Abstract
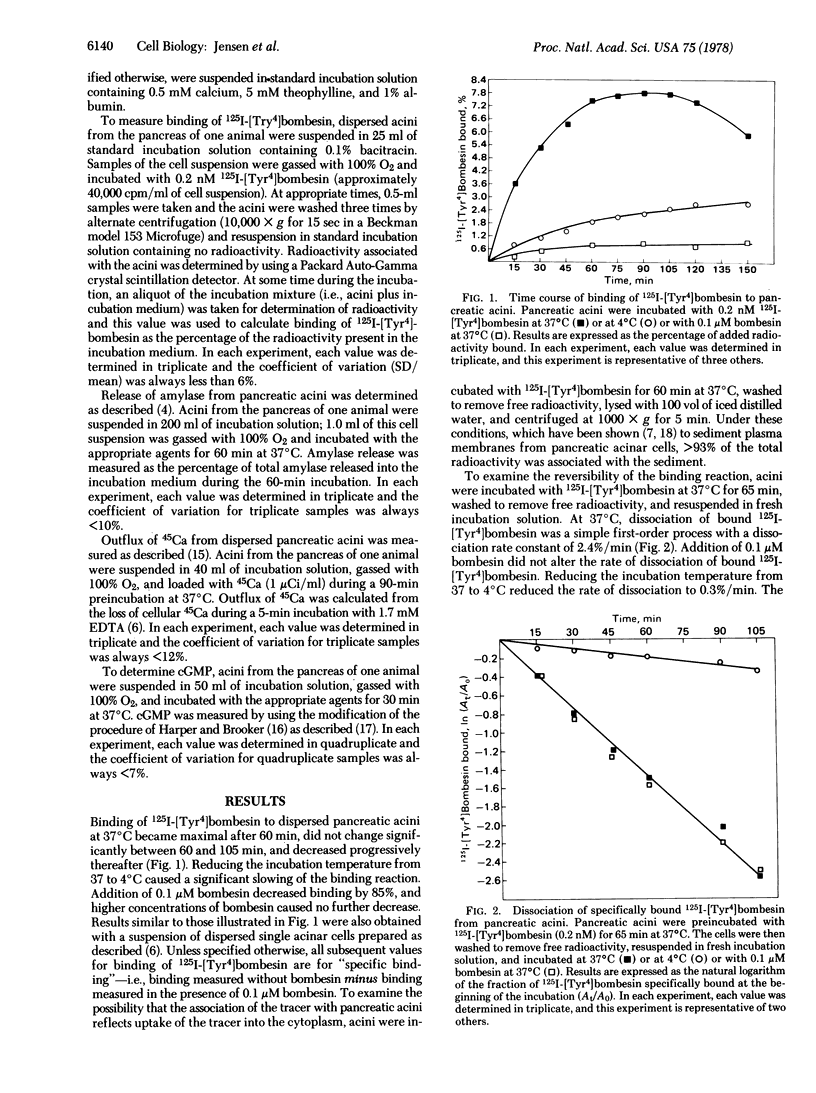
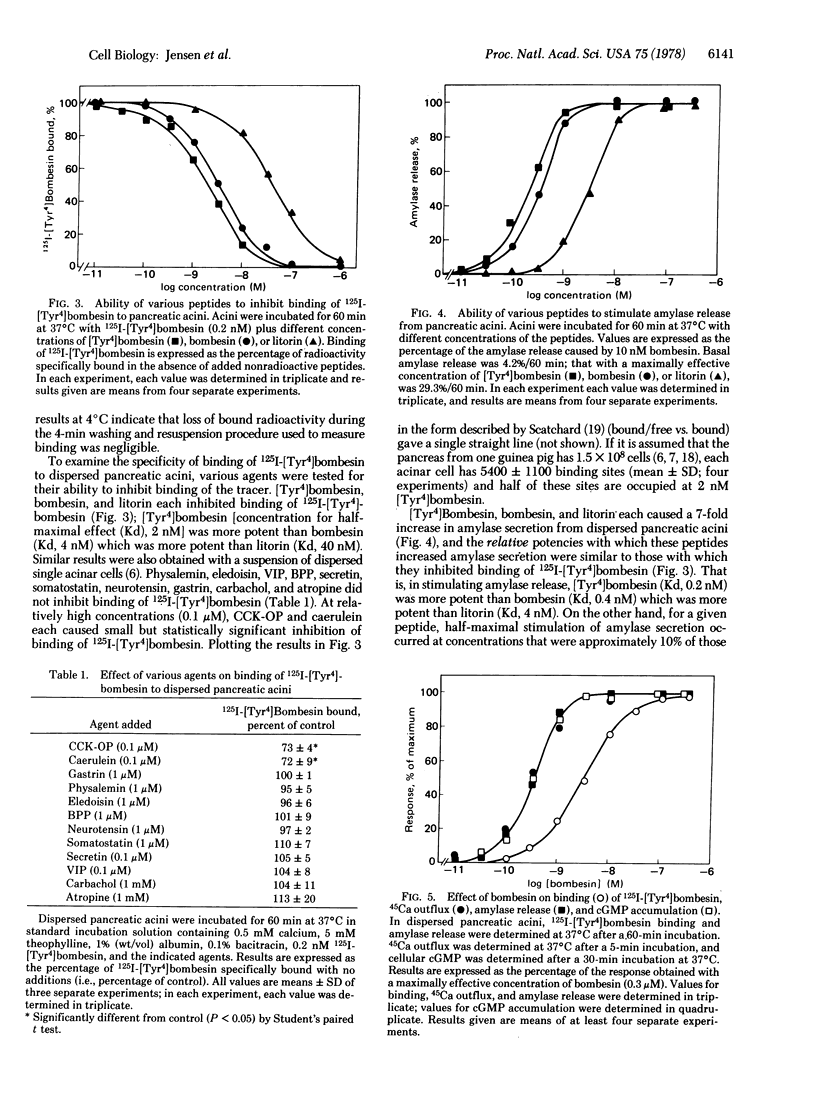
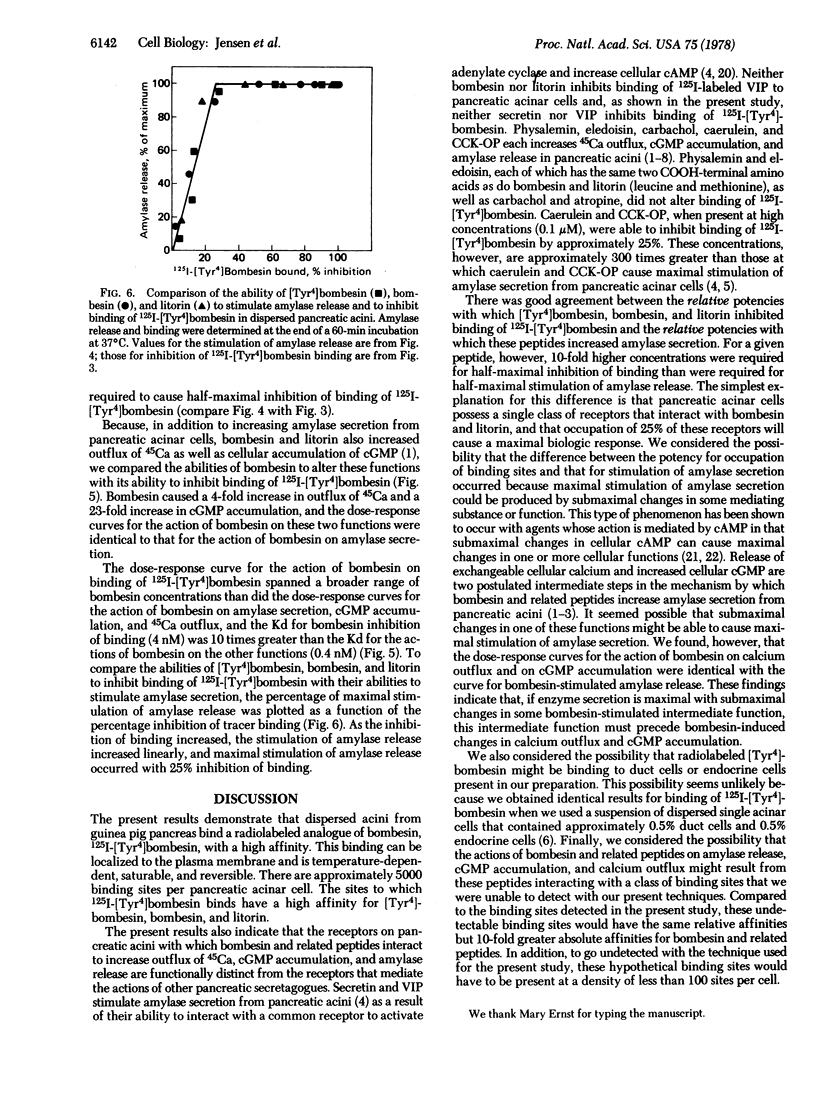
We have prepared 125I-labeled [Tyr4]bombesin and have examined the kinetics, stoichiometry, and chemical specificity with which the labeled peptide binds to dispersed acini from guinea pig pancreas. Binding of 125I-labeled [Tyr4]-bombesin was saturable, temperature-dependent, and reversible and reflected interaction of the labeled peptide with a single class of binding sites on the plasma membrane of pancreatic acinar cells. Each acinar cell possessed approximately 5000 binding sites, and binding of the tracer to these sites could be inhibited by [Tyr4]bombesin [concentration for half-maximal effect (Kd), 2 nM], bombesin (Kd, 4 nM), or litorin (Kd, 40 nM) but not by eledoisin, physalemin, somatostatin, carbachol, atropine, secretin, vasocative intestinal peptide, neurotensin, or bovine pancreatic polypeptide. At high concentrations (>0.1 μM), cholecystokinin and caerulein each caused a small (15-20%) reduction in binding of lableled [Tyr4]bombesin. With bombesin, litorin, and [Tyr4]bombesin, there was a close correlation between the relative potency for inhibition of binding of labeled [Tyr4]bombesin and that for stimulation of amylase secretion. For a given peptide, however, a 10-fold higher concentration was required for half-maximal inhibition of binding than for half-maximal stimulation of amylase secretion, calcium outflux, or cyclic GMP accumulation. These results indicate that dispersed acini from guinea pig pancreas possess a single class of receptors that interact with [Tyr4]bombesin, bombesin, and litorin and that occupation of 25% of these receptors will cause a maximal biological response.
Keywords: pancreatic secretagogues, amylase secretion, calcium transport, cyclic GMP
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANASTASI A., ERSPAMER V., CEI J. M. ISOLATION AND AMINO ACID SEQUENCE OF PHYSALAEMIN, THE MAIN ACTIVE POLYPEPTIDE OF THE SKIN OF PHYSALAEMUS FUSCUMACULATUS. Arch Biochem Biophys. 1964 Nov;108:341–348. doi: 10.1016/0003-9861(64)90395-9. [DOI] [PubMed] [Google Scholar]
- Anastasi A., Erspamer V., Endean R. Isolation and amino acid sequence of caerulein, the active decapeptide of the skin of hyla caerulea. Arch Biochem Biophys. 1968 Apr;125(1):57–68. doi: 10.1016/0003-9861(68)90638-3. [DOI] [PubMed] [Google Scholar]
- Christophe J. P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Binding of radioiodinated peptide. J Biol Chem. 1976 Aug 10;251(15):4629–4634. [PubMed] [Google Scholar]
- Christophe J. P., Frandsen E. K., Conlon T. P., Krishna G., Gardner J. D. Action of cholecystokinin, cholinergic agents, and A-23187 on accumulation of guanosine 3':5'-monophosphate in dispersed guinea pig pancreatic acinar cells. J Biol Chem. 1976 Aug 10;251(15):4640–4645. [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., De Neef P., Lammens M., Christophe J. In vitro action of bombesin and bombesin-like peptides on amylase secretion, calcium efflux, and adenylate cyclase activity in the rat pancreas: a comparison with other secretagogues. J Clin Invest. 1976 Oct;58(4):891–898. doi: 10.1172/JCI108542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Tsuruhara T., Horner K. A., Podesta E., Catt K. J. Intermediate role of adenosine 3':5'-cyclic monophosphate and protein kinase during gonadotropin-induced steroidogenesis in testicular interstitial cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3419–3423. doi: 10.1073/pnas.74.8.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P., Kleveman H. L., Adams T. D., Ondetti M. A. Action of cholecystokinin and cholinergic agents on calcium transport in isolated pancreatic acinar cells. J Clin Invest. 1975 Aug;56(2):366–375. doi: 10.1172/JCI108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Hahne W. F. Calcium transport in dispersed acinar cells from rat pancreas. Biochim Biophys Acta. 1977 Dec 15;471(3):466–476. doi: 10.1016/0005-2736(77)90050-5. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Jackson M. J. Regulation of amylase release from dispersed pancreatic acinar cells. J Physiol. 1977 Sep;270(2):439–454. doi: 10.1113/jphysiol.1977.sp011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Klaeveman H. L., Bilezikian J. P., Aurbach G. D. Effect of beta-adrenergic catecholamines on sodium transport in turkey erythrocytes. J Biol Chem. 1973 Aug 25;248(16):5590–5597. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. In vitro action of bombesin on amylase secretion, membrane potential, and membrane resistance in rat and mouse pancreatic acinar cells. A comparison with other secretagogues. J Clin Invest. 1978 Jan;61(1):41–46. doi: 10.1172/JCI108923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus L. H., Perrin M. H., Brown M. R. Mast cell binding of neurotensin. I. Iodination of neurotensin and characterization of the interaction of neurotensin with mast cell receptor sites. J Biol Chem. 1977 Oct 25;252(20):7174–7179. [PubMed] [Google Scholar]
- May R. J., Conlon T. P., Erspamer V., Gardner J. D. Actions of peptides isolated from amphibian skin on pancreatic acinar cells. Am J Physiol. 1978 Aug;235(2):E112–E118. doi: 10.1152/ajpendo.1978.235.2.E112. [DOI] [PubMed] [Google Scholar]
- Rivier J. E., Brown M. R. Bombesin, bombesin analogues, and related peptides: effects on thermoregulation. Biochemistry. 1978 May 2;17(9):1766–1771. doi: 10.1021/bi00602a030. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Conlon T. P., Gardner J. D. Interaction of porcine vasoactive intestinal peptide with dispersed pancreatic acinar cells from the guinea pig. Structural requirements for effects of vasoactive intestinal peptide and secretin on cellular adenosine 3':5'-monophosphate. J Biol Chem. 1976 Aug 10;251(15):4635–4639. [PubMed] [Google Scholar]
- Shelby H. T., Gross L. P., Lichty P., Gardner J. D. Action of cholecystokinin and cholinergic agents on membrane-bound calcium in dispersed pancreatic acinar cells. J Clin Invest. 1976 Dec;58(6):1482–1493. doi: 10.1172/JCI108605. [DOI] [PMC free article] [PubMed] [Google Scholar]


