hyphae that eventually contain a ladder like structure of Z rings that form simultaneously (Grantcharova et al., 2005). An interesting aspect of this system is the identification of a protein (SsgB) that precedes FtsZ localization (Willemse et al., 2011). How it is localized is still not known but it represents the first case where a protein precedes FtsZ to the division site. This protein is not widespread among bacteria, however, it is possible that analogues exist in other bacteria..
Growth rate regulation of Z ring function
Bacteria have a remarkable ability to adjust their cell size with growth rate. Fast growing cells (τ=20 min) can be up to eight times the volume of slow growing cells (τ>60 min). This increase in cell volume accommodates the increased DNA content of the cell, which results from initiation of DNA replication occurring before the previous round of replication is completed. In B. subtilis the increased size is due to an increase in cell length while in E. coli the increase in size is due to increases in width and length. Levin’s lab found that a mutant in B. subtilis deficient in the synthesis of a nonessential cell wall polymer was unable to increase its cell length upon shift to a faster growth rate (Weart et al., 2007). This result indicated that this pathway is used to communicate information about the growth rate to the division apparatus to delay division at faster growth rates. The effector of this response is the last enzyme in this pathway, UgtP, which interacts directly with FtsZ. Although the mechanism is not clear, it is possible that the metabolic flux through UgtP regulates its interaction with FtsZ. At fast growth rates UgtP may decrease the amount of active FtsZ so that cell division is delayed until more FtsZ accumulates. An E. coli mutant (pgm) corresponding to the original B. subtilis mutant (pgcA) also displays a defect in adjusting cell size to growth rate suggesting that this is a conserved regulatory feature. Furthermore, the small cell size at fast growth rate observed in the pgm mutant is mimicked by a gain of function allele of ftsA (ftsA*) raising the possibility that the ftsA* mutant is resistant to this regulation (Hill et al., 2012). A potential effector protein in E. coli is not clear since it lacks a homologue of UgtP. Despite the isolation of the above mutants a molecular mechanism for this size regulation is not known.
Formation of the divisome
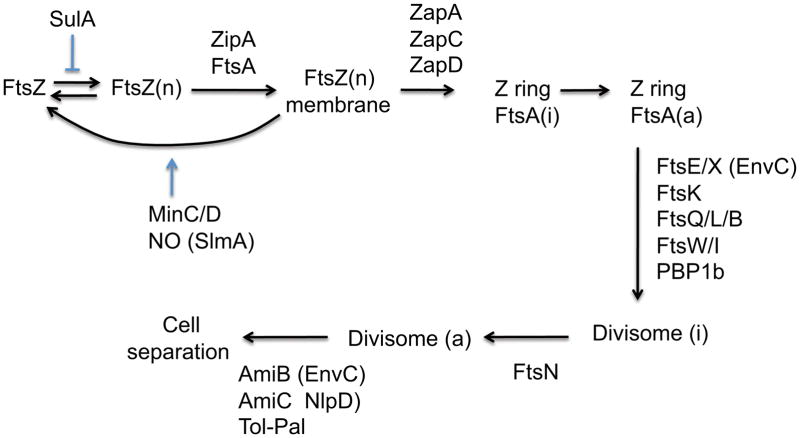
The assembly of the complete divisome follows the assembly of the Z ring and occurs in two temporally distinct steps in E. coli, B. subtilis and Caulobacter (Aarsman et al., 2005; Gamba et al., 2009; Goley et al., 2011). There is a considerable gap between formation of the Z ring and the subsequent assembly of the divisome. In E. coli at least 9 additional essential division proteins are added almost simultaneously to the Z ring (Goehring and Beckwith, 2005) (Fig. 4). How these additional proteins are connected to the Z ring is not clear but FtsA plays a key role. Dependency studies indicate that there is a linear order to the assembly process even though several of the proteins have been shown to exist in complexes even when they are not associated with the Z ring (Goehring et al., 2006). The roles of these additional essential division proteins in E. coli are described below.
Fig. 4.
Diagram of assembly of the Z ring and maturation to the divisome in E. coli. FtsZ polymers (FtsZ[n]) are tethered to the membrane by FtsA and ZipA, which leads to formation of the Z ring. However, FtsA is not immediately available to recruit downstream proteins (FtsA[i]). Although not essential, several FtsZ interacting proteins (ZapA–D) localize to the ring and promote the integrity of the Z ring. Antagonists of FtsZ assembly, MinC/D and SlmA, are positioned away from midcell so that it is permissive for Z ring formation. When enough monomeric FtsA (active [a]) is present at the Z ring the remaining Fts proteins and PBP1b are recruited. The arrival of FtsN signals the divisome is complete and activation of septal PG synthesis occurs (divisome [a]). The septal cross wall is split by AmiB, which is activated by EnvC (recruited earlier), and AmiC, which is activated by NlpD. Although EnvC is recruited early, the arrival of AmiC, AmiB and NlpD depend upon the start of septal PG synthesis induced by the arrival of FtsN. The Tol-Pal complex is needed for efficient invagination of the outer membrane.
FtsE and FtsX encode an ABC transporter with the most homology to the Lol system, which extracts lipoproteins out of the cytoplasmic membrane (Schmidt et al., 2004). FtsX encodes the membrane component and FtsE encodes the ATPase. Since these genes can be deleted under conditions of high osmolarity without a dramatic effect on cell division, they have been given less attention (Reddy, 2007). However, there is a slight division defect under suppressing conditions and recently it was shown that FtsE/FtsX recruit an activator, EnvC, of a septal peptidoglycan amidase (AmiB) to the Z ring (Yang et al., 2011). EnvC is recruited directly by FtsX but the ATPase activity of FtsE is required for EnvC to activate AmiB. In the absence of FtsE/FtsX this activity is missing and splitting of the septum is due to other amidases with partially redundant activity.
FtsK, is a DNA translocase with a membrane domain containing 4 transmembrane spanning segments fused to the DNA translocase domain by a long linker (Begg et al., 1995). When located at the septum this protein is able to translocate DNA away from the septum due to specific sequences (KOPS) located throughout the chromosome, which give directionality to the movement of the DNA (Bigot et al., 2005). Although this protein can rescue DNA trapped at the septum this only comes into play during stress and is not an essential function (Steiner et al., 1999). The essential function of FtsK lies in the 4 transmembrane segments and may play a role resolution of the membrane as cytokinesis is completed (Fleming et al., 2010).
FtsQ, FtsL and FtsB have no known enzymatic activity and appear to function as a link between the Z ring and the peptidoglycan biosynthetic machinery (Goehring and Beckwith, 2005). FtsI and FtsW are part of the peptidoglycan machinery dedicated to septation (Typas et al., 2012). Their orthologues, PBP2 and RodA respectively, are dedicated to peptidoglycan synthesis during cell elongation and have no role in septation. These proteins alone cannot synthesize peptidoglycan, which requires a transglycoslyase. E. coli has several proteins with this activity and it appears that the majority of PG synthesis is carried out by PBP1A and PBP1B. Even though these two synthetases are thought to primarily be involved in cell elongation and division respectively, their function overlaps as only one is necessary for cells to survive.
FtsN is the last essential division protein to arrive at the ring and may signal that the divisome complex is complete and septation should be initiated, basically acting as a trigger for septation (Gerding et al., 2009; Goehring and Beckwith, 2005). FtsN is a bitopic protein with a short cytoplasmic region connected to a larger periplasmic region by a single transmembrane domain (Dai et al., 1993). The most conserved region of FtsN lies at the C-terminus (SPOR domain) and binds a form of peptidoglycan that is only present at the septum. However, this domain is not essential (Gerding et al., 2009). FtsN, however, is required for the recruitment of a host of downstream proteins whose activities are partially redundant. It is unlikely that this recruitment is direct but may rely upon FtsN’s ability to trigger septation (Bernard et al., 2007). At least one allele of ftsA can bypass the requirement for FtsN suggesting that septation can be triggered another way (Bernard et al., 2007).
Role of FtsA in formation of the divisome
FtsA plays two critical roles in cytokinesis. First, along with ZipA, it tethers FtsZ filaments to the membrane (Pichoff and Lutkenhaus, 2002). Second, along with ZipA, it is required for recruitment of all the downstream division proteins (Hale and de Boer, 2002). Importantly, Margolin’s lab isolated an allele of ftsA, ftsA*, that was able to bypass the requirement for ZipA (Geissler et al., 2007). This, along with evidence that FtsA is much more conserved in evolution than ZipA, and interacts with a number of downstream division proteins indicate that FtsA has a more direct role in their recruitment (Corbin et al., 2004). In addition to bypassing ZipA, FtsA* has a number of unusual properties, including resistance to various treatments that destabilize the Z ring, including excess MinC, and allowing cells to divide at a smaller cell size (Bernard et al., 2007). Bacterial two hybrid tests indicated FtsA* interacted more strongly with itself leading to the suggestion that increased interaction between FtsA molecules stabilized the Z ring to destabilizing conditions (Shiomi and Margolin, 2007).
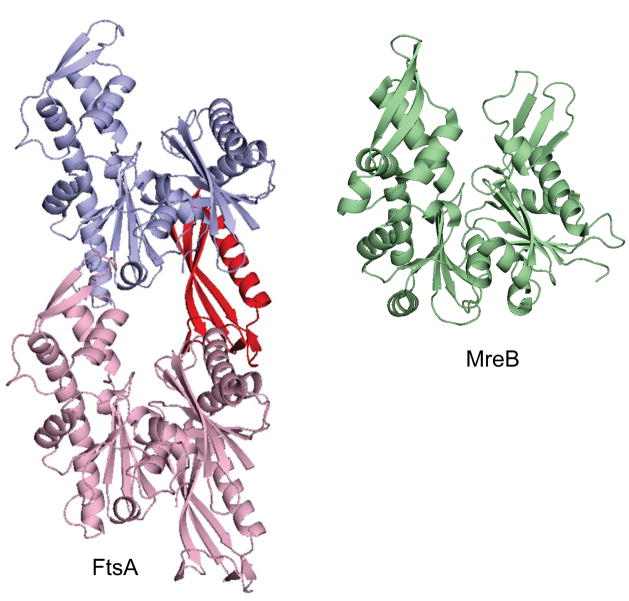
Although FtsA is a member of the actin family, it is missing one of actin’s four subdomains but has an additional subdomain (1C) located elsewhere in the structure (Szwedziak et al., 2012) (Fig. 5). Many bacteria possess another actin homologue (MreB) that has the same domain structure as actin (van den Ent et al., 2001) (Fig. 5), but in E. coli and B. subtilis it is required for the maintenance of the rod shape by organizing peptidoglycan synthesis for lateral wall synthesis (Jones et al., 2001; Typas et al., 2012). Nonetheless, various reports indicated FtsA assembled into filaments in vitro although no reliable ATPase activity accompanied assembly and the filaments were not dynamic (Krupka et al., 2012; Lara et al., 2005). Other reports indicated FtsA could assemble in vivo forming cytoplasmic filaments when overexpressed without its membrane binding domain (Pichoff and Lutkenhaus, 2005). Recently, Lowe’s lab solved the structure of FtsA revealing actin-like protofilaments (Szwedziak et al., 2012). They also observed FtsA polymers on a lipid monolayer and in the cytoplasm of cell (without the membrane binding domain) with the same repeat distance as observed in the crystal structure. Moreover, mutations that would be expected to interfere with polymer contacts were less efficient at division.
Fig. 5.
Structures of FtsA and MreB. Two molecules of FtsA are shown as arranged in a filament. The structure of MreB is similar to conventional actin. FtsA lacks domain 1B but has a new domain 1C (colored red in one FtsA) that interacts with other division proteins.
In a separate study Pichoff et al. (Pichoff et al., 2012) isolated mutations that interfered with the ability of FtsA to form cytoplasmic filaments. Surprisingly, ftsA* was among them and a decrease in self-interaction was confirmed by independent tests. In addition, selection of many additional mutations that bypassed ZipA led to the inescapable conclusion that such mutations decrease FtsA’s self interaction. Consistent with this the altered residues mapped to the 4 major contact points between subunits in the FtsA filament. Whether some low level of FtsA self interaction is essential is not clear. Nonetheless, this finding led to a new model for how FtsA recruits downstream division proteins. In this model FtsA switches between a form that is unable to interact with downstream proteins (polymeric) and a form that is active in recruitment (monomeric). Somehow this switch is regulated by the dynamic interaction of proteins with the tail of FtsZ.
Model for FtsA recruitment of downstream division proteins
Two complementary lines of evidence indicate FtsA, and in particular domain 1C, plays a critical role by in assembly of the divisome. Artificially targeting domain 1C to the poles of the cell leads to polar localization of several late division proteins (FtsN and FtsI) (Corbin et al., 2004). On the other hand, FtsA deleted for the 1C domain localizes efficiently to the Z ring but is unable to recruit the late cell division proteins (Rico et al., 2004). Although the best evidence is for interaction between domain 1C and FtsN (Busiek et al., 2012), some evidence, although indirect, also suggests interaction of this domain with FtsI and FtsQ. These proteins are all single pass bitopic membrane proteins with short cytoplasmic N-termini and large extracytoplasmic domains. A direct interaction with the cytoplasmic FtsA has to be with the short N-termini of one or more of these proteins.
Since ftsA mutations that bypass ZipA result in less self interaction, it was proposed that the essential function of ZipA is to antagonize FtsA self assembly -(Pichoff et al., 2012). Furthermore, FtsA mutants that no longer require ZipA self-interact less well, suggesting that it is the monomer form of FtsA that recruits one or more of the downstream division proteins, presumably because domain 1C becomes available. In this model the Z ring is formed as FtsA and ZipA interact with polymers of FtsZ and tether them to the membrane. In shorter cells, other proteins (such as MinC/MinD) that interact with the FtsZ tail, compete with FtsA and reduce the amount of FtsA’s domain IC available at the Z ring. However, a combination of a build up of FtsA at the Z ring along with conditions favoring monomers increase the availability of domain 1C and the recruitment of downstream proteins commences. During constriction the presence of the downstream division proteins at the septum perpetuates the monomeric form of FtsA through interaction with the 1C domain.
Together the information suggests the following more explicit model. In the polymer form the 1C domain is not available for interaction with a protein such as FtsN, since it is occupied in the filament. In the monomer form, however, the 1C domain is free and the connection to the body of the protein by a hinge allows movement and the acceptance of the N-terminus of FtsN. The structure of proteins from the Type IV pilus from Pseudomonas suggests a likely scenario (Busiek et al., 2012). PilM, which is similar in structure to FtsA, binds to the N-terminus of the bitopic PilN protein (Karuppiah and Derrick, 2011). Comparison of the structures of FtsA with the PilM-PilN complex is consistent with the necessary membrane orientation. The tail of FtsA, which attaches to the membrane, positions FtsA to interact with the FtsN protein. Furthermore, FtsZ is attached to the opposite side of FtsA so that it would be on the cytoplasmic side.
Triggering septation and cell separation
Upon completion of the divisome septation is triggered leading to synthesis of peptidoglycan and its eventual splitting. In E. coli the triggering event coincides with the arrival of FtsN at the divisome (Gerding et al., 2009). FtsN recruitment has two requirements: 1) it requires the immediate preceding protein, FtsI (Wissel and Weiss, 2004); and 2) it requires FtsA (Goehring et al., 2006). Thus, FtsN arrival signals completion of divisome assembly and by activating FtsI, which along with PBP1b synthesizes septal specific peptidoglycan, results in septation. The activation of FtsI leads to the recruitment of additional proteins that metabolize the peptidoglycan and invaginate the outer membrane (Gerding et al., 2007). In E. coli four proteins are recruited to the septum by a conserved SPOR domain that binds septal specific peptidoglycan, presumably glycan chains that have been metabolized by amidases (Arends et al., 2010; Gerding et al., 2009). These SPOR containing proteins make good markers revealing the onset of septation.
E. coli contains three amidases, enzymes that remove the peptide side chains attached to the glycan chains that constitute peptidoglycan. Removal of these peptide side chains, which crosslink the glycan chains, allows cells to be separated (Bernhardt and de Boer, 2003). The activity of these amidases overlap and the presence of any one ensures survival. Two of them, AmiB and AmiC, are localized to the septum (Peters et al., 2011). AmiB requires EnvC and thus, FtsX for recruitment and also FtsE for activation. EnvC localizes early whereas its partner, AmiB requires FtsN for localization and so is localized later. AmiC and its activator (a lipoprotein called NlpD), both require FtsN for localization. The requirement for FtsN means that the amidases won’t be specifically recruited and activated until the switch for peptidoglycan synthesis is thrown. However, in the absence of AmiB and AmiC, AmiA (which does not localize specifically to the septum) is still able to carry out the splitting of the septum through activation by the localized EnvC (Peters et al., 2011).
In a Gram negative bacterium like E coli invagination of the outer membrane follows septal peptidoglycan synthesis. A large set of proteins including a cytoplasmic complex of Tol proteins (TolQAR) interacts with an outer membrane lipoprotein (Pal) to ensure timely and efficient invagination of this layer (Gerding et al., 2007). These proteins are not essential though and in their absence invagination occurs.
Acknowledgments
Work in the author’s laboratory is supported by NIH grant GM29764.
References
- Aarsman ME, Piette A, Fraipont C, Vinkenvleugel TM, Nguyen-Disteche M, den Blaauwen T. Maturation of the Escherichia coli divisome occurs in two steps. Mol Microbiol. 2005;55:1631–1645. doi: 10.1111/j.1365-2958.2005.04502.x. [DOI] [PubMed] [Google Scholar]
- Addinall SG, Bi E, Lutkenhaus J. FtsZ ring formation in fts mutants. J Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addinall SG, Lutkenhaus J. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol Microbiol. 1996;22:231–237. doi: 10.1046/j.1365-2958.1996.00100.x. [DOI] [PubMed] [Google Scholar]
- Anderson DE, Gueiros-Filho FJ, Erickson HP. Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins. J Bacteriol. 2004;186:5775–5781. doi: 10.1128/JB.186.17.5775-5781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu JM, Schaffner-Barbero C, Huecas S, Alonso D, Lopez-Rodriguez ML, Ruiz-Avila LB, Nunez-Ramirez R, Llorca O, Martin-Galiano AJ. The antibacterial cell division inhibitor PC190723 is an FtsZ polymer-stabilizing agent that induces filament assembly and condensation. J Biol Chem. 2010;285:14239–14246. doi: 10.1074/jbc.M109.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends SJ, Williams K, Scott RJ, Rolong S, Popham DL, Weiss DS. Discovery and characterization of three new Escherichia coli septal ring proteins that contain a SPOR domain: DamX, DedD, and RlpA. J Bacteriol. 2010;192:242–255. doi: 10.1128/JB.01244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg KJ, Dewar SJ, Donachie WD. A new Escherichia coli cell division gene, ftsK. J Bacteriol. 1995;177:6211–6222. doi: 10.1128/jb.177.21.6211-6222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehuda S, Losick R. Asymmetric cell division in B. subtilis involves a spiral-like intermediate of the cytokinetic protein FtsZ. Cell. 2002;109:257–266. doi: 10.1016/s0092-8674(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Bernander R, Ettema TJ. FtsZ-less cell division in archaea and bacteria. Curr Opin Microbiol. 2010;13:747–752. doi: 10.1016/j.mib.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Bernard CS, Sadasivam M, Shiomi D, Margolin W. An altered FtsA can compensate for the loss of essential cell division protein FtsN in Escherichia coli. Mol Microbiol. 2007;64:1289–1305. doi: 10.1111/j.1365-2958.2007.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt TG, de Boer PA. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Mol Microbiol. 2003;48:1171–1182. doi: 10.1046/j.1365-2958.2003.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt TG, de Boer PA. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over Chromosomes in E. coli. Mol Cell. 2005;18:555–564. doi: 10.1016/j.molcel.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Lutkenhaus J. FtsZ regulates frequency of cell division in Escherichia coli. J Bacteriol. 1990;172:2765–2768. doi: 10.1128/jb.172.5.2765-2768.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Lutkenhaus J. Isolation and characterization of ftsZ alleles that affect septal morphology. J Bacteriol. 1992;174:5414–5423. doi: 10.1128/jb.174.16.5414-5423.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi EF, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- Bigot S, Saleh OA, Lesterlin C, Pages C, El Karoui M, Dennis C, Grigoriev M, Allemand JF, Barre FX, Cornet F. KOPS: DNA motifs that control E. coli chromosome segregation by orienting the FtsK translocase. EMBO J. 2005;24:3770–3780. doi: 10.1038/sj.emboj.7600835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramkamp M, Emmins R, Weston L, Donovan C, Daniel RA, Errington J. A novel component of the division-site selection system of Bacillus subtilis and a new mode of action for the division inhibitor MinCD. Mol Microbiol. 2008;70:1556–1569. doi: 10.1111/j.1365-2958.2008.06501.x. [DOI] [PubMed] [Google Scholar]
- Busiek KK, Eraso JM, Wang Y, Margolin W. The early divisome protein FtsA interacts directly through its 1c subdomain with the cytoplasmic domain of the late divisome protein FtsN. J Bacteriol. 2012;194:1989–2000. doi: 10.1128/JB.06683-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bjornson K, Redick SD, Erickson HP. A rapid fluorescence assay for FtsZ assembly indicates cooperative assembly with a dimer nucleus. Biophys J. 2005;88:505–514. doi: 10.1529/biophysj.104.044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Erickson HP. FtsZ filament dynamics at steady state: subunit exchange with and without nucleotide hydrolysis. Biochemistry. 2009;48:6664–6673. doi: 10.1021/bi8022653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Milam SL, Erickson HP. SulA inhibits assembly of FtsZ by a simple sequestration mechanism. Biochemistry. 2012;51:3100–3109. doi: 10.1021/bi201669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, McManus HR, Dove SL, Bernhardt TG. Nucleoid occlusion factor SlmA is a DNA-activated FtsZ polymerization antagonist. Proc Natl Acad Sci U S A. 2011;108:3773–3778. doi: 10.1073/pnas.1018674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin BD, Geissler B, Sadasivam M, Margolin W. Z-ring-independent interaction between a subdomain of FtsA and late septation proteins as revealed by a polar recruitment assay. J Bacteriol. 2004;186:7736–7744. doi: 10.1128/JB.186.22.7736-7744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell SC, Robinson EJ, Lowe J. Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proc Natl Acad Sci U S A. 2003;100:7889–7894. doi: 10.1073/pnas.1330742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Xu Y, Lutkenhaus J. Cloning and characterization of ftsN, an essential cell division gene in Escherichia coli isolated as a multicopy suppressor of ftsA12(Ts) J Bacteriol. 1993;175:3790–3797. doi: 10.1128/jb.175.12.3790-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajkovic A, Lutkenhaus J. Z ring as executor of bacterial cell division. J Mol Microbiol Biotechnol. 2006;11:140–151. doi: 10.1159/000094050. [DOI] [PubMed] [Google Scholar]
- Dajkovic A, Mukherjee A, Lutkenhaus J. Investigation of regulation of FtsZ assembly by SulA and development of a model for FtsZ polymerization. J Bacteriol. 2008;190:2513–2526. doi: 10.1128/JB.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajkovic A, Pichoff S, Lutkenhaus J, Wirtz D. Cross-linking FtsZ polymers into coherent Z rings. Mol Microbiol. 2010;78:651–668. doi: 10.1111/j.1365-2958.2010.07352.x. [DOI] [PubMed] [Google Scholar]
- de Boer PA. Advances in understanding E. coli cell fission. Curr Opin Microbiol. 2010;13:730–737. doi: 10.1016/j.mib.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand-Heredia J, Rivkin E, Fan G, Morales J, Janakiraman A. Identification of ZapD as a Cell Division Factor That Promotes the Assembly of FtsZ in Escherichia coli. J Bacteriol. 2012;194:3189–3198. doi: 10.1128/JB.00176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP. Evolution of the cytoskeleton. Bioessays. 2007;29:668–677. doi: 10.1002/bies.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, Anderson DE, Osawa M. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol Mol Biol Rev. 2010;74:504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J, Daniel RA, Scheffers DJ. Cytokinesis in bacteria. Microbiol Mol Biol Rev. 2003;67:52–65. doi: 10.1128/MMBR.67.1.52-65.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswaramoorthy P, Erb ML, Gregory JA, Silverman J, Pogliano K, Pogliano J, Ramamurthi KS. Cellular architecture mediates DivIVA ultrastructure and regulates min activity in Bacillus subtilis. MBio. 2011;2 doi: 10.1128/mBio.00257-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TC, Shin JY, Lee SH, Becker E, Huang KC, Bustamante C, Pogliano K. Dynamic SpoIIIE assembly mediates septal membrane fission during Bacillus subtilis sporulation. Genes Dev. 2010;24:1160–1172. doi: 10.1101/gad.1925210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Shih YL, Zhang Y, Rothfield LI. The MinE ring required for proper placement of the division site is a mobile structure that changes its cellular location during the Escherichia coli division cycle. Proc Natl Acad Sci U S A. 2001;98:980–985. doi: 10.1073/pnas.031549298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba P, Veening JW, Saunders NJ, Hamoen LW, Daniel RA. Two-step assembly dynamics of the Bacillus subtilis divisome. J Bacteriol. 2009;191:4186–4194. doi: 10.1128/JB.01758-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler B, Shiomi D, Margolin W. The ftsA* gain-of-function allele of Escherichia coli and its effects on the stability and dynamics of the Z ring. Microbiology. 2007;153:814–825. doi: 10.1099/mic.0.2006/001834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding MA, Liu B, Bendezu FO, Hale CA, Bernhardt TG, de Boer PA. Self-enhanced accumulation of FtsN at Division Sites and Roles for Other Proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. J Bacteriol. 2009;191:7383–7401. doi: 10.1128/JB.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding MA, Ogata Y, Pecora ND, Niki H, de Boer PA. The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Mol Microbiol. 2007;63:1008–1025. doi: 10.1111/j.1365-2958.2006.05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring NW, Beckwith J. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr Biol. 2005;15:R514–526. doi: 10.1016/j.cub.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Goehring NW, Gonzalez MD, Beckwith J. Premature targeting of cell division proteins to midcell reveals hierarchies of protein interactions involved in divisome assembly. Mol Microbiol. 2006;61:33–45. doi: 10.1111/j.1365-2958.2006.05206.x. [DOI] [PubMed] [Google Scholar]
- Goley ED, Yeh YC, Hong SH, Fero MJ, Abeliuk E, McAdams HH, Shapiro L. Assembly of the Caulobacter cell division machine. Mol Microbiol. 2011;80:1680–1698. doi: 10.1111/j.1365-2958.2011.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantcharova N, Lustig U, Flardh K. Dynamics of FtsZ assembly during sporulation in Streptomyces coelicolor A3(2) J Bacteriol. 2005;187:3227–3237. doi: 10.1128/JB.187.9.3227-3237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JA, Becker EC, Pogliano K. Bacillus subtilis MinC destabilizes FtsZ-rings at new cell poles and contributes to the timing of cell division. Genes Dev. 2008;22:3475–3488. doi: 10.1101/gad.1732408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueiros-Filho FJ, Losick R. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 2002;16:2544–2556. doi: 10.1101/gad.1014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CA, de Boer PA. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell. 1997;88:175–185. doi: 10.1016/s0092-8674(00)81838-3. [DOI] [PubMed] [Google Scholar]
- Hale CA, de Boer PA. ZipA is required for recruitment of FtsK, FtsQ, FtsL, and FtsN to the septal ring in Escherichia coli. J Bacteriol. 2002;184:2552–2556. doi: 10.1128/JB.184.9.2552-2556.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CA, Meinhardt H, de Boer PA. Dynamic localization cycle of the cell division regulator MinE in Escherichia coli. EMBO J. 2001;20:1563–1572. doi: 10.1093/emboj/20.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CA, Shiomi D, Liu B, Bernhardt TG, Margolin W, Niki H, de Boer PA. Identification of Escherichia coli ZapC (YcbW) as a component of the division apparatus that binds and bundles FtsZ polymers. J Bacteriol. 2011;193:1393–1404. doi: 10.1128/JB.01245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon DJ, Stokes NR, Ure R, Galbraith G, Bennett JM, Brown DR, Baker PJ, Barynin VV, Rice DW, Sedelnikova SE, et al. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science. 2008;321:1673–1675. doi: 10.1126/science.1159961. [DOI] [PubMed] [Google Scholar]
- Hill NS, Kadoya R, Chattoraj DK, Levin PA. Cell size and the initiation of DNA replication in bacteria. PLoS Genet. 2012;8:e1002549. doi: 10.1371/journal.pgen.1002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, Kruse K. Cellular organization by self-organization: mechanisms and models for Min protein dynamics. J Cell Biol. 2005;168:533–536. doi: 10.1083/jcb.200411122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Lutkenhaus J. Topological regulation of cell division in Escherichia coli involves rapid pole to pole oscillation of the division inhibitor MinC under the control of MinD and MinE. Mol Microbiol. 1999;34:82–90. doi: 10.1046/j.1365-2958.1999.01575.x. [DOI] [PubMed] [Google Scholar]
- Hu Z, Mukherjee A, Pichoff S, Lutkenhaus J. The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc Natl Acad Sci U S A. 1999;96:14819–14824. doi: 10.1073/pnas.96.26.14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huecas S, Llorca O, Boskovic J, Martin-Benito J, Valpuesta JM, Andreu JM. Energetics and geometry of FtsZ polymers: nucleated self-assembly of single protofilaments. Biophys J. 2008;94:1796–1806. doi: 10.1529/biophysj.107.115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O, D’Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981;290:797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Inoue I, Ino R, Nishimura A. New model for assembly dynamics of bacterial tubulin in relation to the stages of DNA replication. Genes Cells. 2009;14:435–444. doi: 10.1111/j.1365-2443.2009.01280.x. [DOI] [PubMed] [Google Scholar]
- Ivanov V, Mizuuchi K. Multiple modes of interconverting dynamic pattern formation by bacterial cell division proteins. Proc Natl Acad Sci U S A. 2010;107:8071–8078. doi: 10.1073/pnas.0911036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Lackner LL, de Boer PA. Targeting of (D)MinC/MinD and (D)MinC/DicB complexes to septal rings in Escherichia coli suggests a multistep mechanism for MinC-mediated destruction of nascent FtsZ rings. J Bacteriol. 2002;184:2951–2962. doi: 10.1128/JB.184.11.2951-2962.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LJ, Carballido-Lopez R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell. 2001;104:913–922. doi: 10.1016/s0092-8674(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Karuppiah V, Derrick JP. Structure of the PilM-PilN inner membrane type IV pilus biogenesis complex from Thermus thermophilus. J Biol Chem. 2011;286:24434–24442. doi: 10.1074/jbc.M111.243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiekebusch D, Michie KA, Essen LO, Lowe J, Thanbichler M. Localized Dimerization and Nucleoid Binding Drive Gradient Formation by the Bacterial Cell Division Inhibitor MipZ. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupka M, Rivas G, Rico AI, Vicente M. Key role of two terminal domains in the bidirectional polymerization of FtsA protein. J Biol Chem. 2012;287:7756–7765. doi: 10.1074/jbc.M111.311563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara B, Rico AI, Petruzzelli S, Santona A, Dumas J, Biton J, Vicente M, Mingorance J, Massidda O. Cell division in cocci: localization and properties of the Streptococcus pneumoniae FtsA protein. Mol Microbiol. 2005;55:699–711. doi: 10.1111/j.1365-2958.2004.04432.x. [DOI] [PubMed] [Google Scholar]
- Levin PA, Losick R. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- Li Z, Trimble MJ, Brun YV, Jensen GJ. The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J. 2007;26:4694–4708. doi: 10.1038/sj.emboj.7601895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose M, Fischer-Friedrich E, Herold C, Kruse K, Schwille P. Min protein patterns emerge from rapid rebinding and membrane interaction of MinE. Nat Struct Mol Biol. 2011;18:577–583. doi: 10.1038/nsmb.2037. [DOI] [PubMed] [Google Scholar]
- Loose M, Fischer-Friedrich E, Ries J, Kruse K, Schwille P. Spatial regulators for bacterial cell division self-organize into surface waves in vitro. Science. 2008;320:789–792. doi: 10.1126/science.1154413. [DOI] [PubMed] [Google Scholar]
- Lowe J, Amos LA. Tubulin-like protofilaments in Ca2+-induced FtsZ sheets. EMBO J. 1999;18:2364–2371. doi: 10.1093/emboj/18.9.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Reedy M, Erickson HP. Straight and curved conformations of FtsZ are regulated by GTP hydrolysis. J Bacteriol. 2000;182:164–170. doi: 10.1128/jb.182.1.164-170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem. 2007;76:539–562. doi: 10.1146/annurev.biochem.75.103004.142652. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. The ParA/MinD family puts things in their place. Trends Microbiol. 2012 doi: 10.1016/j.tim.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Ehrhardt DW, Margolin W. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc Natl Acad Sci U S A. 1996;93:12998–13003. doi: 10.1073/pnas.93.23.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Margolin W. Genetic and functional analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J Bacteriol. 1999;181:7531–7544. doi: 10.1128/jb.181.24.7531-7544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannik J, Wu F, Hol FJ, Bisicchia P, Sherratt DJ, Keymer JE, Dekker C. Robustness and accuracy of cell division in Escherichia coli in diverse cell shapes. Proc Natl Acad Sci U S A. 2012;109:6957–6962. doi: 10.1073/pnas.1120854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston AL, Thomaides HB, Edwards DH, Sharpe ME, Errington J. Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes Dev. 1998;12:3419–3430. doi: 10.1101/gad.12.21.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie KA, Lowe J. Dynamic filaments of the bacterial cytoskeleton. Annu Rev Biochem. 2006;75:467–492. doi: 10.1146/annurev.biochem.75.103004.142452. [DOI] [PubMed] [Google Scholar]
- Migocki MD, Freeman MK, Wake RG, Harry EJ. The Min system is not required for precise placement of the midcell Z ring in Bacillus subtilis. EMBO Rep. 2002;3:1163–1167. doi: 10.1093/embo-reports/kvf233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraldi ER, Thomas PJ, Romberg L. Allosteric models for cooperative polymerization of linear polymers. Biophys J. 2008;95:2470–2486. doi: 10.1529/biophysj.107.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima SY. Mechanism of plastid division: from a bacterium to an organelle. Plant Physiol. 2011;155:1533–1544. doi: 10.1104/pp.110.170688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan LG, Robinson A, Harry EJ. Lateral FtsZ association and the assembly of the cytokinetic Z ring in bacteria. Mol Microbiol. 2009;74:1004–1017. doi: 10.1111/j.1365-2958.2009.06914.x. [DOI] [PubMed] [Google Scholar]
- Mosyak L, Zhang Y, Glasfeld E, Haney S, Stahl M, Seehra J, Somers WS. The bacterial cell-division protein ZipA and its interaction with an FtsZ fragment revealed by X-ray crystallography. EMBO J. 2000;19:3179–3191. doi: 10.1093/emboj/19.13.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Lutkenhaus J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J Bacteriol. 1994;176:2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Lutkenhaus J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 1998;17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Saez C, Lutkenhaus J. Assembly of an FtsZ mutant deficient in GTPase activity has implications for FtsZ assembly and the role of the Z ring in cell division. J Bacteriol. 2001;183:7190–7197. doi: 10.1128/JB.183.24.7190-7197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales E, Downing KH, Amos LA, Lowe J. Tubulin and FtsZ form a distinct family of GTPases. Nat Struct Biol. 1998;5:451–458. doi: 10.1038/nsb0698-451. [DOI] [PubMed] [Google Scholar]
- Osawa M, Anderson DE, Erickson HP. Reconstitution of contractile FtsZ rings in liposomes. Science. 2008;320:792–794. doi: 10.1126/science.1154520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Erickson HP. FtsZ from divergent foreign bacteria can function for cell division in Escherichia coli. J Bacteriol. 2006;188:7132–7140. doi: 10.1128/JB.00647-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Erickson HP. Inside-out Z rings--constriction with and without GTP hydrolysis. Mol Microbiol. 2011;81:571–579. doi: 10.1111/j.1365-2958.2011.07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW. Organelle fission in eukaryotes. Curr Opin Microbiol. 2001;4:639–646. doi: 10.1016/s1369-5274(01)00263-6. [DOI] [PubMed] [Google Scholar]
- Park KT, Wu W, Battaile KP, Lovell S, Holyoak T, Lutkenhaus J. The Min oscillator uses MinD-dependent conformational changes in MinE to spatially regulate cytokinesis. Cell. 2011;146:396–407. doi: 10.1016/j.cell.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JE, Kearns DB. MinJ (YvjD) is a topological determinant of cell division in Bacillus subtilis. Mol Microbiol. 2008;70:1166–1179. doi: 10.1111/j.1365-2958.2008.06469.x. [DOI] [PubMed] [Google Scholar]
- Peters NT, Dinh T, Bernhardt TG. A fail-safe mechanism in the septal ring assembly pathway generated by the sequential recruitment of cell separation amidases and their activators. J Bacteriol. 2011;193:4973–4983. doi: 10.1128/JB.00316-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. EMBO J. 2002;21:685–693. doi: 10.1093/emboj/21.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol Microbiol. 2005;55:1722–1734. doi: 10.1111/j.1365-2958.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- Pichoff S, Shen B, Sullivan B, Lutkenhaus J. FtsA mutants impaired for self-interaction bypass ZipA suggesting a model in which FtsA’s self-interaction competes with its ability to recruit downstream division proteins. Mol Microbiol. 2012;83:151–167. doi: 10.1111/j.1365-2958.2011.07923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin DM, de Boer PA. MinDE-dependent pole-to-pole oscillation of division inhibitor MinC in Escherichia coli. J Bacteriol. 1999;181:6419–6424. doi: 10.1128/jb.181.20.6419-6424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. Role of FtsEX in cell division of Escherichia coli: viability of ftsEX mutants is dependent on functional SufI or high osmotic strength. J Bacteriol. 2007;189:98–108. doi: 10.1128/JB.01347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico AI, Garcia-Ovalle M, Mingorance J, Vicente M. Role of two essential domains of Escherichia coli FtsA in localization and progression of the division ring. Mol Microbiol. 2004;53:1359–1371. doi: 10.1111/j.1365-2958.2004.04245.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues CD, Harry EJ. The Min system and nucleoid occlusion are not required for identifying the division site in Bacillus subtilis but ensure its efficient utilization. PLoS Genet. 2012;8:e1002561. doi: 10.1371/journal.pgen.1002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg L, Simon M, Erickson HP. Polymerization of Ftsz, a bacterial homolog of tubulin. is assembly cooperative? J Biol Chem. 2001;276:11743–11753. doi: 10.1074/jbc.M009033200. [DOI] [PubMed] [Google Scholar]
- Rothfield L, Taghbalout A, Shih YL. Spatial control of bacterial division-site placement. Nat Rev Microbiol. 2005;3:959–968. doi: 10.1038/nrmicro1290. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Peterson ND, Kustusch RJ, Wissel MC, Graham B, Phillips GJ, Weiss DS. A predicted ABC transporter, FtsEX, is needed for cell division in Escherichia coli. J Bacteriol. 2004;186:785–793. doi: 10.1128/JB.186.3.785-793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Lutkenhaus J. The conserved C-terminal tail of FtsZ is required for the septal localization and division inhibitory activity of MinC(C)/MinD. Mol Microbiol. 2009;72:410–424. doi: 10.1111/j.1365-2958.2009.06651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Lutkenhaus J. Examination of the interaction between FtsZ and MinCN in E. coli suggests how MinC disrupts Z rings. Mol Microbiol. 2010;75:1285–1298. doi: 10.1111/j.1365-2958.2010.07055.x. [DOI] [PubMed] [Google Scholar]
- Shiomi D, Margolin W. Dimerization or oligomerization of the actin-like FtsA protein enhances the integrity of the cytokinetic Z ring. Mol Microbiol. 2007;66:1396–1415. doi: 10.1111/j.1365-2958.2007.05998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JK, Makde RD, Kumar V, Panda D. A membrane protein, EzrA, regulates assembly dynamics of FtsZ by interacting with the C-terminal tail of FtsZ. Biochemistry. 2007;46:11013–11022. doi: 10.1021/bi700710j. [DOI] [PubMed] [Google Scholar]
- Singh JK, Makde RD, Kumar V, Panda D. SepF increases the assembly and bundling of FtsZ polymers and stabilizes FtsZ protofilaments by binding along its length. J Biol Chem. 2008;283:31116–31124. doi: 10.1074/jbc.M805910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner W, Liu G, Donachie WD, Kuempel P. The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol Microbiol. 1999;31:579–583. doi: 10.1046/j.1365-2958.1999.01198.x. [DOI] [PubMed] [Google Scholar]
- Stricker J, Maddox P, Salmon ED, Erickson HP. Rapid assembly dynamics of the Escherichia coli FtsZ-ring demonstrated by fluorescence recovery after photobleaching. Proc Natl Acad Sci U S A. 2002;99:3171–3175. doi: 10.1073/pnas.052595099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwedziak P, Wang Q, Freund SM, Lowe J. FtsA forms actin-like protofilaments. EMBO J. 2012;31:2249–2260. doi: 10.1038/emboj.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanbichler M, Shapiro L. MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell. 2006;126:147–162. doi: 10.1016/j.cell.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Tonthat NK, Arold ST, Pickering BF, Van Dyke MW, Liang S, Lu Y, Beuria TK, Margolin W, Schumacher MA. Molecular mechanism by which the nucleoid occlusion factor, SlmA, keeps cytokinesis in check. EMBO J. 2011;30:154–164. doi: 10.1038/emboj.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typas A, Banzhaf M, Gross CA, Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol. 2012;10:123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Bernhardt TG. More than just lysins: peptidoglycan hydrolases tailor the cell wall. Curr Opin Microbiol. 2011;14:698–703. doi: 10.1016/j.mib.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ent F, Amos LA, Lowe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001;413:39–44. doi: 10.1038/35092500. [DOI] [PubMed] [Google Scholar]
- Vicente M, Rico AI. The order of the ring: assembly of Escherichia coli cell division components. Mol Microbiol. 2006;61:5–8. doi: 10.1111/j.1365-2958.2006.05233.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Lutkenhaus J. FtsZ ring: the eubacterial division apparatus conserved in archaebacteria. Mol Microbiol. 1996;21:313–319. doi: 10.1046/j.1365-2958.1996.6421360.x. [DOI] [PubMed] [Google Scholar]
- Weart RB, Lee AH, Chien AC, Haeusser DP, Hill NS, Levin PA. A metabolic sensor governing cell size in bacteria. Cell. 2007;130:335–347. doi: 10.1016/j.cell.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemse J, Borst JW, de Waal E, Bisseling T, van Wezel GP. Positive control of cell division: FtsZ is recruited by SsgB during sporulation of Streptomyces. Genes Dev. 2011;25:89–99. doi: 10.1101/gad.600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissel MC, Weiss DS. Genetic analysis of the cell division protein FtsI (PBP3): amino acid substitutions that impair septal localization of FtsI and recruitment of FtsN. J Bacteriol. 2004;186:490–502. doi: 10.1128/JB.186.2.490-502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Errington J. Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell. 2004;117:915–925. doi: 10.1016/j.cell.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Ishikawa S, Kawai Y, Oshima T, Ogasawara N, Errington J. Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. EMBO J. 2009;28:1940–1952. doi: 10.1038/emboj.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DC, Peters NT, Parzych KR, Uehara T, Markovski M, Bernhardt TG. An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proc Natl Acad Sci U S A. 2011;108:E1052–1060. doi: 10.1073/pnas.1107780108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XC, Margolin W. FtsZ ring clusters in min and partition mutants: role of both the Min system and the nucleoid in regulating FtsZ ring localization. Mol Microbiol. 1999;32:315–326. doi: 10.1046/j.1365-2958.1999.01351.x. [DOI] [PubMed] [Google Scholar]