Abstract
Background and Aims
Intestinal alkaline phosphatase (IAP) is a gut mucosal defense factor known to dephosphorylate lipopolysaccharide (LPS); however, the role of IAP in the gut response to luminal bacteria remains poorly defined. We investigated immune responses of wild-type (WT) and IAP-knockout (IAP-KO) mice to LPS and Salmonella typhimurium challenges.
Methods
Cryostat sectioning and standard indirect immunohistochemical staining for major histocompatibility complex (MHC) class II molecules were performed on liver tissue from WT and IAP-KO mice. WT and IAP-KO mice were orally gavaged with S. typhimurium; bacterial translocation to mesenteric nodes, liver, and spleen was determined by tissue homogenization and plating. In other experiments, WT and IAP-KO mice received intraperitoneal injections of LPS, with subsequent quantification of complete blood counts and serum interleukin (IL)-6 by enzyme-linked immunosorbent assay (ELISA). WT and IAP-KO whole blood were plated and stimulated with LPS and Pam-3-Cys, followed by cytokine assays.
Results
Immunohistologic liver examinations showed increased expression of MHC class II molecules in IAP-KO mice. Following S. typhimurium challenge, WT mice appeared moribund compared with IAP-KO mice, with increased bacterial translocation. WT mice had [50% decrease (P \ .005) in platelets and 1.8-fold (P \ .05) increased serum IL-6 compared with IAP-KO mice in response to LPS injections. IAP-KO whole-blood stimulation with LPS and Pam-3-Cys resulted in increased IL-6 and tumor necrosis factor (TNF)-alpha secretion compared with WT.
Conclusions
IAP-KO mice exhibit characteristics consistent with local LPS tolerance. Whole-blood response of IAP-KO mice did not reflect systemic tolerance. These data suggest that IAP is a local immunomodulating factor, perhaps regulating LPS–toll-like receptor 4 (TLR4) interaction between commensal microflora and intestinal epithelium.
Keywords: Mucosal gut defense factor, Lipopolysaccharide, Tolerance, TLR4
Introduction
The precise physiological role for the small intestinal brush border enzyme intestinal alkaline phosphatase (IAP) remains incompletely understood. To date, IAP is best understood as a gut mucosal defense factor. We and others have shown that IAP has the ability to detoxify Gram-negative bacterial endotoxin lipopolysaccharides (LPS) through a dephosphorylation process [1–4]. IAP expression is silenced during times of fasting, which is concordant with a decrease in LPS dephosphorylating activity [5], and is associated with increased bacterial translocation and gut-derived sepsis [6, 7]. In fact, we have demonstrated that IAP-knockout (IAP-KO) mice experience increased bacterial translocation when subjected to direct or indirect gut injury [1].
Although IAP appears to function in gut mucosal defense, under normal conditions IAP-KO mice survive without apparent difficulty or increased infection rates. In fact, the only phenotype initially described for IAP-KO mice was obesity when fed a high-fat diet, an effect thought to be due to accelerated fat absorption [8, 9]. However, there is some evidence that IAP plays a role in gut immune function; for example, inflammatory bowel disease appears to result from a dysregulated mucosal immune response to bacterial components such as LPS. Furthermore, presence of luminal bacteria is necessary for development of spontaneous murine colitis [10, 11]. Low IAP activity has been documented in patients with active inflammatory bowel disease [12, 13]; in fact, IAP levels in even noninflamed tissues in IBD patients are decreased as well [13]. It is conceivable that IAP may play a role in dephosphorylating LPS from commensal microflora and decoupling its interaction with TLR4 receptors in epithelial cells or resident gut macrophages, thus keeping the immune system in check. Although IAP-KO mice do not exhibit spontaneous colitis, we have shown that they are more susceptible to experimental colitis (unpublished observations), and Tuin et al. showed a protective effect of oral IAP supplementation in the setting of dextran sodium sulfate (DSS)-induced murine colitis [13].
Given these data on IAP function, we sought to further explore its role in regard to gut immunity. We speculated that WT mice would be able to normally dephosphorylate and detoxify intraluminal intestinal LPS, thus limiting gut/ portal exposure to intact LPS. In contrast, since IAP-KO mice have decreased capacity to detoxify LPS, these animals would be continuously exposed to active, intact LPS from endogenous intestinal flora. We hypothesized that the excessive chronic exposure to intact LPS in these IAP-KO mice would result in development of tolerance to endotoxin.
The concept of LPS tolerance is complex; on the one hand, some degree of tolerance is desirable as a way to limit the host inflammatory response, whereas extreme suppression of the host immune response would be detrimental. Mice with induced endotoxin tolerance have alternately been shown to be more resistant to certain infections, such as
S. typhimurium and Cryptococcus infections [14, 15], yet more susceptible to others, such as E. coli [16]. With these observations in mind, characterization of the immune responses of IAP-KO mice compared with their WT litter-mates was undertaken to delineate the role of endogenous IAP in the gut responses to bacteria and LPS. We show here that lack of IAP has a demonstrable impact on LPS responsiveness, and that IAP-KO mice appear to develop tolerance to LPS compared with their WT counterparts.
Materials and Methods
Chemicals
Mouse IL-6 and TNF-alpha OptEIA ELISA kits and reagents (BD Biosciences, San Diego, CA) were used for serum cytokine analyses. Ultra pure E. coli LPS (Invivogen, San Diego, CA) was obtained as a lyophilized pellet and reconstituted in sterile water as directed for mouse intraperitoneal injection studies. For whole-blood stimulation studies, E. coli LPS (List Biologicals, Campbell, CA) and Pam-3-Cys (EMC Microcollections GMBH, Tuebingen, Germany) were used. Streptomycin sulfate (Gibco, San Diego, CA) was obtained as a powder and reconstituted in Luria–Bertani broth as needed.
Bacterial Culture
Salmonella typhimurium was obtained as a kind gift from Hans Christian Reinecker’s laboratory (MGH, Boston, MA). Bacteria were grown in culture in Luria–Bertani media at 37°C overnight as needed.
Animals
IAP-KO mice (Mus musculus C57BL/6) construction has been previously described [8]. Heterozygous mice were obtained from the Burnham Institute for Medical Research (La Jolla, CA) and subsequently bred at the MGH animal facility to acquire homozygous IAP-KO and WT C57BL/6 littermates. Genotype was confirmed by polymerase chain reaction (PCR) analysis. Animals in this work were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School (Boston, MA) and those prepared by the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Resources and the National Institutes of Health [17].
Hepatic Immunohistological Examination
WT (n = 6) and IAP-KO (n = 9) mice were sacrificed by carbon dioxide inhalation, and liver cryostat sectioning was performed (thickness 4 lm). Liver sections were fixed in acetone at 4°C. A rat monoclonal antibody against a subunit of mouse MHC class II molecule was used as a primary antibody (sc-59318; Santa Cruz Biotechnology Inc, Santa Cruz, CA); rabbit anti-rat IgG was used as a secondary antibody (Vector Laboratories, Burlingame, CA), followed by hybridization with horseradish peroxidase-conjugated goat anti-rabbit IgG (Vector Laboratories). Diaminobenzidine (DAB; Sigma–Aldrich, St. Louis, MO) was used as the peroxidase substrate for chromogenic visualization of the target protein.
Salmonella Gavage in WT and IAP-KO Mice
WT and IAP-KO mice (n = 5 per group) were fasted for 4 h, and then given an oral gavage of streptomycin (100 mg/kg). Food and water were then returned ad libitum. Twenty-four hours later, food and water were again withheld for 4 h, and each mouse was then orally gavaged with 106 colony forming units (CFU) of S. typhimurium; controls from each WT and IAP-KO group were gavaged with an equal volume of Luria–Bertani broth. Food and water were then resumed ad libitum. Daily weights and appearances of these mice were recorded. Six days after gavage of S. typhimurium, all mice were sacrificed. Mesenteric lymph nodes, spleen, and liver were harvested and weighed. Each organ was homogenized in 1 mL phosphate-buffered saline (PBS); samples were serially diluted and plated on Luria–Bertani agar plates overnight at 37°C. Bacterial CFUs were counted the following morning; these values are expressed as mean CFU/g tissue.
In Vivo Response to LPS
WT and IAP-KO mice (male, 9–12 weeks, n = 5) were injected intraperitoneally (IP) with 100 lg LPS. After 2 h, mice were sacrificed. Blood was withdrawn by cardiac puncture and collected in a microcentrifuge tube. Twenty-five microliters of whole blood was submitted to the MGH Center for Comparative Medicine (CCM) for complete blood count analysis. The remainder of the blood was centrifuged at 13,200 rpm (16,300g) for 5min in a microcentrifuge, and the serum fraction was collected for quantification of IL-6 by ELISA.
Whole-Blood Stimulation with LPS
WT and IAP-KO mice (male, n = 8 and 9, respectively) were exsanguinated using heparinized syringes. Blood was diluted 1:4 in Roswell Park Memorial Institute (RPMI) media and plated for 1 h. Blood was then stimulated overnight by addition of LPS (20–2,000 lg) or Pam-3-Cys (20–2,000 lg). Plates were centrifuged, and supernatants were assayed for IL-6 and TNF-alpha by ELISA.
Statistical Analysis
Cytokine response experiments in WT and IAP-KO mice in response to intraperitoneal injection of LPS were performed three times with consistent results. Whole-blood stimulation with LPS and Pam-3-Cys was performed with blood samples from eight WT and nine IAP-KO mice, respectively. S. typhimurium gavage experiments were performed four times, with varying ages and time to sacrifice after gavage (n = 3–5 and 5 for WT and IAP-KO mice, respectively), but the results were consistent from experiment to experiment. The data presented are for a representative experiment. Statistical significance was calculated by performing an unpaired, one-tailed Student’s t-test between WT and IAP-KO groups. The significance level was set to P \ .05.
Results
MHC Class II Molecules Are Overexpressed in IAP-KO Mouse Liver
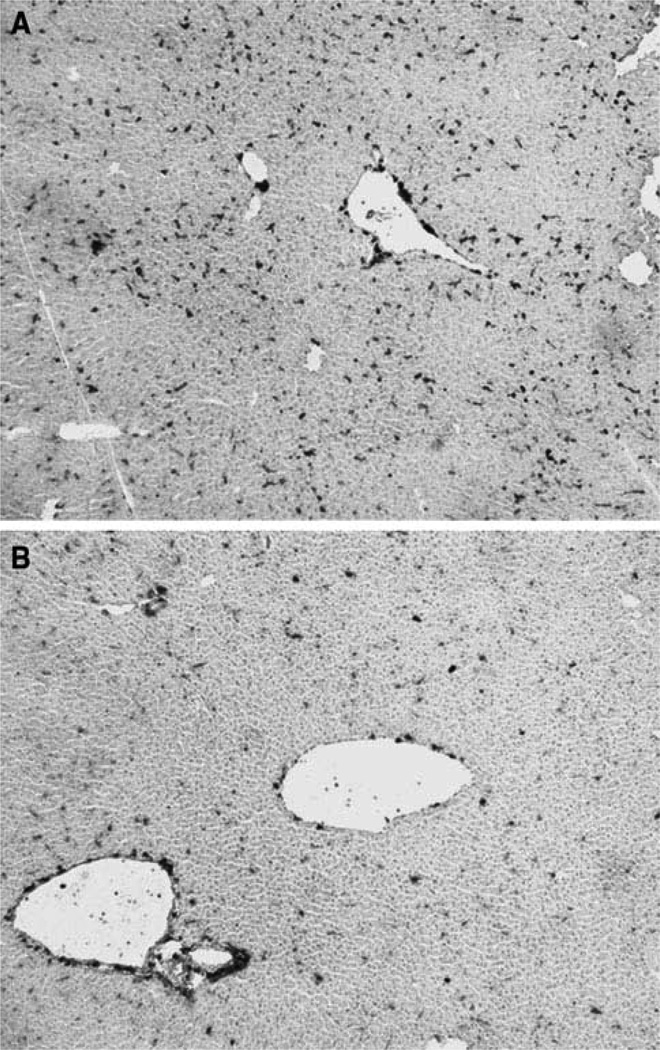
Because enteric antigens, including LPS, are cleared through the liver, we were first interested in examining MHC class II protein expression in WT and IAP-KO mice. Consistent with our speculation that IAP-KO mice are exposed to more active enteric endotoxin, immunohistology performed on the livers showed upregulation of MHC class II molecules in IAP-KO mice compared with WT mice (Fig. 1). The staining pattern was consistent with localization of Kupffer cells (KC) in the parenchyma. There was no difference in the number of KC present in these livers as reflected by F4/80 staining (results not shown).
Fig. 1.
MHCII molecules are upregulated in IAP-KO mice (a) com-pared with WT mice (b)
IAP-KO Mice Exhibit Resistance to Systemic Dissemination of S. typhimurium
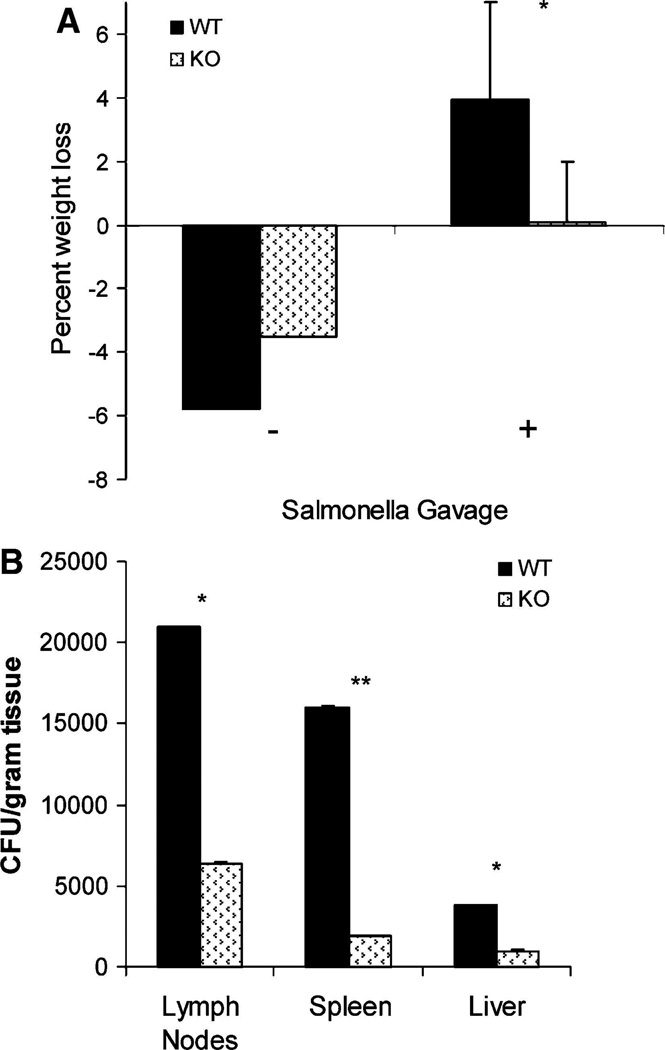
WT and IAP-KO mice were then compared in regard to their sensitivity to an oral bacterial challenge. We chose S. typhimurium because mice are readily susceptible to oral challenge with this bacterium. Furthermore, animal responses to S. typhimurium infection have been well described in the setting of LPS tolerance, with endotoxin-tolerant mice exhibiting prolonged survival and decreased bacterial loads [14]. When WT and IAP-KO mice were gavaged with S. typhimurium, both groups exhibited weight loss; however, WT mice on average lost significantly more weight and appeared sicker than IAP-KO mice (Fig. 2a). Furthermore, WT mice had increased bacterial translocation to the mesenteric nodes, spleen, and liver, with three-, eight-, and fourfold increases in CFUs compared with IAP-KO mice (P \ .05; Fig. 2b).
Fig. 2.
WT and IAP-KO mice that did not receive S. typhimurium gavage (−) did not experience weight loss. However, with S. typhimurium gavage (?), WT mice had an associated greater percentage weight loss compared with IAP-KO mice (a), and also exhibited [2–fold increased bacterial translocation to mesenteric nodes and remote organs (b). (**P \.005, *P \.05)
IAP-KO Mice Are Tolerant to LPS-Mediated Immune Response
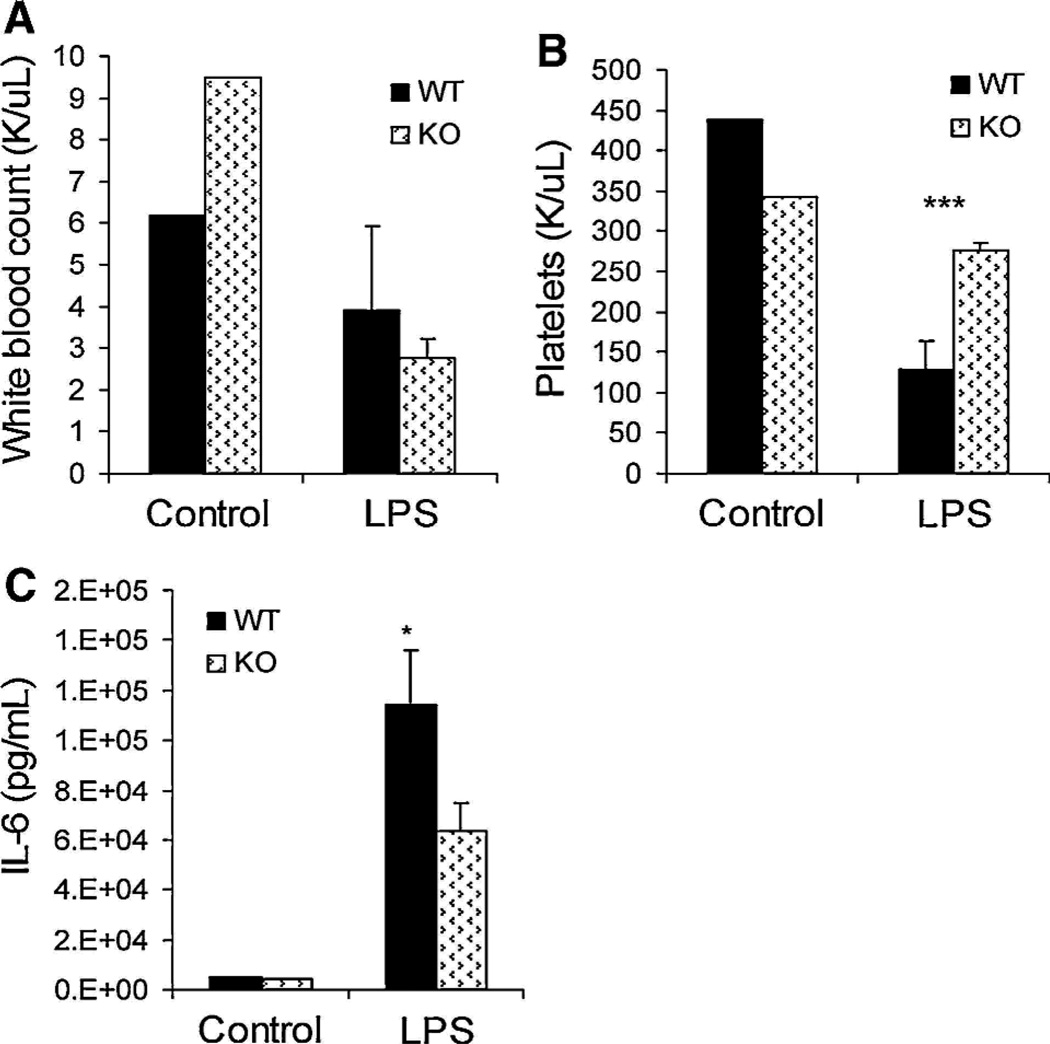
The observation that IAP-KO mice were relatively resistant to S. typhimurium provided evidence for the development of endotoxin tolerance in IAP-KO mice. We sought to further define this immune response by exposing the mice to systemic LPS. In our experiments (unpublished), we found that serum IL-6 peaks at 2 h after IP injection of LPS, and cytokine and whole-blood studies were performed accordingly. There was no difference in serum IL-6 levels between WT and IAP-KO mice injected with normal saline. However, following IP injection of LPS, WT mice demonstrated increased immune response compared with IAP-KO mice. Platelet counts were decreased by [50% (P \ .005) in WT mice compared with IAP-KO mice (Fig. 3b). Serum IL-6 was also increased by 1.8-fold (P \ .05) in WT mice compared with IAP-KO mice (Fig. 3c). There was no difference in white blood cell (WBC) counts between the two groups, although both groups exhibited [40% decrease in WBC compared with their controls (Fig. 3a).
Fig. 3.
There was no significant difference in white blood cell count after intraperitoneal LPS injection (a) but a 50% decrease in platelet count in WT mice (b). Serum IL-6 was significantly increased (1.8-fold) in WT compared with IAP-KO mice (c). (***P \ .0005, *P \ .05)
Whole-Blood Experiments to Assess Systemic Tolerance
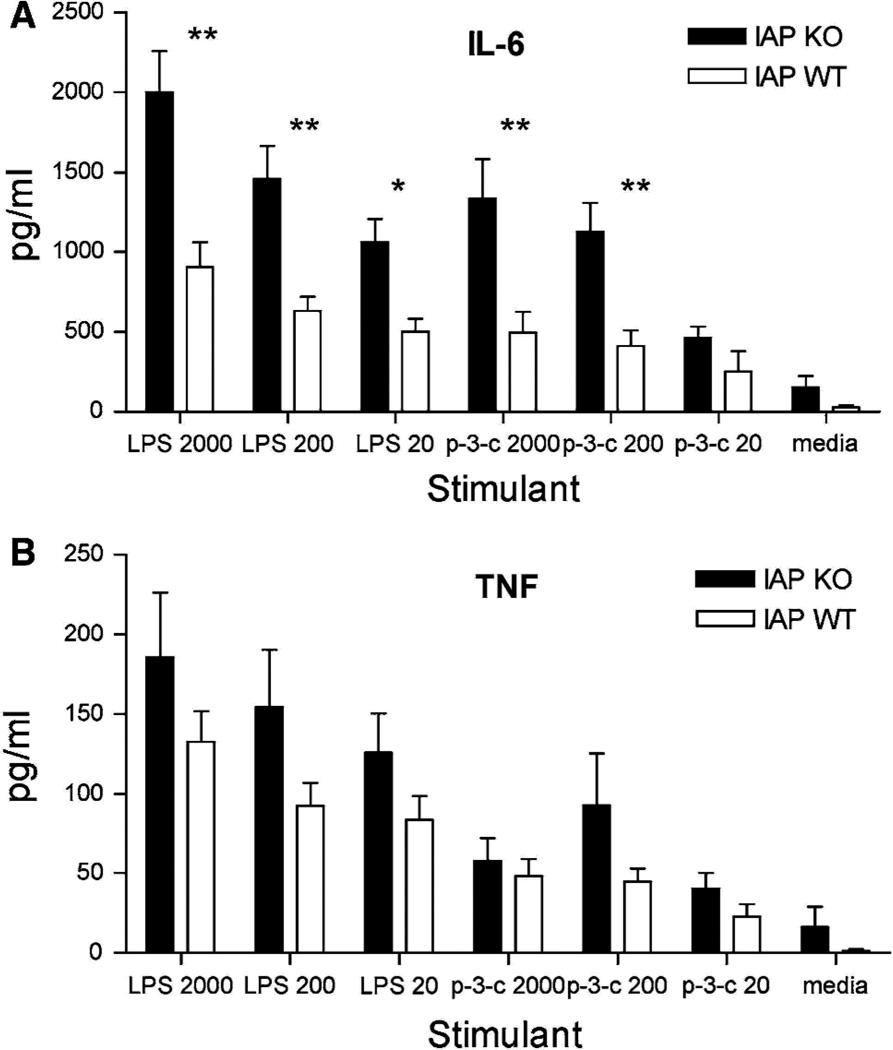
Finally, we performed whole-blood experiments to determine whether the LPS tolerance in IAP-KO mice was confined to the gut/portal system or was systemically evident. Whole blood was stimulated by both LPS and Pam-3-Cys, and resulted in [2–fold increases (P \ .005) in IL-6 secretion in IAP-KO samples compared with the WT blood (Fig. 4). TNF-alpha release was also increased in IAP-KO samples, although this did not reach statistical significance. This result indicates that the LPS tolerance exhibited by IAP-KO mice is confined to the gut/portal system.
Fig. 4.
Whole-blood stimulation with varying amounts of LPS (lL) and Pam-3-Cys (lL) resulted in significantly decreased IL-6 (a) and a trend towards decreased TNF-alpha secretion in WT mice compared with IAP-KO mice (b). (**P \ .005, *P \ .05)
Discussion
Intestinal alkaline phosphatase is well known to have the ability to dephosphorylate LPS, and we have previously demonstrated that its expression in the intestine correlates directly with LPS dephosphorylating activity in the gut [1]. When IAP expression is decreased in the setting of starvation, LPS dephosphorylating activity is also diminished. In addition, IAP-KO mice have less LPS dephosphorylating activity compared with their WT littermates [1]. Based upon these observations, we have speculated that IAP has a role in regulating the host response to active endotoxin.
Commensal microflora serves important functions in regard to vitamin synthesis, nutrient digestion, and competitive inhibition of pathogen colonization. However, these bacteria also represent a major antigen burden within the intestinal lumen. IAP, which is both membrane bound and secreted into the lumen, may serve as a mucosal defense factor by dephosphorylating and detoxifying this burden. Breakdown of the gut mucosal barrier can result in increased endotoxemia and LPS-induced hepatitis and Kupffer cell activation [18, 19]. Accordingly, we hypothesized that, by limiting exposure to intact endotoxin, the IAP enzyme could be providing an important protective function to the host.
Under normal conditions in WT mice, the LPS that crosses the intestinal brush border would be expected to be in a dephosphorylated (inactive) form, having been exposed to IAP. In contrast, IAP-KO mice lack the ability to detoxify LPS in this manner, and these mice would therefore be continuously exposed to intact and active LPS. As such, over time, the IAP-KO mice would be expected to become tolerant to LPS. We designed a series of experiments to test this hypothesis.
We found that Kupffer cell MHC class II molecules are upregulated in IAP-KO mice. LPS is known to activate MHC class II molecules in liver tissue, especially Kupffer cells [20–24]. In regard to bacterial infection, endotoxin-tolerant mice have generally been shown to be resistant to various aspects of sepsis, including vasomotor collapse and fever [25, 26]. In particular, it has been shown that mice in which LPS tolerance has been induced are resistant to Salmonella infection [14, 27], which is what we observed in the IAP-KO mice. The IAP-KO mice had decreased weight loss, appeared healthier, and, following oral inoculation of S. typhimurium, had decreased bacterial translocation to mesenteric nodes and distant organs compared with WT mice. Lastly, we demonstrated LPS tolerance in IAP-KO mice in the classical sense of the definition, which is described as hyporesponsiveness to endotoxin following previous sublethal exposure to endotoxin [28, 29]. Previous studies have shown that monophosphoryl LPS is unable to induce an equivalent tolerance to that provoked by intact LPS [30, 31], and that increased concentrations of monophosphoryl LPS are needed to match the initial cytokine response induced by that of intact LPS. In accordance with this idea of tolerance, we observed that IAP-KO mice had blunted immune response to intraperitoneal injections of LPS; the platelet drop was not as dramatic as in WT mice, nor was the increase in serum IL-6 as high. Taken together, these data suggest that the IAP-KO mice are indeed exposed to active endotoxin across the gut barrier and, as a consequence, have developed a tolerance to LPS and bacterial pathogens.
In contrast to the gut/portal system LPS tolerance noted in the IAP-KO mice, we found that whole-blood responsiveness of the IAP-KO mice was not less than that seen in WT mice. It appears, therefore, that the tolerance we observe in IAP-KO mice is confined to the local gut/ portal system environment. Following LPS injection into the bloodstream, the majority of the LPS is cleared in the liver [32, 33]; this, along with other LPS binding factors in whole blood, inhibits the contact between LPS and circulating leukocytes [34–37]. Serum IL-6 production after LPS injection is largely driven by the portal system; LPS levels following intraperitoneal LPS injection are higher in the portal system than in the arterial system, with subsequent hepatically derived TNF-alpha release [38, 39]. Furthermore, serum IL-6 following LPS injection is driven by TNF-alpha production as well [40]. Thus, gut tolerance to LPS may not translate to systemic tolerance to LPS, as demonstrated by whole-blood responsiveness in the present studies. Interestingly, Fitting et al. demonstrated that endotoxin tolerance can be compartmentalized [41]. Following IP LPS injections in mice, they observed that alveolar leukocytes did not demonstrate the same level of tolerance as peritoneal and bone marrow cells or splenocytes. Furthermore, they found using whole-blood studies that in vivo tolerance did not translate into prolonged ex vivo tolerance. Our findings with IAP-KO mice are consistent with these observed differences between in vivo and ex vivo LPS tolerance. Lastly, we note that, with our whole-blood studies, not only was LPS tolerance not demonstrated, but IAP-KO whole blood in fact exhibited increased cytokine production over that of WT whole blood when stimulated with LPS and Pam-3-Cys. The reason for this is not clear. Possibilities could include increased sensitivity of circulating leukocytes, decreased suppressive plasma proteins, increased facilitating plasma proteins, or some combination. Further studies will be required to elucidate the underlying mechanisms.
Based on our present data, we believe that IAP functions as a local immunomodulating factor in the intestine. Several recent papers support the idea that TLR pathways in the intestinal epithelium are integral to maintaining intestinal-commensal microflora homeostasis [42–44]; in essence, some degree of TLR signaling by commensal bacteria is necessary to maintain normal intestinal surface structure and the capacity for repair in response to injury. However, a balance is required, as hyperactive TLR signaling can lead to increased intestinal inflammation, such as that seen in inflammatory bowel disease. Although in our present studies we demonstrate an advantage to LPS tolerance in terms of salmonellosis, this observation does not necessarily correlate with an advantage in terms of commensal microflora. S. typhimurium is a particular pathogen that is not dependent on LPS for invasion or recognition by host defenses (in fact, LPS-deficient Salmonella are cleared more rapidly from the bloodstream, which may explain why LPS tolerance is beneficial in this case) [44]. In contrast to S. typhimurium, we have previously demonstrated that IAP-KO mice are more susceptible to bacterial translocation from the gut in response to mesenteric and hind-limb ischemia [1]. With our present data and previous observations, we believe that IAP plays a role in LPS–TLR4 interaction, limiting host tolerance to commensal microflora and thereby helping to maintain the balance and integrity of the gut mucosal barrier.
Acknowledgments
Grant support: (1) National Institutes of Health Grant R01DK050623; (2) National Institutes of Health Grant R01DK047186; (3) National Institutes of Health Grant T32DK007754.
Footnotes
Conflicts of interest No conflicts of interest are reported.
Contributor Information
Kathryn T. Chen, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA Department of Surgery, University of Minnesota, 420 Delaware Street SE, Mayo Mail Code 195, Minneapolis, MN 55455, USA.
Madhu S. Malo, Email: mmalo@partners.org, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Laura Kline Beasley-Topliffe, Email: lbeasleytopliffe@gmail.com, Division of Infectious Disease, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Klaas Poelstra, Email: k.poelstra@rug.nl, Department of Pharmacokinetics, Toxicology and Targeting, University of Groningen, Groningen, The Netherlands.
Jose Luis Millan, Email: millan@burnham.org, Sanford Children’s Health Research Center, Burnham Institute for Medical Research, La Jolla, CA 92037, USA.
Golam Mostafa, Email: gmostafa@partners.org, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Sayeda N. Alam, Email: snalam@partners.org, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Sundaram Ramasamy, Email: ramasamy.sundaram@mgh.harvard.edu, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
H. Shaw Warren, Email: warren@helix.mgh.harvard.edu, Division of Infectious Disease, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Elizabeth L. Hohmann, Email: ehohmann@partners.org, Division of Infectious Disease, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Richard A. Hodin, Email: rhodin@partners.org, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
References
- 1.Goldberg RF, Austen WG, Jr, Zhang X, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008;105(9):3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2(6):371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poelstra K, Bakker WW, Klok PA, Kamps JA, Hardonk MJ, Meijer DK. Dephosphorylation of endotoxin by alkaline phosphatase in vivo. Am J Pathol. 1997;151(4):1163–1169. [PMC free article] [PubMed] [Google Scholar]
- 4.Poelstra K, Bakker WW, Klok PA, Hardonk MJ, Meijer DK. A physiologic function for alkaline phosphatase: endotoxin detoxification. Lab Invest. 1997;76(3):319–327. [PubMed] [Google Scholar]
- 5.Hodin RA, Graham JR, Meng S, Upton MP. Temporal pattern of rat small intestinal gene expression with refeeding. Am J Physiol. 1994;266(1 Pt 1):G83–G89. doi: 10.1152/ajpgi.1994.266.1.G83. [DOI] [PubMed] [Google Scholar]
- 6.McClure RJ, Newell SJ. Randomised controlled study of clinical outcome following trophic feeding. Arch Dis Child Fetal Neonatal Ed. 2000;82(1):F29–F33. doi: 10.1136/fn.82.1.F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma—a prospective, randomized study. J Trauma. 1986;26(10):874–881. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Narisawa S, Huang L, Iwasaki A, Hasegawa H, Alpers DH, Millan JL. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol Cell Biol. 2003;23(21):7525–7530. doi: 10.1128/MCB.23.21.7525-7530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakano T, Inoue I, Koyama I, et al. Disruption of the murine intestinal alkaline phosphatase gene Akp3 impairs lipid transcytosis and induces visceral fat accumulation and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2007;292(5):G1439–G1449. doi: 10.1152/ajpgi.00331.2006. [DOI] [PubMed] [Google Scholar]
- 10.Rath HC, Schultz M, Freitag R, et al. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun. 2001;69(4):2277–2285. doi: 10.1128/IAI.69.4.2277-2285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elson CO. Experimental models of intestinal inflammation: new insights into mechanisms of mucosal homeostasis. In: M EL, Ogro PL, Bienenstock J, Mestecky J, Strober W, McGhee JR, editors. Mucosal Immunology. San Diego: Academic Press; 1999. pp. 1007–1024. [Google Scholar]
- 12.Torres MI, Lorite P, Lopez-Casado MA, Rios A. A new approach using tissue alkaline phosphatase histochemistry to identify Crohn’s disease. Pathol Res Pract. 2007;203(6):485–487. doi: 10.1016/j.prp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Tuin A, Poelstra K, de Jager-Krikken A, et al. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58(3):379–387. doi: 10.1136/gut.2007.128868. [DOI] [PubMed] [Google Scholar]
- 14.Lehner MD, Ittner J, Bundschuh DS, van Rooijen N, Wendel A, Hartung T. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar typhimurium infection despite attenuated cytokine response. Infect Immun. 2001;69(1):463–471. doi: 10.1128/IAI.69.1.463-471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rayhane N, Fitting C, Lortholary O, Dromer F, Cavaillon JM. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infect Immun. 2000;68(6):3748–3753. doi: 10.1128/iai.68.6.3748-3753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu M, Varley AW, Ohta S, Hardwick J, Munford RS. Host inactivation of bacterial lipopolysaccharide prevents prolonged tolerance following gram-negative bacterial infection. Cell Host Microbe. 2008;4(3):293–302. doi: 10.1016/j.chom.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.N.I.o. Health, editor. Department of Health E.a.H.S., Publication no. 85–23. 1985 [Google Scholar]
- 18.Enomoto N, Ikejima K, Bradford BU, et al. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(Suppl):20–25. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 19.Thurman RG., II Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275(4 Pt 1):G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 20.Jephthah-Ochola J, Urmson J, Farkas S, Halloran PF. Regulation of MHC expression in vivo. Bacterial lipopolysaccharide induces class I and II MHC products in mouse tissues by a T cell-independent, cyclosporine-sensitive mechanism. J Immunol. 1988;141(3):792–800. [PubMed] [Google Scholar]
- 21.Kaufman JF, Auffray C, Korman AJ, Shackelford DA, Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984;36(1):1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, Ogasawara K, Takeda K, et al. LPS induces NK1.1? alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J Immunol. 1996;156(7):2436–2442. [PubMed] [Google Scholar]
- 23.Franco A, Barnaba V, Natali P, Balsano C, Musca A, Balsano F. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. 1988;8(3):449–454. doi: 10.1002/hep.1840080302. [DOI] [PubMed] [Google Scholar]
- 24.Wiegard C, Wolint P, Frenzel C, et al. Defective T helper response of hepatocyte-stimulated CD4 T cells impairs antiviral CD8 response and viral clearance. Gastroenterology. 2007;133(6):2010–2018. doi: 10.1053/j.gastro.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Coffee KA, Halushka PV, Wise WC, Tempel GE, Cook JA. Endotoxin tolerance differentially alters hemodynamic responses to a thromboxane A2 mimetic and phenylephrine. J Cardiovasc Pharmacol. 1991;17(1):20–26. doi: 10.1097/00005344-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Beeson PB. Development of tolerance to typhoid bacterial pyrogen and its abolition by reticulo-endothelial blockade. Proc Soc Exp Biol Med. 1946;61:248–250. doi: 10.3181/00379727-61-15291p. [DOI] [PubMed] [Google Scholar]
- 27.Garg S, Bal V, Rath S, George A. Effect of multiple antigenic exposures in the gut on oral tolerance and induction of antibacterial systemic immunity. Infect Immun. 1999;67(11):5917–5924. doi: 10.1128/iai.67.11.5917-5924.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favorite GO, Morgan HR. Effects produced by the intravenous injection in man of a toxic antigenic material derived from eberthella typhosa: clinical, hematological, chemical and serological studies. J Clin Invest. 1942;21(5):589–599. doi: 10.1172/JCI101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greisman SE, Woodward WE. Mechanisms of endotoxin tolerance. 3. The refractory state during continuous intravenous infusions of endotoxin. J Exp Med. 1965;121:911–933. doi: 10.1084/jem.121.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madonna GS, Peterson JE, Ribi EE, Vogel SN. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun. 1986;52(1):6–11. doi: 10.1128/iai.52.1.6-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henricson BE, Benjamin WR, Vogel SN. Differential cytokine induction by doses of lipopolysaccharide and monophosphoryl lipid A that result in equivalent early endotoxin tolerance. Infect Immun. 1990;58(8):2429–2437. doi: 10.1128/iai.58.8.2429-2437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathison JC, Ulevitch RJ. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979;123(5):2133–2143. [PubMed] [Google Scholar]
- 33.Munford RS, Hall CL, Lipton JM, Dietschy JM. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J Clin Invest. 1982;70(4):877–888. doi: 10.1172/JCI110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munford RS, Hall CL, Dietschy JM. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect Immun. 1981;34(3):835–843. doi: 10.1128/iai.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vesy CJ, Kitchens RL, Wolfbauer G, Albers JJ, Munford RS. Lipopolysaccharide-binding protein and phospholipid transfer protein release lipopolysaccharides from gram-negative bacterial membranes. Infect Immun. 2000;68(5):2410–2417. doi: 10.1128/iai.68.5.2410-2417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180(3):1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitchens RL, Thompson PA, Viriyakosol S, O’Keefe GE, Munford RS. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest. 2001;108(3):485–493. doi: 10.1172/JCI13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asari Y, Majima M, Sugimoto K, Katori M, Ohwada T. Release site of TNF alpha after intravenous and intraperitoneal injection of LPS from Escherichia coli in rats. Shock. 1996;5(3):208–212. doi: 10.1097/00024382-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Sugimoto K, Kawamura M, Katori M, Shindo M, Ohwada T. Transmigration routes and a delayed systemic hypotension in rats after intraperitoneal injection of endotoxin from Escherichia coli. Circ Shock. 1993;41(3):185–196. [PubMed] [Google Scholar]
- 40.Ghezzi P, Sacco S, Agnello D, Marullo A, Caselli G, Bertini R. LPS induces IL-6 in the brain and in serum largely through TNF production. Cytokine. 2000;12(8):1205–1210. doi: 10.1006/cyto.2000.0697. [DOI] [PubMed] [Google Scholar]
- 41.Fitting C, Dhawan S, Cavaillon JM. Compartmentalization of tolerance to endotoxin. J Infect Dis. 2004;189(7):1295–1303. doi: 10.1086/382657. [DOI] [PubMed] [Google Scholar]
- 42.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Madara J. Building an intestine—architectural contributions of commensal bacteria. N Engl J Med. 2004;351(16):1685–1686. doi: 10.1056/NEJMcibr042621. [DOI] [PubMed] [Google Scholar]
- 44.Ohno A, Isii Y, Tateda K, et al. Role of LPS length in clearance rate of bacteria from the bloodstream in mice. Microbiology. 1995;141(Pt 10):2749–2756. doi: 10.1099/13500872-141-10-2749. [DOI] [PubMed] [Google Scholar]