Abstract
OBJECTIVE
To test cognitive behavioral therapy for adherence and depression (CBT-AD) in type 2 diabetes. We hypothesized that CBT-AD would improve adherence; depression; and, secondarily, hemoglobin A1c (A1C).
RESEARCH DESIGN AND METHODS
Eighty-seven adults with unipolar depression and uncontrolled type 2 diabetes received enhanced treatment as usual (ETAU), including medication adherence, self-monitoring of blood glucose (SMBG), and lifestyle counseling; a provider letter documented psychiatric diagnoses. Those randomized to the intervention arm also received 9–11 sessions of CBT-AD.
RESULTS
Immediately after acute treatment (4 months), adjusting for baseline, CBT-AD had 20.7 percentage points greater oral medication adherence on electronic pill cap (95% CI −31.14 to −10.22, P = 0.000); 30.2 percentage points greater SMBG adherence through glucometer downloads (95% CI −42.95 to −17.37, P = 0.000); 6.44 points lower depression scores on the Montgomery-Asberg Depression Rating Scale (95% CI 2.33–10.56, P = 0.002); 0.74 points lower on the Clinical Global Impression (95% CI 0.16–1.32, P = 0.01); and 0.72 units lower A1C (95% CI 0.29–1.15, P = 0.001) relative to ETAU. Analyses of 4-, 8-, and 12-month follow-up time points indicated that CBT-AD maintained 24.3 percentage points higher medication adherence (95% CI −38.2 to −10.3, P = 0.001); 16.9 percentage points greater SMBG adherence (95% CI −33.3 to −0.5, P = 0.043); and 0.63 units lower A1C (95% CI 0.06–1.2, P = 0.03) after acute treatment ended. For depression, there was some evidence of continued improvement posttreatment, but no between-group differences.
CONCLUSIONS
CBT-AD is an effective intervention for adherence, depression, and glycemic control, with enduring and clinically meaningful benefits for diabetes self-management and glycemic control in adults with type 2 diabetes and depression.
Introduction
Despite clear evidence linking glycemic control and risk for complications (1) ∼50% of adults with diabetes achieve glycemic control targets (A1C <7%) (2). Patient nonadherence to medications prescribed to treat diabetes is common (3) and clearly related to poor glycemic control, risk for hospitalization, and mortality (4). Clinical depression is highly prevalent in diabetes, being up to two times more common among patients with diabetes than those without (5). Depression in diabetes is not only distressing in and of itself but also consistently associated with poor adherence to self-care behaviors (6), worse glycemic control (7), complications (8–10), and mortality (11).
Although a few trials have tested the efficacy of treatments for depression in adults with diabetes with generally positive effects on depression, effects on health outcomes such as glycemic control and adherence are, at best, mixed (12). An early small trial of cognitive behavioral therapy (CBT) demonstrated an improvement in glycemic control (13), but subsequent larger trials of collaborative care have failed to impact glycemic control (14,15) or self-care and medication adherence (16). Accordingly, treating depression alone may not result in changes in health behaviors or outcomes; hence, an integrative approach may be necessary.
Adapting an approach used successfully in adults with HIV/AIDS (17,18), we integrated the treatment of depression and nonadherence (19–22) using CBT intervention strategies. The objective of the current study was to test, in a two-arm randomized controlled trial, CBT for adherence and depression (CBT-AD) combined with a series of diabetes self-management and adherence interventions, which we call enhanced treatment as usual (ETAU), versus ETAU alone in patients with uncontrolled type 2 diabetes and depression. We had two major hypotheses. First, we hypothesized that patients assigned to CBT-AD would have better adherence, decreased depression, and improved glucose control than those assigned to ETAU at immediately posttreatment (4 months). Second, we hypothesized that observed posttreatment between-group differences in these outcomes would be sustained over 8- and 12-month follow-up.
RESEARCH DESIGN AND METHODS
Design and Procedures
This was a 12-month single-blind randomized trial. All participants had ETAU. Accordingly, they met once with a nurse educator to set goals for self-monitoring blood glucose (SMBG), twice with a dietitian to set individualized diet and physical activity goals, and once with an adherence counselor to help with these self-management goals. There were two arms: 1) CBT-AD plus ETAU and 2) ETAU alone. Assessments were at baseline and 4 (immediately posttreatment), 8, and 12 months and took place at Massachusetts General Hospital (MGH) (Diabetes Center, Behavioral Medicine Service, and/or Clinical Research Program, Boston, MA). The period of recruitment was June 2007 to March 2011, with 1-year follow-up lasting until March 2012.
All procedures were reviewed and approved by the Institutional Review Board at MGH. All participants completed an informed consent process with a study clinician, including signing an informed consent form.
Participants
Eighty-seven adults between the ages of 18 and 70 with suboptimally controlled type 2 diabetes (physician defined as A1C >≈7% [53 mmol/mol]) despite treatment with an oral hypoglycemic and who also met DSM-IV (23) criteria for depression (66 current major depressive episode, 11 current dysthymic disorder, 10 major depression in partial remission and prescribed antidepressant treatment) were enrolled. Antidepressants for depression, oral hypoglycemic medications, and insulin all needed to be stable for 2 months. The Mini International Neuropsychiatric Inventory (24) was used to establish baseline psychiatric diagnoses. The assessment of depression during baseline was conducted by a study clinician (master- or doctoral-level psychologist trained through audiotape supervision in the treatment and assessment protocols). Other treatments for depression, such as medications, were allowed to be continued or started by usual care providers, if indicated. If at any visit treatment for depression beyond what the study provided was clinically indicated or if self-report depression scores increased by 25% compared with the average of their past two scores, participants were given appropriate referrals (e.g., additional therapy, medication evaluation) and continued to be followed per the assessment schedule. Participants who experienced severe depression (i.e., requiring intensive treatment such as hospitalization) were dropped from the study. Participants who had active untreated major mental illness (e.g., untreated psychosis), bipolar disorder, eating disorder, mental retardation, dementia, or active suicidality; were unable or unwilling to provide informed consent; or had a history of or were undergoing current CBT for depression were excluded.
Interventions
ETAU (All Participants)
Provider Letter and Monitoring of Depression
After the baseline assessment, a letter was sent to each patient’s health-care provider regarding any psychiatric diagnoses for which the patient met criteria. The letter stated that participation in the study should not alter the provider’s normal course of assessment or treatment for these conditions. At follow-up assessments, if clinically indicated, referrals for depression treatment were also provided. Referrals were made if the participant’s self-reported depression scores increased by 25% compared with the average of the prior two and were also determined on a case-by-case basis, including but not limited to situations such as suicidal ideation.
Nurse and Dietitian Visits
Before randomization, the nurse diabetes educator met with each patient for diabetes self-management education and counseling. The goal of these visits was to establish tailored goals for diabetes self-care, including medication adherence (for both oral medications and insulin), glucose monitoring, exercise, and foot care. The dietitian conducted a nutritional assessment and then set two individualized nutrition goals and one activity goal that were selected based on the likelihood of impacting glucose control and each participant’s self-confidence in his or her ability to achieve them. These activities were evaluated and revised as necessary at the second visit after randomization.
Adherence Counseling
All participants had one session of Life-Steps (25,26), a stand-alone CBT intervention designed to improve adherence to medical recommendations and individualized diabetes self-management goals set by the dietitian and nurse. In this intervention, after brief discussion about patient-generated reasons for engaging in treatment, 11 cognitive and behavioral steps to adherence are discussed (e.g., setting a daily schedule, having reminder cues for medications, managing getting to appointments), and the interventionist and patient generate a plan and backup plan for each (see Supplementary Data).
CBT-AD
The intervention group then participated in CBT-AD delivered across 9–12 sessions, with the Life-Steps intervention delivered as session 1. For additional details, see the Supplementary Data and/or published treatment manuals (19,20). The subsequent modules included 1) introducing the patient to the nature of CBT and motivational interviewing for behavior change (approximately one session); 2) increasing pleasurable activities and mood monitoring (approximately one session); 3) thought monitoring and cognitive restructuring (adaptive thinking) (approximately five sessions); 4) problem-solving as a skill to aid in decision-making processes, particularly those related to diabetes self-care (approximately two sessions); and 5) relaxation training (approximately two sessions). The therapist and participant were able to structure the number of sessions spent on each module to meet the participant’s individual needs. For all modules, participants were encouraged to apply these skills generally, but they were linked to diabetes self-care whenever possible. Participants were offered up to two booster sessions, which usually happened at the time of the 8- and 12-month follow-up assessments. For additional details, see the Supplementary Data and/or published treatment manuals (19,20).
Outcomes
Medication Adherence
Each patient was given a medication event monitoring system (MEMS; AARDEX Inc.) electronic pill cap, which fits on a medication bottle and registers each time the patient’s medication bottle is opened. This system allowed us to calculate the percentage of doses taken. A corrected score was used if participants could recall times when they took pills but did not use the bottle (17,18,27–29). Participants informed study staff of changes to medications during the course of the study, and this was accounted for in the calculation of these adherence scores.
Adherence to Glucose Monitoring
One Touch Ultra meters (LifeScan, Inc.) for daily glucose control also provided frequency of self-monitoring, which compared with the individualized goals from the nurse visits, also yielded a percentage (30,31) adherence score.
The Montgomery-Asberg Depression Rating Scale
The Montgomery-Asberg Depression Rating Scale (MADRS) (32) is a structured, validated, 10-item interview that was completed by the blinded assessor.
Clinical Global Impression
The blinded assessor also used the Clinical Global Impression (CGI) (1 = not ill, 7 = extremely ill) (33), a single-item, valid, and reliable measure of the severity of global impairment and distress related to depression.
Assessment of Diabetes Control
Assessment of diabetes control was determined by measurement of A1C, at the MGH laboratory, one of the reference laboratories for the National Glycohemoglobin Standardization Program (34,35), using a Bio-Rad Variant II Turbo (normal range 3.8–6.4% [18–46 mmol/mol]). No point-of-care A1C tests were used.
Sample Size
The study was powered for adherence and depression outcomes according to our initial work in HIV (17), which had effect sizes in excess of 1.0 for these outcomes. The recruitment goal was 100 completers, which would have allowed for an excess of 95% power to detect an effect size of 1.0 for adherence and depression with a general linear model (GLM) ANOVA approach for the posttreatment effect. We experienced slower-than-anticipated recruitment but still had sufficient power to conduct the analyses with 87 participants.
Randomization
We stratified randomization based on prescription of medication (oral medication, insulin, or both), sex, and CGI score (≥3 cutoff) in blocks of four. The randomization sequence was generated by the data manager; participants were assigned to a study arm by the research assistant. Study interventionists conducted the enrollment visits.
Blinding
Participants, study assessors, interventionists, and dietitians were blinded to study assignment during the baseline visit, the three nurse/dietitian visits, and the adherence counseling visit.
Statistical Methods
The two sets of analyses corresponded to the two study hypotheses: 1) CBT-AD will result in superior adherence, depression, and glucose control, and 2) the benefits will be maintained over 8 months. All analyses used SPSS version 19 or 20 statistical software and followed intention-to-treat principles. For significant differences, parameter estimates (B) are in the units of the measure that best describe the clinical implication of the results.
The first set of analyses corresponds to the first hypothesis that the CBT-AD condition would have superior outcomes at the end of acute treatment. Accordingly, we evaluated between-group differences at the 4-month assessment (i.e., immediately posttreatment), controlling for baseline scores. To allow for intention-to-treat principles, we used GLMs with multiple imputation to provide conservative estimates for missing data. (Completer analyses revealed a similar pattern of results, although with stronger P values and larger parameter estimates.) For the first set of analyses, we hypothesized that the CBT-AD condition would have lower depression, higher adherence, and lower A1C than the ETAU condition.
The second set of analyses corresponds to the second hypothesis that those who participated in the treatment would maintain their benefit. Accordingly, we evaluated the follow-up data by mixed-effects modeling with the 4-, 8-, and 12-month data. These models did not include baseline values. They contained a term for treatment condition, which measured the difference between the two conditions over the entire follow-up period; a term for time, which measured the extent to which the values decreased or increased over time averaged over both conditions; and an interaction term, which measured whether the change with time, after acute treatment was discontinued, was different in the two treatments over the follow-up period. The purpose of these analyses was to examine whether there was an effect for the treatment condition term. Accordingly, we hypothesized that there would be a significant main effect for treatment condition such that after acute treatment discontinuation, the CBT-AD condition would maintain lower depression, higher adherence, and lower A1C than the comparison condition. Additionally, we hypothesized that there would not be an effect for time after acute treatment discontinuation, improvements in the CBT condition would not wane, and the comparison condition would not make changes after the 4-month assessment. Finally, we hypothesized that there would not be a significant effect for the interaction of time by condition during this follow-up period such that both groups would maintain scores similar to those at the 4-month assessment.
Results
Participant Characteristics and Flow
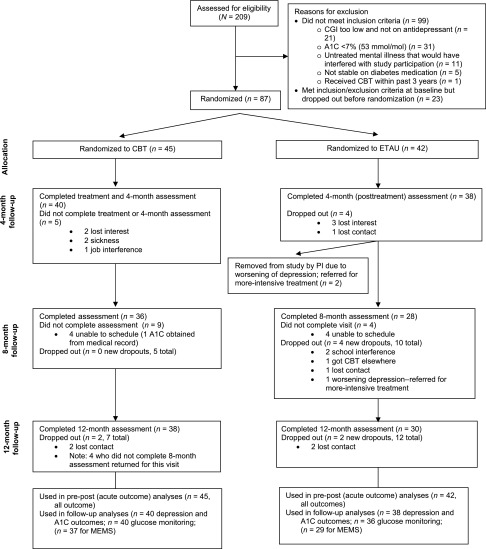
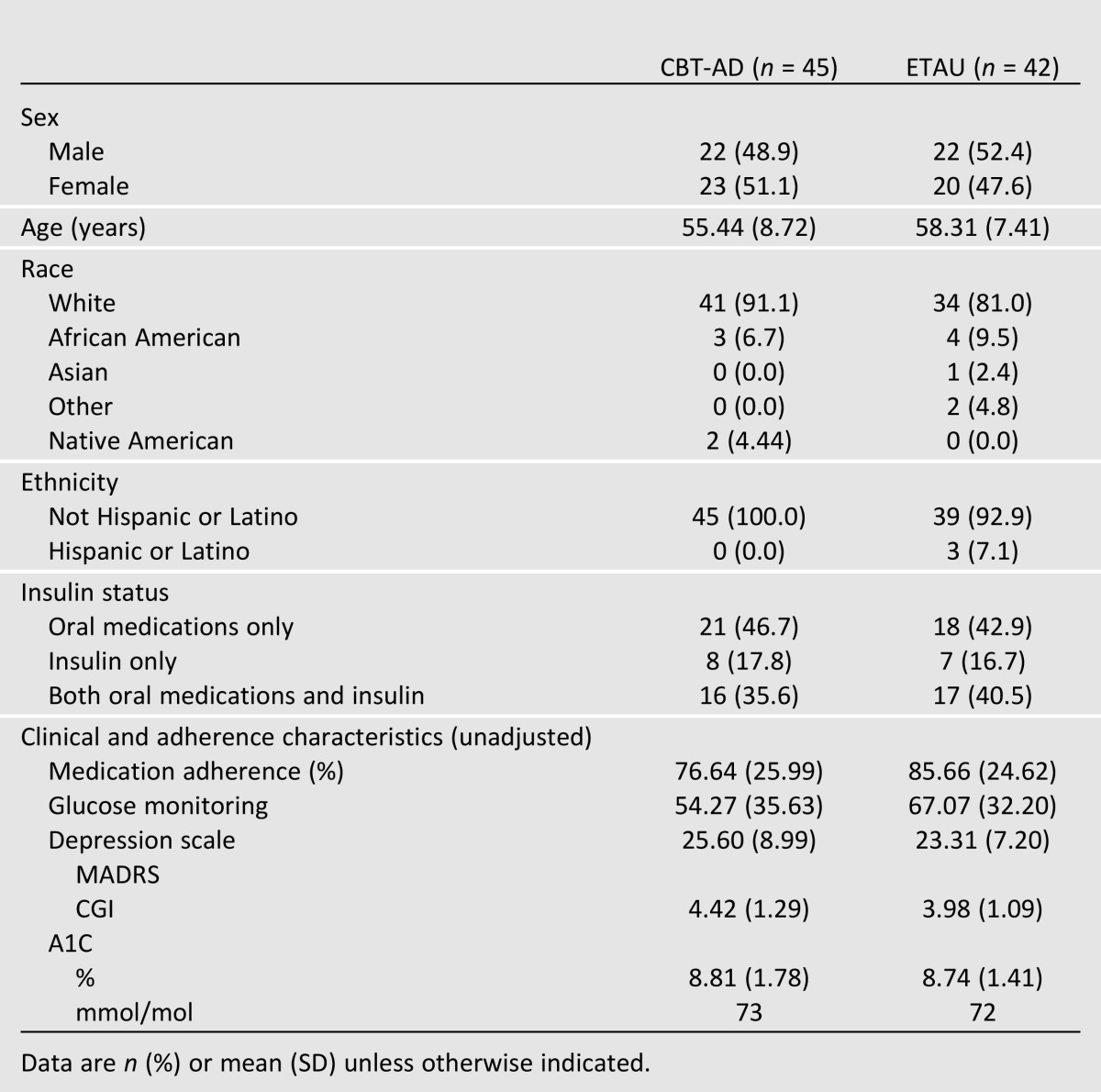
A CONSORT (Consolidated Standards of Reporting Trials) diagram of participant flow is shown in Fig. 1. Table 1 lists the baseline characteristics for those randomized. None of the demographic or outcome data differed by treatment arm. For the posttreatment outcome, retention was 90%, and 83% of participants returned for either the 8- or 12-month follow-up. Three participants (all in ETAU) were dropped because of severe depression requiring more-intensive treatment (two at 4 months and one at 8 months). There were no study-related adverse events.
Figure 1.
Participant flow diagram. PI, principal investigator.
Table 1.
Baseline demographics and outcomes by study condition
Posttreatment (Acute/4-Month) Outcomes
Adherence
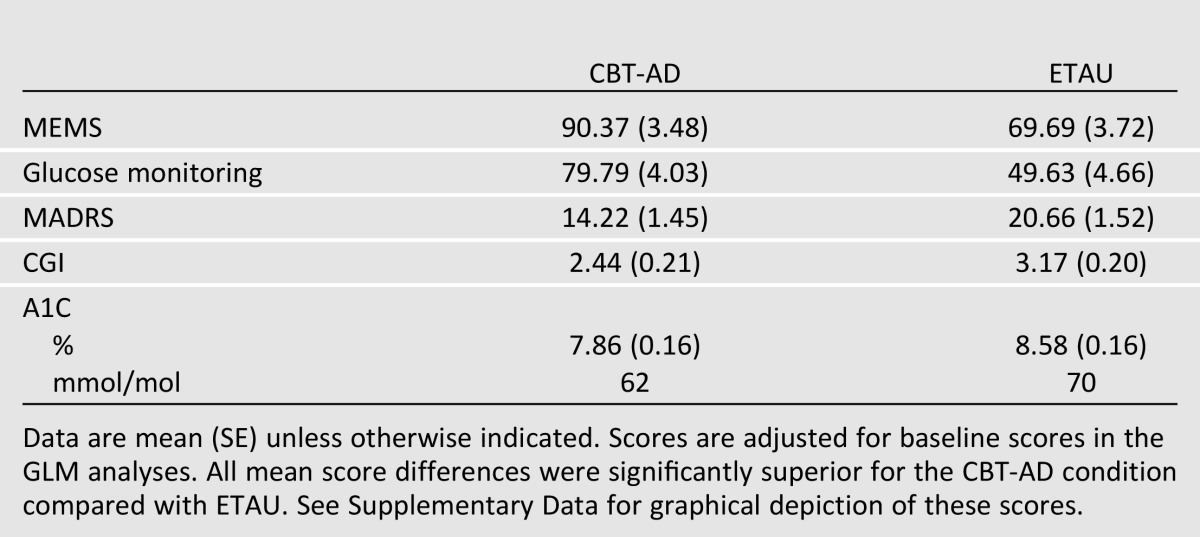
At posttreatment, controlling for baseline, the CBT-AD arm had 20.7 percentage points higher adherence to medications as assessed by electronic pill cap (95% CI −31.14 to −10.22, P = 0.000) and 30.2 percentage points higher electronically assessed 2-week SMBG (95% CI −42.95 to −17.37, P = 0.000) than the ETAU arm (Table 2).
Table 2.
Adjusted posttreatment (4-month) outcome scores by treatment arm
Depression
Controlling for baseline, the CBT-AD arm had 6.22 lower depression scores on the MADRS than the ETAU arm (95% CI 2.33–10.56, P = 0.002) and 0.74 lower ratings on the CGI (95% CI 0.16–1.32, P = 0.01), where lower scores indicate less depression on both scales (Table 2).
Diabetes Control
Controlling for baseline, the CBT-AD arm had superior glycemic control as indicated by a 0.72 difference in A1C compared with the ETAU group (95% CI 0.29–1.15, P = 0.001).
Follow-up/Maintenance of Gains
Adherence
For medication adherence, a significant main effect for study arm indicated that the CBT-AD arm maintained 24.3 percentage points higher medication adherence than the ETAU arm during the follow-up period (95% CI −38.2 to −10.3, P = 0.001) (Table 3). The main effect for time was not significant (P = 0.14), indicating that adherence gains did not significantly decline or improve from the time of treatment discontinuation to the follow-ups. The interaction of time by study arm was not significant (P = 0.09), indicating that the nonsignificant main effect for time over the follow-up period was not different by study arm; after acute treatment ended, both groups did not differ at months 8 or 12 from the scores they had right after the acute treatment ended (i.e., month 4).
Table 3.
Adjusted follow-up outcome scores by study condition
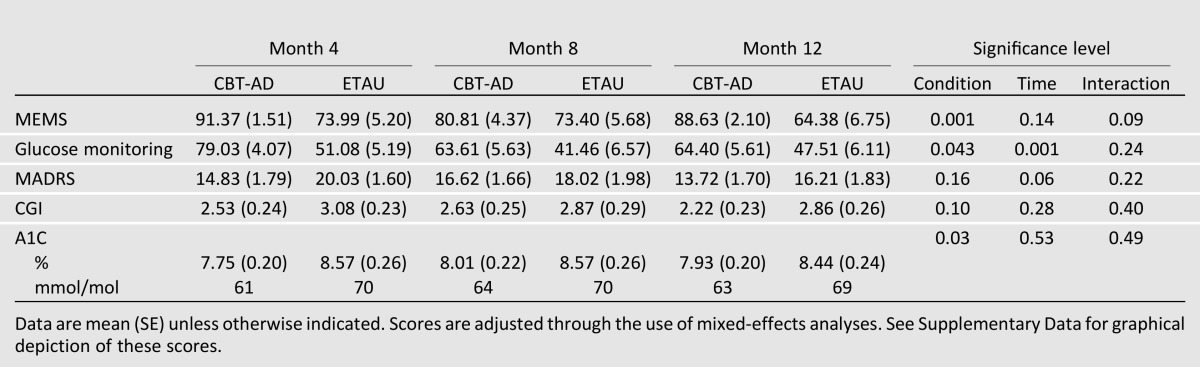
For adherence to glucose monitoring goals, a significant main effect for study arm indicated that the CBT-AD arm maintained 16.9 percentage points better glucose monitoring adherence than the ETAU arm during the follow-up period (95% CI −33.3 to −0.5, P = 0.043). A significant effect for time (P = 0.001), however, indicated some decline over follow-up, with scores at month 8 being 12.5 percentage points lower than at month 4 (95% CI 5.9–19.1, P = 0.001) and 9.1 percentage points lower at month 12 than at month 4 (95% CI 2.7–15.5, P = 0.006), without significant differences between 8 and 12 months (B = −3.4, 95% CI −9.5 to 2.7, P = 0.27). The interaction effect between time by study arm was not significant (P = 0.24), indicating that these declines over time after acute treatment ended (at month 4) did not differ by study arm (i.e., both groups had decreased adherence to SMBG goals over time, but the CBT-AD arm remained superior).
Depression
For the MADRS, there were no significant effects for study arm (P = 0.16) during the follow-up period. There was a trend, however, toward continued improvement over follow-up for both study arms (P = 0.06). Depression scores were 2.4 points lower at month 12 than at month 8 (95% CI 0.23–4.5, P = 0.03) and 2.5 points lower at month 12 than at month 4 (95% CI 0.10–4.8, P = 0.04), without significant differences between 4 and 8 months (B = 0.10, 95% CI −2.1 to 2.3, P = 0.9). The interaction was not significant, indicating that these time point improvements did not differ by study arm (P = 0.22). Accordingly, although the trend for improvement occurred in both conditions, the better scores in the CBT-AD condition were not sustained after acute treatment discontinuation.
For the CGI, there was an absence of main effects for study arm (P = 0.10) during the follow-up period. There also were not effects for time (P = 0.28), indicating that scores did not significantly decline or improve after the treatment ended. The interaction effect was also not significant (P = 0.40), indicating that the nonsignificant effects for time did not differ by study arm during follow-up. Accordingly, although the CBT-AD condition did not have significantly worsening of scores after acute treatment discontinuation, the superiority over the control condition was no longer significant in the follow-up period.
Diabetes Control
The CBT-AD arm maintained superior glycemic control compared with the ETAU arm, as indicated by 0.63 lower A1C values (95% CI 0.06–1.2, P = 0.03), over the follow-up. The main effects for time were not significant (P = 0.53), indicating maintenance of gains from the time of treatment discontinuation to month 8 or month 12. The interaction effect was not significant (P = 0.49), indicating that the nonsignificant effects for time did not differ by study arm during follow-up. Accordingly, the CBT-AD condition retained superior A1C scores during the follow-up period.
Conclusions
After completing 4 months of CBT-AD, patients with uncontrolled type 2 diabetes and depression had lower A1C (0.72-unit reduction), 21 percentage points better medication adherence, and 30 percentage points better adherence to SMBG than patients assigned to ETAU. For glycemic control, the effect of the intervention was comparable to the addition of a weak hypoglycemic medication (36). These gains were maintained over the 8 months of follow-up (after the acute delivery of intervention), with between-group differences evident for glycemia and the two adherence outcomes.
For depression, right after acute treatment ended at the 4-month assessment, those who received CBT-AD had scores that were 6.44 points better on the MADRS and 0.74 points better on the CGI for depression than the ETAU participants, indicating significantly lower levels of depression in the CBT-AD group. Although mean scores over time revealed continued improvement for both conditions, there was a lack of between-group difference in the postintervention follow-up period. At least two possible conclusions can be drawn from these data. The first is that the effects of CBT-AD on depression were not robust enough to be maintained over time relative to alerting providers of depression diagnoses. However, depression scores in the intervention condition did not worsen during the follow-up, and data showed a trend for continued improvement in scores on the MADRS. Furthermore, scores did not change differently across the two conditions during the follow-up. Therefore, the second possible conclusion is that this pattern of results could be related to the study design. Whenever clinically indicated, participants were referred for further care for depression. At baseline, referring primary care providers were informed that depression had been detected in ETAU participants. Despite this, there were significant group differences on both depression outcomes. At follow-up, study staff (not primary care providers) directly referred ETAU participants with clinically significant depression for treatment outside the study. Additionally, participants who were severely depressed (n = 3, all in the ETAU group) were dropped from the study at month 4 (or any other time point, but it only happened at month 4) for ethical reasons and referred to more-intensive care. This ethical consideration may have affected the distribution of scores in the ETAU group, with the most severe scores for depression no longer being present. If it were not for this needed design, we may have seen stronger effects for depression. The maintenance of clinically significant effects on diabetes self-management and glycemic control over the follow-up, despite the lack of maintenance of the depression effects, suggests some degree of independence between these outcomes. This may also explain why previous trials that were successful in treating depression in diabetes failed to demonstrate corresponding benefits for self-management or glycemic control (14–16).
One difference between this study and earlier investigations of treatments for depression in diabetes (12–16) is that the current study integrated adherence counseling into the psychosocial treatment of depression versus treating depression without also specifically targeting adherence. The rationale for this integrative approach was based on the documented association of depression and poor diabetes treatment adherence (6) and the failure of previous trials to consistently impact glycemic control or diabetes self-management (12). Since the initiation of this trial, we are aware of two others that have taken a similarly integrative approach. One was successful in improving depression, medication adherence, and glycemic control for patients already prescribed antidepressants (37), and the second failed to impact self-management or medication adherence but did affect glycemic control and depression (38). However, the second trial differentially provided medications for depression, glycemic control, blood pressure, and lipids to experimental participants relative to controls, which may have accounted for glycemic benefits observed in the absence of improvements in self-management (39). The current results, therefore, add to the evidence base for the conclusion that treating depression may be necessary but not sufficient to improve diabetes outcomes in depressed adults with type 2 diabetes. Treating depression may allow patients to maximally benefit from adherence/self-care interventions such as those provided to both the experimental intervention and the comparison arms of the current study. The magnitude of the present effects suggests that such an approach could have clinically meaningful benefits for glycemic control.
The study results have several limitations to note. First, the study was meant to examine whether treating depression with an evidenced-based and comprehensive approach would be superior to ETAU, which included nurse and dietitian counseling and adherence counseling. We chose this approach because CBT is a widely studied treatment for depression, and well-designed depression treatment trials in diabetes had already demonstrated that depression treatment did not consistently result in improved glycemic control or diabetes self-management (14,15,39). Therefore, we sought to test an integrative approach in a design that could evaluate whether a more time-intensive intervention would have greater efficacy on a comprehensive set of depression and diabetes outcomes relative to less-intensive, but still significant enhancements to usual care. This design allowed for greater translation of the findings to clinical practice in that there were no artificial restrictions on additional treatment in either arm, and the comparison group we evaluated more closely represents models of care that could be implemented in practice. However, our design cannot address questions about the role of increased attention and nonspecific support provided as part of the intervention. Second, the MEMS cap used for the medication adherence outcome may have underestimated true adherence in that participants could take pills and not use the cap. To correct for this, we asked participants at study visits whether they recalled taking the pills without using the cap and used a corrected adherence score (27–29). Third, the sample was 86% white; hence, additional study is needed to extend findings to racial or ethnic minorities. Fourth, although brief for psychotherapy, the treatment was intensive, and the study design required participants to meet criteria for a depressive disorder. Subsequent work suggests that the relationship between depression and worse outcomes in diabetes is incremental and that patients with subclinical depression could also benefit from treatment (40). Finally, we were not able to include use of antidepressants as a variable in the analyses because of variability in dose, type of medicine, timing of any changes, and lack of measuring adherence. Including antidepressant use systematically may have increased the precision of the estimate of the effect of the intervention over and above ETAU, which can include the use of antidepressants.
In conclusion, given the high prevalence of depression in patients with diabetes and the association of depression with poor adherence and outcomes, interventions to treat depression and improve adherence could improve diabetes care. Overall, these results suggest that a behavioral intervention for depression and diabetes treatment nonadherence (CBT-AD) (19,20) is effective for managing depression and treatment nonadherence and improving glycemic control in depressed adults with type 2 diabetes.
Author Notes
Of the 87 participants randomized, 84 had A1C levels of ≥7.0 (53 mmol/mol). Three participants had A1C approaching 7.0 on study blood draw but a self-reported A1C >7.0 (6.8 [51 mmol/mol], 6.9 [52 mmol/mol], and 6.9 [52 mmol/mol]). These participants were entered into the study with a protocol exception because the study blood draw A1C values were considered essentially equivalent to 7.0.
Although we originally planned to also use average blood glucose values downloaded from participants’ glucometers as an additional indicator of glycemic control, experience with the initial participants and in the pilot demonstrated that this procedure was not feasible because values depend on timing of monitoring and whether the participant was fasting. In light of the burden to participants and the potential for error of reporting of fasting/not fasting, we dropped this outcome in favor of A1C, which is a superior indicator of glycemic control over time.
We monitored progress on depression at study visits with the Center for Epidemiologic Studies Depression Scale.
The full protocol may be accessed by request to the corresponding author.
Supplementary Material
Article Information
Acknowledgments. The authors thank the following individuals who assisted with the project: 1) Research assistants who are were at MGH when working on the study and are now at different institutions: Nicholas Perry, University of Utah; Matthew Carpenter, Temple University; Jesse Wilkinson, Stony Brook University, State University of New York; Luis Serpa, The University of North Carolina at Chapel Hill; Erin Marie Conklin, Emory University; and Lauren McCarl-Dutra, Harvard School of Public Health. 2) Study nurses and nutritionists: Tiffany Soper and Valerie Goldman, MGH Diabetes Center. 3) Patrick Lustman, Washington University School of Medicine, for consultation on the study design and implementation. 4) Additional study staff (therapists, assessors, data manager): Jonathan Lerner, Lara Traeger, C. Andres Bedoya, and Allison Labbe, MGH/Harvard Medical School; Adam Gonzalez, MGH/Harvard Medical School and Stony Brook University, The State University of New York; Laura Knouse, MGH/Harvard Medical School and University of Richmond; Sarah Markowitz, MGH/Harvard Medical School and Wells College; and Ellen Hendriksen, MGH/Harvard Medical School and Stanford University.
Funding. This project was supported by National Institute of Mental Health grant R01-MH-078571 (principal investigator: S.A.S.). Additional support came from the Harvard Catalyst, Harvard Clinical and Translational Science Center, by National Institutes of Health grant 1UL1-RR-025758-03 for a portion of the nurse and dietitian study visits. J.S.G. is partially supported by grant DK-020541. D.J.W. was supported by a National Institute of Diabetes and Digestive and Kidney Diseases Career Development Award (K23-DK-080228). A.J.B. is supported by grant 1K23-MH-096647.
Duality of Interest. Additional support came from the Investigator-Initiated Study Program of LifeScan, Inc., in the form of donated glucometers and glucose test strips. S.A.S. and J.S.G. receive royalties from Oxford University Press, and S.A.S. receives royalties from Guilford Publications for authored books. C.P. has served as a consultant to Bracket Global, specifically, providing independent reviews of assessment measures completed as part of clinical trials and training on structured clinical interviews for depression. L.M.D. is a consultant for Eli Lilly and Pfizer. This work is unrelated to the submitted manuscript. No other potential conflicts of interest relevant to this article were reported.
The sponsor had no role in the collection, management, analysis, interpretation of data, or preparation of the manuscript. LifeScan, Inc. reviewed and approved the manuscript before submission.
Author Contributions. S.A.S., principal investigator, contributed to the study concept, acute outcome analyses, drafting of the manuscript, and integration of coauthor comments and edits. J.S.G. contributed to the study concept, shaping of the CBT-AD intervention, clinical supervision of study therapists, and editing of the manuscript, particularly the Introduction and Conclusions sections. D.J.W. contributed to the background research, study operations, and editing of the manuscript. C.P. contributed greatly in study operations and implementation, drafted the Research Design and Methods section, and contributed to editing the manuscript. L.M.D. contributed to the study design, particularly that of the nutritionist- and nurse-delivered interventions, and editing of the manuscript. A.J.B. contributed to the follow-up analyses and writing of the Results section. A.I.M. contributed to editing the manuscript and provided support throughout the submission process. E.C. contributed to the biomedical conceptualization of the study, study design operations and interface with the diabetes clinic, and editing of the manuscript. S.A.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Acute outcomes were presented at the Association for Behavioral and Cognitive Therapies Convention, National Harbor, MD, 15–18 November 2012. Follow-up outcomes were presented at the 34th Annual Meeting & Scientific Sessions of the Society of Behavioral Medicine, San Francisco, CA, 20–23 March 2013.
Footnotes
Clinical trial reg. no. NCT00564070, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0816/-/DC1.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health/National Institute of Mental Health; LifeScan, Inc.; or the Harvard Catalyst.
References
- 1.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013;36:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008;31:81–86 [DOI] [PubMed] [Google Scholar]
- 3.Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with type 2 diabetes: a retrospective cohort study. Diabet Med 2002;19:279–284 [DOI] [PubMed] [Google Scholar]
- 4.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836–1841 [DOI] [PubMed] [Google Scholar]
- 5.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2006;23:1165–1173 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care 2008;31:2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000;23:934–942 [DOI] [PubMed] [Google Scholar]
- 8.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001;63:619–630 [DOI] [PubMed] [Google Scholar]
- 9.Lin EHB, Heckbert SR, Rutter CM, et al. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med 2009;7:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez JS, Vileikyte L, Ulbrecht JS, et al. Depression predicts first but not recurrent diabetic foot ulcers. Diabetologia 2010;53:2241–2248 [DOI] [PubMed] [Google Scholar]
- 11.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care 2005;28:2668–2672 [DOI] [PubMed] [Google Scholar]
- 12.Markowitz SM, Gonzalez JS, Wilkinson JL, Safren SA. A review of treating depression in diabetes: emerging findings. Psychosomatics 2011;52:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 1998;129:613–621 [DOI] [PubMed] [Google Scholar]
- 14.Williams JW, Jr, Katon W, Lin EHB, et al. IMPACT Investigators The effectiveness of depression care management on diabetes-related outcomes in older patients. Ann Intern Med 2004;140:1015–1024 [DOI] [PubMed] [Google Scholar]
- 15.Katon WJ, Von Korff M, Lin EHB, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042–1049 [DOI] [PubMed] [Google Scholar]
- 16.Lin EHB, Katon W, Rutter C, et al. Effects of enhanced depression treatment on diabetes self-care. Ann Fam Med 2006;4:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol 2009;28:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: a randomized controlled trial. J Consult Clin Psychol 2012;80:404–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safren S, Gonzalez J, Soroudi N. Coping with Chronic Illness: A Cognitive-Behavioral Therapy Approach for Adherence and Depression Workbook ed. New York, Oxford University Press, 2007. [Google Scholar]
- 20.Safren S, Gonzalez J, Soroudi N. Coping with Chronic Illness: A Cognitive-Behavioral Approach for Adherence and Depression Therapist Guide. 1st ed. New York, : Oxford University Press, 2007 [Google Scholar]
- 21.Gonzalez JS, McCarl LA, Wexler D DD, et al. Cognitive behavioral therapy for adherence and depression (CBT-AD) in type 2 diabetes. J Cogn Psychother 2010;24:329–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz SM, Carper MM, Gonzalez JS, Delahanty LM, Safren SA. Cognitive-behavioral therapy for the treatment of depression and adherence in patients with type 1 diabetes: pilot data and feasibility. Prim Care Companion CNS Disord. 2012;14(2). [DOI] [PMC free article] [PubMed]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision Washington, DC, American Psychiatric Association, 2000. [Google Scholar]
- 24.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(Suppl. 20):22–33; quiz 34–57 [PubMed] [Google Scholar]
- 25.Safren SA, Otto MW, Worth JL. Life-Steps: applying cognitive behavioral therapy to HIV medication adherence. Cognit Behav Pract 1999;6:332–341 [Google Scholar]
- 26.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther 2001;39:1151–1162 [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med 2001;134:968–977 [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Miller LG, Hays RD, et al. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr 2006;41:315–322 [DOI] [PubMed] [Google Scholar]
- 29.Llabre MM, Weaver KE, Durán RE, Antoni MH, McPherson-Baker S, Schneiderman N. A measurement model of medication adherence to highly active antiretroviral therapy and its relation to viral load in HIV-positive adults. AIDS Patient Care STDS 2006;20:701–711 [DOI] [PubMed] [Google Scholar]
- 30.Goodall TA, Halford WK. Self-management of diabetes mellitus: a critical review. Health Psychol 1991;10:1–8 [DOI] [PubMed] [Google Scholar]
- 31.Kurtz SM. Adherence to diabetes regimens: empirical status and clinical applications. Diabetes Educ 1990;16:50–59 [DOI] [PubMed] [Google Scholar]
- 32.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389 [DOI] [PubMed] [Google Scholar]
- 33.Guy W. Clinical global impressions. In ECDEU Assessment Manual for Psychopharmacology Rockville, MD, U.S. Department of Health, Education, and Welfare, 1976, p. 217–222
- 34.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med 1984;310:341–346 [DOI] [PubMed] [Google Scholar]
- 35.Singer DE, Coley CM, Samet JH, Nathan DM. Tests of glycemia in diabetes mellitus. Their use in establishing a diagnosis and in treatment. Ann Intern Med 1989;110:125–137 [DOI] [PubMed] [Google Scholar]
- 36.Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care 2010;33:1859–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogner HR, Morales KH, de Vries HF, Cappola AR. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med 2012;10:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katon WJ, Lin EHB, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin EH, Von Korff M, Ciechanowski P, et al. Treatment adjustment and medication adherence for complex patients with diabetes, heart disease, and depression: a randomized controlled trial. Ann Fam Med 2012;10:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care 2007;30:2222–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.