Abstract
Ticks serve as both vectors and the reservoir hosts capable of transmitting spotted fever group Rickettsia by horizontal and vertical transmission. Persistent maintenance of Rickettsia species in tick populations is dependent on the specificity of the tick and Rickettsia relationship that limits vertical transmission of particular Rickettsia species, suggesting host-derived mechanisms of control. Tick-derived molecules are differentially expressed in a tissue-specific manner in response to rickettsial infection; however, little is known about tick response to specific rickettsial species. To test the hypothesis that tissue-specific tick-derived molecules are uniquely responsive to rickettsial infection, a bioassay to characterize the tick tissue-specific response to different rickettsial species was used. Whole organs of Dermacentor variabilis (Say) were exposed to either Rickettsia montanensis or Rickettsia amblyommii, two Rickettsia species common, or absent, in field-collected D. variabilis, respectively, for 1 and 12 h and harvested for quantitative real time-polymerase chain reaction assays of putative immune-like tick-derived factors. The results indicated that tick genes are differently expressed in a temporal and tissue-specific manner. Genes encoding glutathione S-transferase 1 (dvgst1) and Kunitz protease inhibitor (dvkpi) were highly expressed in midgut, and rickettsial exposure downregulated the expression of both genes. Two other genes encoding glutathione S-transferase 2 (dvgst2) and β-thymosin (dvβ-thy) were highly expressed in ovary, with dvβ-thy expression significantly downregulated in ovaries exposed to R. montanensis, but not R. amblyommii, at 12-h postexposure, suggesting a selective response. Deciphering the tissue-specific molecular interactions between tick and Rickettsia will enhance our understanding of the key mechanisms that mediate rickettsial infection in ticks.
Keywords: tissue-specific, tick immunity, backless tick, Dermacentor variabilis, Rickettsia
Tick-borne rickettsial diseases (TBRD) are caused by several species of bacteria within the genera Anaplasma, Ehrlichia, and Rickettsia. In the United States, human infection by Rickettsia spp. now accounts for ≈50% of the cases of TBRD reported in 2008 (Dumler 2010). The spotted fever group (SFG), Rickettsia, contains well-recognized rickettsial pathogens, such as Rickettsia rickettsii, the agent of Rocky Mountain spotted fever, as well as more recently recognized pathogens, including Rickettsia parkeri (Paddock et al. 2004). These rickettsial pathogens are transmitted to vertebrate host during tick bloodmeal acquisition. Often any feeding life cycle stage (larva, nymph, and adult) is capable of transmitting SFG Rickettsia, as the infection in ticks is also vertically maintained throughout the entire life cycle. Interestingly, intensive field surveys have coincided with increased recognition of a number of SFG Rickettsia in ticks that are not associated with human infection (Stromdahl et al. 2010). Some of these species were reported to function in an endosymbiotic manner (Simser et al. 2005), and their presence in tick populations may influence pathogen transmission by that population. For example, in Dermacentor andersoni, the vertically maintained rickettsial endosymbiont, Rickettsia peacockii (formerly East Side agent), influenced introduction of R. rickettsii into tick populations and limited transovarial transmission of R. rickettsii (Burgdorfer et al. 1981). Subsequently, laboratory experiments demonstrated that Dermacentor variabilis (Say) infected with Rickettsia montanensis, a Rickettsia species commonly associated with wild-caught D. variabilis, was refractory to secondary infection with Rickettsia rhipicephali, an SFG Rickettsia infrequently associated with D. variabilis (Macaluso et al. 2002). In addition, laboratory-infected D. variabilis were unable to maintain R. rhipicephali through multiple generations via transovarial transmission. Combined, these studies suggest that successful transovarial transmission of Rickettsia is dependent on the nature of the tick and Rickettsia relationship (Macaluso et al. 2002); however, contributing elements that mediate vector competence of ticks for rickettsial transmission are undetermined.
A molecular response has been described in SFG Rickettsia infections of ticks with a number of putative tick-derived immune molecules being identified in R. montanensis-infected D. variabilis (Macaluso et al. 2003, Mulenga et al. 2003, Macaluso et al. 2006). For example, glutathione S-transferase 1 and 2 genes (dvgst1 and dvgst2) were identified from R. montanensis-infected D. variabilis using subtractive hybridization (Mulenga et al. 2003) and homolog cloning (Dreher-Lesnick et al. 2006). Transcription of dvgst1 and dvgst2 was upregulated during blood feeding, or R. montanensis-challenge, and downregulated when ticks were challenged with Escherichia coli (Mulenga et al. 2003, Dreher-Lesnick et al. 2006). More recently, D. variabilis Kunitz protease inhibitor (DvKPI) was identified as a novel antimicrobial molecule that limits rickettsial colonization in tick midgut during initial infection by an intimate association with rickettsiae (Ceraul et al. 2008, 2011). A D. variabilis mRNA sequence for β-thymosin (Dvβ-Thy), an invertebrate-antimicrobial molecule (Gai et al. 2009, Schillaci et al. 2010), is available in GenBank; however, the function of β-thymosin (β-Thy) during rickettsial infection has not been characterized. Although most factors were identified in a model representing a commonly reported natural tick and Rickettsia pairing, little is known about the specific response of ticks to uncommon rickettsial infection.
Despite the sympatric distribution of multiple tick and SFG Rickettsia species, the persistent transmission of Rickettsia appears limited to species-specific tick and Rickettsia relationships. In contrast, cell culture systems (both tick and vertebrate derived) demonstrate a wide host range for many species of SFG Rickettsia (Kurtti et al. 2005, Baldridge et al. 2010), suggesting that infection is not a limitation of an individual rickettsial species’ ability to infect a host cell. Thus, the underlying molecular mechanisms involved in successful rickettsial infection of ticks are potentially tick derived. Tissue-specific responses in ticks correlated to successful horizontal and vertical transmission of SFG Rickettsia in tick populations may be a critical element of successful transmission. Toward an understanding of this interaction, the objective of the current study was to quantify the differential tick response to SFG Rickettsia in a temporal and tissue-specific manner. A quantitative real time-polymerase chain reaction (qRT-PCR) was developed to estimate expression of D. variabilis putative immune-like genes (dvgst1, dvgst2, dvkpi, and dvβ-thy) in a rickettsial infection bioassay using Rickettsia species common (R. montanensis) and absent (Rickettsia amblyommii) in D. variabilis in nature. Using a whole tick model, both tissue-specific and SFG Rickettsia-specific responses were identified.
Materials and Methods
Ticks
Rickettsia-free D. variabilis colony was routinely maintained using rats (Rattus norvegicus) for immature tick feeding and New Zealand White rabbits (Oryctolagus cunniculus) for adult feeding. All animals were handled according to the Old Dominion University’s Institutional Animal Care and Use Committee.
Rickettsia
R. amblyommii strain Darkwater (provided by Dr. C. Paddock, CDC Atlanta, GA) and R. montanensis strain M5/6 were grown and maintained in Vero E6 cells in Dulbecco’s modified medium supplemented with 5% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT) at 34°C and 5% CO2. For rickettsial purification, infected Vero E6 cells were detached using a cell scraper and transferred to Erlenmeyer flasks containing sterile 3-mm borosilicate glass beads (Sigma, St. Louis, MO). The infected cells were lysed by vortex at high speed for 3 min, and the cell lysate was filtered through a sterile two micron syringe filter (Whatman, Clifton, NJ). Viability of rickettsiae was determined using a BacLight viability stain kit (Molecular Probes, Carlsbad, CA), and rickettsiae were counted using a Petroff–Hausser bacteria counting chamber (Kurtti et al. 2005, Sunyakumthorn et al. 2008).
Tissue-Specific Expression of Tick Immune Genes During Rickettsial Infection
To determine the tissue-specific expression of tick immune genes (dvgst1, dvgst2, dvkpi, and dvβ-thy) in unfed D. variabilis, five female ticks were dissected to collect salivary glands, midgut, and ovary. The tick tissues were kept in 100 μl RNALater (Ambion, Austin, TX) at −20°C until RNA extraction.
An ex vivo bioassay of D. variabilis tissues (backless tick explant) was modified from previously described protocols (Bell 1980, Sunyakumthorn et al. 2012). Briefly, unfed female D. variabilis were cleaned with 70% ethanol and 10% benzalkonium chloride solution, and rinsed with sterile water three times. The ticks were placed on sterile filter paper in a biological safety cabinet. To remove the tick’s dorsal cuticle, ticks were excised along the perimeter of alloscutum (Fig. 1). The backless ticks were incubated 34°C in 200 μl of complete L15B medium in a 96-well plate (Corning, Corning, NY). After 24 h, backless ticks were divided to three groups (10 ticks per group); the first group, unexposed, was incubated in 200 μl L15B medium, the second and third groups were exposed to R. amblyommii or R. montanensis (1.2 × 106 rickettsiae/μl), respectively. After 1- and 12-h postexposure (hpe), the tick tissues (five ticks per group per time point) were collected, and similar tissues were pooled into 100 μl RNALater (Ambion, Austin, TX) and kept at −20°C until RNA extraction. Two independent experiments were performed.
Fig. 1.

Ex vivo tick tissue culture (backless tick explant). Unfed female D. variabilis were cleaned, air-dried, and transversely cut along the perimeter of alloscutum. Tick dorsal cuticle was removed, and backless ticks were placed in 96-well plates containing 200 μl complete L15B medium for 24 h before rickettsial exposure. (Online figure in color.)
RNA Extraction and qRT-PCR Assay
Total RNA was extracted using the RNasey Mini kit (Qiagen, Valencia, CA) and digested with (4 U per reaction) DNase Turbo (Ambion, Austin, TX). Total RNA (62.5 ng) was used for cDNA synthesis in 25 μl total volume of iScript reverse transcription kit (Bio-Rad, Hercules, CA). A no-RT reaction (distilled water was added instead of reverse transcriptase) was included to confirm the absence of genomic DNA. PCR reaction reagents were mixed in 96-well plates containing 2 μl of cDNA template, 2X iTaq SYBR Green Supermix ROX (Bio-Rad, Hercules, CA), 100 μM each forward and reverse primers (Table 1) in a total volume of 35 μl per reaction. Ten microliters of each reaction mixture were transferred into three wells of 384-well plates and amplification occurred in an ABI 7900HT unit (Applied Biosystems, Foster City, CA) using SDS v2.3 software. Actin gene was used as internal control gene as previously described (Sunyakumthorn et al. 2012). Data for each sample were calculated as the difference in threshold cycle (CT) value (ΔCT = CTdvactin − CTtick immune gene).
Table 1.
Primers for qRT-PCR amplification of tick immune genes
| Primer | Sequence (5′-3′) | Reference |
|---|---|---|
| DvKPIFor | CGAAGAATCAGAGTGCTGGAGAAC | Ceraul et al. 2007 |
| DvKPIRev | CCGAGGTGGTTTTTAGGTCCTG | |
| DvGST1-416For | TATTTCCGGCCAAAGTGGTT | This study |
| DvGST1-590Rev | CCCAATCGCTACTCCCAGAG | |
| DvGST2-484For | AAGGCTGGAGCTCCTCATTG | This study |
| DvGST2-600Rev | ACAGGGTCCGCTGCAGTATT | |
| DvBthy-538For | CACAACCGATGCCAAGAGAA | This study |
| DvBthy-718Rev | GTTGATGAAAGGCTGCCACA | |
| DvActin-1424For | CTTTGTTTTCCCGAGCAGAG | Sunyakumthorn et al. 2012 |
| DvActin-1572Rev | CCAGGGCAGTAGAAGACGAG |
Statistical Analysis
The analysis of variance (ANOVA) was conducted using the SAS statistical package (version 9.2) GLM procedure. For tissue-specific gene expression, the relative expression of tick immune genes in different tick tissues was examined for potential differences. For the analysis of tick immune response during rickettsial infection, the time effect was absorbed from a model. When overall significance was found, Tukey’s honestly significant difference (HSD) post hoc test was performed to determine the pairwise difference of means of main effects of relative gene expression among R. amblyommii- and R. montanensis-exposed and unexposed backless ticks. P value of <0.05 was considered significant.
Results
Tissue-Specific Expression of Tick Immune Genes During Rickettsial Infection
The initial analysis of tissue-specific gene expression of tick immune genes (dvgst1, dvkpi, dvgst2, and dvβ-thy) in unfed D. variabilis tissues including salivary glands, midgut, and ovary were performed before rickettsial exposure. The results demonstrated that expression of dvgst1 was significantly higher in tick midgut compared with salivary glands, and that the expression of dvkpi was significantly greater in the midgut, compared with salivary glands and ovary. However, dvgst2 and dvβ-thy expression was significantly higher in tick ovary, compared with other tissues (Fig. 2).
Fig. 2.
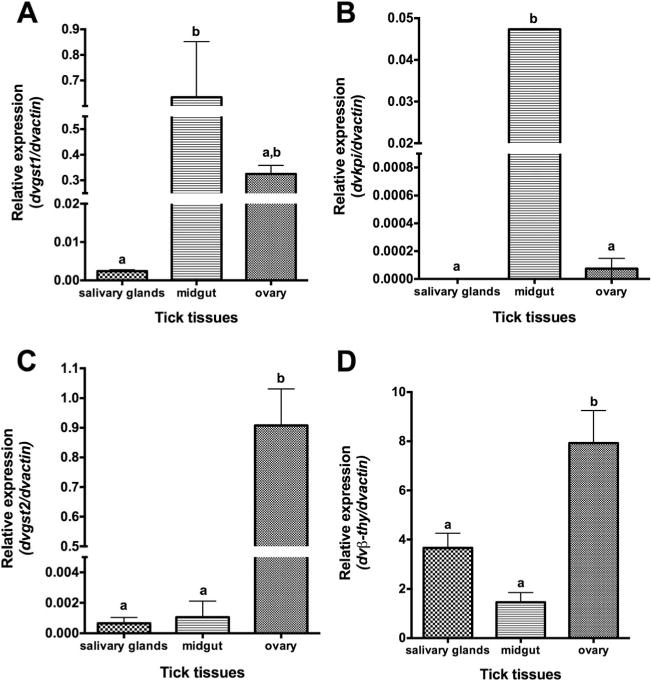
Tissue-specific expression of tick immune genes in unexposed ticks (A) dvgst1, (B) dvkpi, (C) dvgst2, and (D) dvβ-thy. Total RNA from unfed female D. variabilis tissues (salivary glands, midgut, and ovary) was subjected to qRT-PCR assay using specific primers. Transcription level of tick immune genes was normalized to dvactin. Data shown are mean relative expression. Error bar represents standard error of means, and the bars with same letter are not significantly different (P < 0.05).
In the tick salivary glands at 1 and 12 hpe, the expression of dvkpi, dvgst1, dvgst2, and dvβ-thy was low, compared with midgut and ovary, and no significant difference in dvkpi, dvgst1, dvgst2, and dvβ-thy expression was demonstrated when ticks were exposed to rickettsiae.
In the tick midgut, dvgst1 expression was decreased in Rickettsia-exposed ticks compared with unexposed ticks; however, the decrease is only significantly different at one hpe when dvgst1 expression was reduced 80 and 78% in R. amblyommii- and R. montanensis-exposed ticks, respectively (Fig. 3A). In addition, the expression of dvkpi in midgut was significantly decreased in R. amblyommii- (72.5% decrease) and R. montanensis-exposed ticks (63.4% decrease) at one hpe, and likewise at 12 hpe dvkpi expression was significantly downregulated when exposed to R. amblyommii (83.8% decrease) and R. montanensis (86.2% decrease) (Fig. 3B). However, there was no difference in dvkpi and dvgst1 expression between R. amblyommii-and R. montanensis-exposed ticks, and no difference in dvgst2 and dvβ-thy gene expression by tick midgut was shown during rickettsial exposure.
Fig. 3.
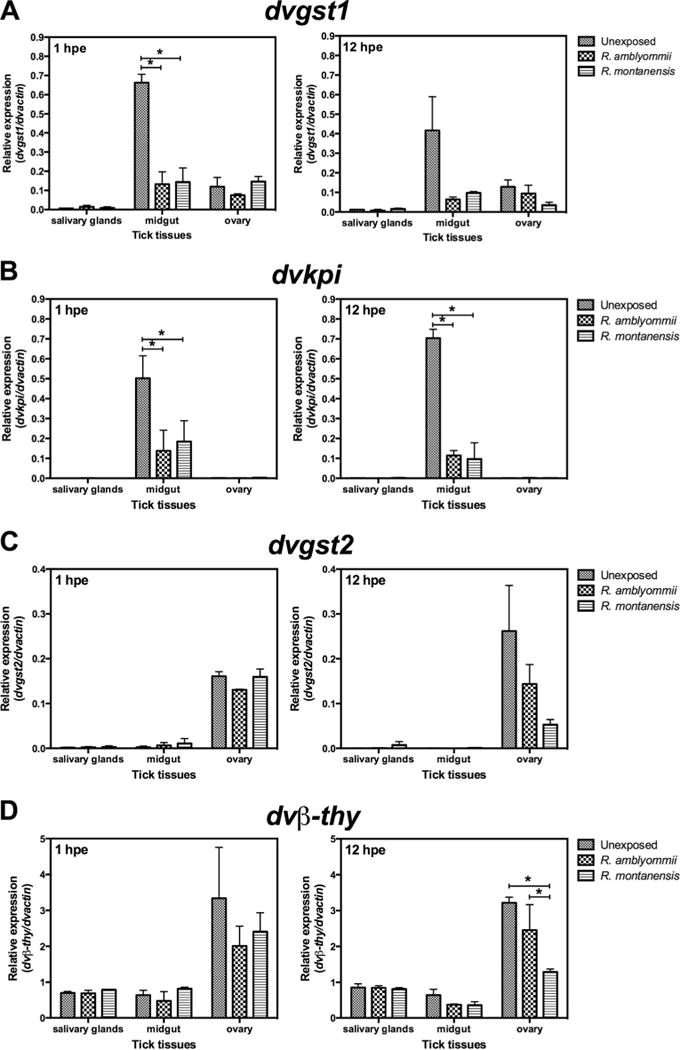
Tissue-specific response of tick immune genes in Rickettsia exposed ticks. (A) dvgst1, (B) dvkpi, (C) dvgst2, and (D) dvβ-thy. Backless ticks were exposed to R. amblyommii or R. montanensis for 1 and12 h, and tick tissues (salivary glands, midgut, and ovary) were dissected and subjected to qRT-PCR assay using specific primers. Transcription level of tick immune genes was normalized to dvactin. Data shown are mean relative expression. Error bar represents standard error of means, and the asterisk indicates significant difference (P < 0.05).
In the tick ovary, at 1 hpe, there was no difference in dvgst2 and dvβ-thy expression when exposed to Rickettsia. The expression of dvgst2 and dvβ-thy was decreased at 12 hpe in R. montanensis-exposed ticks compared with unexposed ticks; however, a significant difference was demonstrated only in dvβ-thy expression (Fig. 3C and D). Sixty percent of dvβ-thy expression was decreased when ticks were exposed to R. montanensis compared with unexposed ticks, and when compared with R. amblyommii-exposed ticks the dvβ-thy expression was 47% lower than in R. montanensis-exposed ticks. Although we were able to detect the dvkpi and dvgst1 mRNA in tick ovary, a significant difference in expression postexposure and between rickettsial species was not observed in Rickettsia-exposed ticks, compared with unexposed ticks.
Discussion
Ticks serve as vectors and reservoirs for both pathogenic and nonpathogenic SFG Rickettsia; however, the role of tick immunity in vector competence for Rickettsia has not been well characterized. In this study, we determined tissue-specific expression of selected tick immune genes and the tick response to R. amblyommii and R. montanensis, which represented Rickettsia species absent and common in D. variabilis, respectively, in a tissue-specific manner using an ex vivo tick model (Sunyakumthorn et al. 2012). The results demonstrated differential expression of target genes in a temporal and tissue-specific manner, and in some cases, as a Rickettsia species-specific response.
Tick midgut is the first site of contact between tick internal organs and host blood that contain rickettsiae. Many tick molecules related to blood digestion and immunity are highly expressed in tick midgut including antimicrobial peptides, protease inhibitors, proteases, and lectins (Sonenshine and Hynes 2008). In this study, dvkpi and dvgst1 expression was significantly downregulated in tick midguts during the exposure to either R. amblyommii or R. montanensis, with no specific differential expression of dvkpi and dvgst1 between the different rickettsial species. This suggests that infection with either Rickettsia species can result in downregulated tick immune molecules in unfed tick midguts, and that the initial midgut response to rickettsiae is nonspecies specific when exposed to R. amblyommii or R. montanensis. Exposure to either Rickettsia species suppresses transcription of glutathione S-transferase and Kunitz protease inhibitor. In contrast, the previous study of Rickettsia-challenged ticks demonstrated that the expression of dvgst1was increased in R. montanensis-infected D. variabilis compared with uninfected ticks (Mulenga et al. 2003), and dvkpi expression was upregulated in D. variabilis challenged with R. montanensis for 72 h (Ceraul et al. 2008). The differences between the current results and these two previous studies may lie in the use of partially fed ticks in which dvkpi and dvgst1expression may be induced during bloodmeal acquisition. Many immune tick molecules are upregulated during bloodmeal acquisition, for example, defensin1 (Ceraul et al. 2007), defensin2 (Ceraul et al. 2007), lysozyme (Ceraul et al. 2007), and GST1 and GST2 (Dreher-Lesnick et al. 2006) in D. variabilis. As the current study sought to capture the early tissue response to rickettsial exposure, independent of a bloodmeal, newly molted unfed ticks were used in the bioassay. Further assessment of the tick response at the midgut interface should use actual animal models of rickettsial infection to mimic natural acquisition of rickettsiae. However, for most rickettsial species, including the two used in the current study, a viable animal model for rickettsial transmission or acquisition by ticks is not available.
Horizontal and vertical transmission of SFG Rickettsia by ticks requires infection of organs, associated with egg production and feeding, ovary and salivary glands, respectively. Combined field observations and laboratory studies suggest a specific association between SFG Rickettsia and their tick hosts (Macaluso et al. 2002, Ammerman et al. 2004, Smith et al. 2010, Stromdahl et al. 2010), which in-part may be influenced by a transmission organ-specific response. A survey to ascertain the molecular basis of specificity identified a number of tick-derived molecules from tick ovaries infected with R. montanensis using differential-display (Macaluso et al. 2003) and subtractive hybridization PCR (Mulenga et al. 2003) including receptor or adhesion molecules, tick immune and stress response factors, and tick–host interaction molecules. To determine the specific immune response of tick ovaries during exposure to Rickettsia species commonly identified, or not identified, in D. variabilis, the gene expression of selected tick molecules was determined. During R. montanensis exposure, dvβ-thy expression was significantly downregulated compared with unexposed and an equivalent dose of R. amblyommii. Although not specifically tested in the current study, a suppressed immune response in ovary during R. montanensis infection may contribute to successful transovarial transmission of this Rickettsia by D. variabilis. Similar to the results of the current study, Rickettsia species-specific differential transcript regulation was demonstrated for D. variabilis α-catenin in R. montanensis-exposed ticks at 12 hpe, but not in R. amblyommii-exposed ticks (Sunyakumthorn et al. 2012). The specific mechanism by which these two genes are regulated during rickettsial exposure and how they directly influence rickettsial infection requires further characterization.
During bloodmeal acquisition, tick salivary glands facilitate blood feeding and likely enhance pathogen transmission to vertebrate hosts. As ticks feed, many tick-derived genes are upregulated to produce factors that enhance blood flow and counter the host immune response (Francischetti et al. 2009, Anatriello et al. 2010, Zivkovic et al. 2010). In the current study, expression of tick immune genes in salivary glands of unfed ticks before and after SFG Rickettsia exposure was examined. The results demonstrated the low expression of all four genes—dvkpi, dvgst1, dvgst2, and dvβ-thy—in salivary glands compared with midgut and ovary. In addition, in contrast to what was identified in the ovary samples, no differential expression was observed when exposed to either species of SFG Rickettsia. Previous examination of gene expression in partially fed D. variabilis chronically infected with R. montanensis demonstrated that mRNA expression of three putative tick proteins including Ena or vasodilator-stimulated protein (VASP), tubulin α-chain, and Cu2+–transporting ATPase were upregulated in salivary glands, compared with uninfected ticks (Macaluso et al. 2003). The distinct pattern(s) of differential regulation of tick factors associated with acute and chronic rickettsial infection may provide a better understanding of maintenance of Rickettsia spp. by ticks and the ecology of tick-borne Rickettsia.
To study the tick tissue-specific response during rickettsial infection, a modified rickettsial infection bioassay using tick tissue culture of backless ticks (Bell 1980) and primary tick tissue culture (Mosqueda et al. 2008) was used. Both techniques allow for tissue-specific analysis during rickettsial infection; however, in our hands, the backless tick technique proved advantageous, as there was higher recovery of tick tissues and an absence of nonspecific microbial contamination. In summary, this study demonstrated that tick transcription in response to rickettsial exposure can be tissue-specific and differential depending on the Rickettsia species. Deciphering the orchestration of tick immune molecules during rickettsial infection of ticks may provide insight into the molecular constituents of vector competence and requires further study.
Acknowledgments
We thank Chutima Thepparit, Supanee Hirunkanokpun, and Mark Guillotte for their technical assistance. We also thank Jacqueline Macaluso for helpful comments. This research was supported by the National Institutes of Health (1R01 AI077784). This work was also part of P. Sunyakumthorn’s doctoral dissertation.
References Cited
- Ammerman NC, Swanson KI, Anderson JM, Schwartz TR, Seaberg EC, Glass GE, Norris DE. Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg Infect Dis. 2004;10:1478–1481. doi: 10.3201/eid1008.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anatriello E, Ribeiro JM, de Miranda–Santos IK, Brandao LG, Anderson JM, Valenzuela JG, Maruyama SR, Silva JS, Ferreira BR. An insight into the sialotranscriptome of the brown dog tick, Rhipicephalus sanguineus. BMC Genomics. 2010;11:450. doi: 10.1186/1471-2164-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge GD, Burkhardt NY, Labruna MB, Pacheco RC, Paddock CD, Williamson PC, Billingsley PM, Felsheim RF, Kurtti TJ, Munderloh UG. Wide dispersal and possible multiple origins of low-copy-number plasmids in rickettsia species associated with blood-feeding arthropods. Appl Environ Microbiol. 2010;76:1718–1731. doi: 10.1128/AEM.02988-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell LJ. Organ culture of Rhipicephalus appendiculatus with maturation of Theileria parva in tick salivary glands in vitro. Acta Trop. 1980;37:319–325. [PubMed] [Google Scholar]
- Burgdorfer W, Hayes EB, Marvos AJ. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and Rickettsial Diseases. Acedemic Press, Inc; New York, NY: 1981. pp. 585–594. [Google Scholar]
- Ceraul SM, Dreher-Lesnick SM, Gillespie JJ, Rahman MS, Azad AF. New tick defensin isoform and antimicrobial gene expression in response to Rickettsia montanensis challenge. Infect Immunol. 2007;75:1973–1983. doi: 10.1128/IAI.01815-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceraul SM, Dreher-Lesnick SM, Mulenga A, Rahman MS, Azad AF. Functional characterization and novel rickettsiostatic effects of a Kunitz-type serine protease inhibitor from the tick Dermacentor variabilis. Infect Immunol. 2008;76:5429–5435. doi: 10.1128/IAI.00866-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceraul SM, Chung A, Sears KT, Popov VL, Beier–Sexton M, Rahman MS, Azad AF. A Kunitz protease inhibitor from Dermacentor variabilis, a vector for spotted fever group rickettsiae, limits Rickettsia montanensis invasion. Infect Immunol. 2011;79:321–329. doi: 10.1128/IAI.00362-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher-Lesnick SM, Mulenga A, Simser JA, Azad AF. Differential expression of two glutathione S-transferases identified from the American dog tick, Dermacentor variabilis. Insect Mol Biol. 2006;15:445–453. doi: 10.1111/j.1365-2583.2006.00657.x. [DOI] [PubMed] [Google Scholar]
- Dumler JS. Fitness and freezing: vector biology and human health. J Clin Invest. 2010;120:3087–3090. doi: 10.1172/JCI44402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai Y, Zhao J, Song L, Wang L, Qiu L, Ning X, Zheng X, Zhang Y, Mu C, Zhang Y, et al. Two thymosin-repeated molecules with structural and functional diversity coexist in Chinese mitten crab Eriocheir sinensis. Dev Comp Immunol. 2009;33:867–876. doi: 10.1016/j.dci.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kurtti TJ, Simser JA, Baldridge GD, Palmer AT, Munderloh UG. Factors influencing in vitro infectivity and growth of Rickettsia peacockii (Rickettsiales: Rickettsiaceae), an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni (Acari, Ixodidae) J Invertebr Pathol. 2005;90:177–186. doi: 10.1016/j.jip.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J Med Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- Macaluso KR, Mulenga A, Simser JA, Azad AF. Differential expression of genes in uninfected and rickettsia-infected Dermacentor variabilis ticks as assessed by differential-display PCR. Infect Immun. 2003;71:6165–6170. doi: 10.1128/IAI.71.11.6165-6170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso KR, Mulenga A, Simser JA, Azad AF. Characterization of Dermacentor variabilis molecules associated with Rickettsial infection. Ann N Y Acad Sci. 2006;1078:384–388. doi: 10.1196/annals.1374.076. [DOI] [PubMed] [Google Scholar]
- Mosqueda J, Cossio–Bayugar R, Rodriguez E, Falcon A, Ramos A, Figueroa JV, Alvarez A. Primary midgut, salivary gland, and ovary cultures from Boophilus microplus. Ann N Y Acad Sci. 2008;1149:49–52. doi: 10.1196/annals.1428.050. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Macaluso KR, Simser JA, Azad AF. Dynamics of Rickettsia-tick interactions: identification and characterization of differentially expressed mRNAs in uninfected and infected Dermacentor variabilis. Insect Mol Biol. 2003;12:185–193. doi: 10.1046/j.1365-2583.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Schillaci D, Arizza V, Parrinello N, Di Stefano V, Fanara S, Muccilli V, Cunsolo V, Haagensen JJ, Molin S. Antimicrobial and antistaphylococcal biofilm activity from the sea urchin Paracentrotus lividus. J Appl Microbiol. 2010;108:17–24. doi: 10.1111/j.1365-2672.2009.04394.x. [DOI] [PubMed] [Google Scholar]
- Simser JA, Rahman MS, Dreher-Lesnick SM, Azad AF. A novel and naturally occurring transposon, ISRpe1 in the Rickettsia peacockii genome disrupting the rick A gene involved in actin-based motility. Mol Microbiol. 2005;58:71–79. doi: 10.1111/j.1365-2958.2005.04806.x. [DOI] [PubMed] [Google Scholar]
- Smith MP, Ponnusamy L, Jiang J, Ayyash LA, Richards AL, Apperson CS. Bacterial pathogens in ixodid ticks from a Piedmont County in North Carolina: prevalence of rickettsial organisms. Vector Borne Zoonotic Dis. 2010;10:939–952. doi: 10.1089/vbz.2009.0178. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, Hynes WL. Molecular characterization and related aspects of the innate immune response in ticks. Front Biosci. 2008;13:7046–7063. doi: 10.2741/3209. [DOI] [PubMed] [Google Scholar]
- Stromdahl EY, Jiang J, Vince M, Richards AL. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector Borne Zoonotic Dis. 2010;11:969–977. doi: 10.1089/vbz.2010.0099. [DOI] [PubMed] [Google Scholar]
- Sunyakumthorn P, Bourchookarn A, Pornwiroon W, David C, Barker SA, Macaluso KR. Characterization and growth of polymorphic Rickettsia felis in a tick cell line. Appl Environ Microbiol. 2008;74:3151–3158. doi: 10.1128/AEM.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyakumthorn P, Petchampai N, Kearney MT, Sonenshine DE, Macaluso KR. Molecular characterization and tissue-specific gene expression of Dermacentor variabilis alpha-catenin in response to rickettsial infection. Insect Mol Biol. 2012;21:197–204. doi: 10.1111/j.1365-2583.2011.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic Z, Esteves E, Almazan C, Daffre S, Nijhof AM, Kocan KM, Jongejan F, de la Fuente J. Differential expression of genes in salivary glands of male Rhipicephalus (Boophilus) microplus in response to infection with Anaplasma marginale. BMC Genomics. 2010;11:186. doi: 10.1186/1471-2164-11-186. [DOI] [PMC free article] [PubMed] [Google Scholar]


