Abstract
OBJECTIVE
Controversy exists about the coronary artery disease (CAD) risk conveyed by diabetes in young and middle-aged women. We investigated sex differences in CAD by diabetes status among healthy individuals with different underlying risks of heart disease.
RESEARCH DESIGN AND METHODS
We examined subjects aged <60 years without CAD at enrollment in the high-risk GeneSTAR Study (n = 1,448; follow-up ∼12 years), Multi-Ethnic Study of Atherosclerosis (MESA; n = 3,072; follow-up ∼7 years), and National Health and Nutrition Examination Survey III (NHANES III) Mortality Follow-up Study (n = 6,997; follow-up ∼15 years). Diabetes was defined by report, hypoglycemic use, and/or fasting glucose ≥126 mg/dL. The outcome was any CAD event during follow-up (fatal CAD in NHANES).
RESULTS
In the absence of diabetes, CAD rates were lower among women in GeneSTAR, MESA, and NHANES (4.27, 1.66, and 0.40/1,000 person-years, respectively) versus men (11.22, 5.64, and 0.88/1,000 person-years); log-rank P < 0.001 (GeneSTAR/MESA) and P = 0.07 (NHANES). In the presence of diabetes, CAD event rates were similar among women (17.65, 7.34, and 2.37/1,000 person-years) versus men (12.86, 9.71, and 1.83/1,000 person-years); all log-rank P values > 0.05. Adjusting for demographics, diabetes was associated with a significant four- to fivefold higher CAD rate among women in each cohort, without differences in men. In meta-analyses of three cohorts, additionally adjusted for BMI, smoking, hypertension, HDL, and non-HDL cholesterol, antihypertensive and cholesterol-lowering medication use, the hazard ratio of CAD in men versus women among nondiabetes was 2.43 (1.76–3.35) and diabetes was 0.89 (0.43–1.83); P = 0.013 interaction by diabetes status.
CONCLUSIONS
Though young and middle-aged women are less likely to develop CAD in the absence of diabetes, the presence of diabetes equalizes the risk by sex. Our findings support aggressive CAD prevention strategies in women with diabetes and at similar levels to those that exist in men.
Introduction
Type 2 diabetes is a potent risk factor for coronary artery disease (CAD) over the life span. The Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) describes diabetes as a CAD risk equivalent (1) that increases the rate of first CAD events by two- to threefold in both men and women (2). This rate is similar to recurrent events in persons without diabetes who have a prior CAD event (3). The presence of diabetes thus represents a guidelines imperative for aggressive CAD prevention strategies.
More recent studies suggest that type 2 diabetes increases the risk of CAD mortality more in women than men (4–6), by up to 50% (7). Further, diabetes may signify a greater risk of CAD events than having prior CAD in women but not men (8,9). These sex differences may be due to a greater adverse effect of diabetes on cardiovascular risk factors in women (10). Conversely, another meta-analysis found no significant sex differences in the relative risk associated with diabetes for CAD mortality after adjusting for traditional cardiovascular risk factors, but instead a greater absolute CAD mortality rate in men with diabetes at every age except the very old (11). Likely, different population characteristics account for the sex differences observed in these various studies. Specifically, inconsistent results could be due to the inclusion of a larger number of older persons, for whom different absolute risks exist for CAD than in younger persons, even without diabetes. In older age groups, women clearly have a high rate of CAD relative to their risk at younger ages. Thus, age may obviate the impact of diabetes on CAD risk in women. However, previous studies have not focused specifically on sex differences in CAD risk among younger and middle-aged persons with diabetes.
The importance of understanding sex differences becomes apparent when examining current guidelines for primary prevention of CAD in diabetes (12–14), which in general favor more aggressive therapy in men than women. For instance, initiation of aspirin is recommended in men a decade earlier than women with diabetes in the most recent position statement by the American Diabetes Association on this topic (14). Previous studies in the general population suggest that CAD has a later onset in women (15,16). The American Diabetes Association Standards of Medical Care (2013) also cites studies demonstrating that the effectiveness of aspirin for primary prevention in diabetes may be greater in men (12,17) and acknowledges that the risk of gastrointestinal bleeding in those at low CAD risk may outweigh any potential benefit of aspirin preventive therapy (18). Consequently, understanding any excess risk conveyed by diabetes in women has important public health and clinical practice implications. Thus, this study was designed to determine the relative and absolute risk of CAD among young and middle-aged adults with diabetes in three different large populations with different underlying risks for heart disease.
Research Design and Methods
Our study included participants from GeneSTAR (enrolled 1993–2005), the Multi-Ethnic Study of Atherosclerosis (MESA) (enrolled 2000–2011), and the National Health and Nutrition Examination Survey III (NHANES III) Mortality Follow-up (enrolled 1988–2006). Participants with history of CAD or early CAD during the study (who may have other risk factors) or lost to follow-up before 35 years of age were excluded. Specifically, in GeneSTAR, of 1,478 participants enrolled, all were under the age of 60 years without CAD at baseline. Exclusions were as follows: early CAD during the study (n = 6), missing baseline fasting glucose measurement (n = 19), and no follow-up after baseline (n = 5), leaving a final sample of 1,448 participants from GeneSTAR for the current study. In MESA, of 6,814 participants enrolled, 3,714 participants were aged ≥60 years and excluded. None had CAD at baseline or early CAD. Other exclusions included: missing baseline fasting glucose (n = 15) or no follow-up after baseline (n = 13), leaving 3,072 participants from MESA for the current study. In NHANES III, of 10,492 participants enrolled, 3,286 were aged ≥60 years and excluded. Additional exclusions included: CAD at baseline (n = 204) and early CAD during the study (n = 5), leaving 6,997 participants from NHANES III for the current analyses.
We selected these studies because they reported physician-diagnosed diabetes, measured fasting glucose, assessed diabetes medication use, and included a comprehensive set of cardiovascular and metabolic risk factors. In addition, they had different underlying risks of CAD given varying demographic characteristics of participants (i.e., age and ethnicity) and pre-existing burden of risk factors across cohorts, allowing us to explore consistency of associations. All three studies had longitudinal follow-up for CAD events in young and middle-aged adults.
In GeneSTAR, European American and African American probands with early-onset CAD events (<60 years) were identified at the time of hospitalization for a documented event (19). Their siblings aged <60 years and apparently free of CAD were recruited for screening and followed at 5-year intervals by trained telephone interviewers. CAD at baseline was ascertained using self-report, physician diagnosis, exercise treadmill testing, and nuclear perfusion imaging. All completed a standardized health status and CAD event questionnaire.
In MESA, men and women 45–84 years of age, who identified themselves as European American, African American, Hispanic American, or Chinese American, were recruited from six U.S. communities: Baltimore City and County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan and the Bronx, NY; and St. Paul, MN (20). Individuals with physician-diagnosed clinical cardiovascular disease (i.e., heart attack, angina, stroke, congestive heart failure, and atrial fibrillation) or cardiovascular-related procedures at baseline were excluded. A telephone interviewer contacted each participant periodically to inquire about interim hospital admissions, cardiovascular outpatient diagnoses, related procedures, and deaths.
In NHANES III, a survey based on a complex, multistage, stratified, clustered, probability sample design, was used to obtain a representative sample of the U.S. population. The survey included an interview, physical examination, and laboratory measurements (21). The Mortality Follow-up study was a prospective study of the vital status of all adult participants followed in NHANES III. The National Center for Health Statistics linked participants in NHANES III to death certificate data found in the National Death Index through 31 December 2006 (21,22). The presence of CAD at baseline was assessed by self-reported history of heart attack or congestive failure or physician-diagnosed clinical cardiovascular disease.
Institutional review boards approved the research, and all participants provided written informed consent at baseline and each examination.
Diabetes
Diabetes was defined as a self-reported physician’s diagnosis, current insulin or hypoglycemic medication use, and/or a measured fasting (≥8 h) glucose of ≥126 mg/dL.
Ascertainment of CAD
Participants were followed from their baseline examination until they experienced a CAD end point (acute coronary syndromes including myocardial infarction or angina with revascularization, stable angina, sudden cardiac death, or known CAD death). The presence of angina was confirmed in GeneSTAR and MESA with additional criteria that required evidence of significant coronary obstruction, ischemia, revascularization, physician diagnosis, symptoms, and/or medical treatment. Events were determined periodically using similar cohort-specific surveillance protocols. Unbiased investigators independently adjudicated medical records and death certificates in each cohort to obtain diagnoses. Nonconcordant classifications among reviewers were adjudicated by an external committee that determined final event classification using standardized coding schema (19–21).
For NHANES III participants, information on CAD death only was available. We used publicly available linked mortality data files, which identify the cause of death using the Underlying Cause of Death-113 groups based on the ICD-10 (22). We defined cardiovascular disease mortality as deaths with underlying cause of death codes ICD-I20–I25 and I70.
Covariates
Information on demographics, health behaviors, comorbid conditions, and medication use for each cohort was obtained using standardized questionnaires and protocols (19–21). Family history of early-onset CAD was defined as history of myocardial infarction, acute coronary syndrome, or stroke in a parent or sibling, and early onset was defined as occurrence in the proband <60 years of age. Standardized measurements of height, weight, and systolic and diastolic blood pressure were also obtained. BMI was defined as weight in kilograms/height in meters squared. Laboratory values were obtained for fasting lipid profile (to assess HDL and non-HDL cholesterol) and glucose using well-described methods for each cohort (19,20,23).
Statistical Analyses
Standard univariable methods were used to examine the baseline study characteristics within each cohort. Kaplan-Meier survival curves with log rank χ2 are presented to compare CAD event rates by sex and diabetes category by cohort. Because the number of participants aged >70 years was small, particularly in the high-risk GeneSTAR study, we truncated the analysis to 70 years of age for all studies. Person-years were calculated beginning at the age of 35 years. Following confirmation of proportional hazards using log-log survival plots, we used Cox regression models to estimate the CAD hazards comparing participants with diabetes to without diabetes (reference), or women to men (reference), and used age as the time scale. We first performed cohort-specific analyses using two models with sequential adjustment: model 1 adjusted for age, race, and education and model 2 further adjusted for smoking, BMI, hypertension, HDL cholesterol, non-HDL cholesterol, use of antihypertensives, and use of lipid-lowering agents. In GeneSTAR, SE estimates and hypothesis testing were done using bootstrapping methods to account for within-family correlations. We performed sensitivity analyses, further adjusting for use of hormone replacement therapy (HRT) in regression models in women only and also exploring for birth cohort effects. Birth cohorts were defined based on decade of birth as follows: 1926–1935 (five individuals born between 1924 and 1925 were also included in this cohort), 1936–1945, 1946–1955, 1956–1965, and 1966–1975. We then generated pooled estimates of the three studies in meta-analysis. We used the Stata metan command to derive an inverse-variance weighted (fixed-effects) meta-analysis. We also performed sensitivity analysis excluding NHANES III, in which only CAD mortality was assessed, from pooled analysis.
All analyses were carried out using Stata version 11/12 (College Station, TX). Statistical significance was determined as a P value <0.05 (two-sided).
Results
Demographics and clinical characteristics at baseline are shown by sex and study in Table 1. Across cohorts, 54% of participants were women. Overall, the average length ± SD of follow-up was longest in NHANES (14.7 ± 2.3 years) followed by GeneSTAR (12.8 ± 5.7 years) and MESA (7.2 ± 1.4 years). The proportion of European American persons was highest in NHANES (74%), followed by GeneSTAR (56%) and MESA (37%). Family history of CAD was greatest in GeneSTAR by design (100%), followed by MESA (19%) and NHANES (18%). Participants in NHANES were the youngest (range 17–60 years), followed by GeneSTAR (24–60 years) and MESA (44–60 years). In general, systolic blood pressure, diastolic blood pressure, and total and LDL cholesterol were highest in GeneSTAR. In contrast, BMI and antihypertensive medication use were lowest in NHANES. The proportion of smokers was lowest and cholesterol medication users highest in MESA. The prevalence of diabetes ranged between ∼7 and 10% in cohorts. Among those with diabetes, the proportion on glucose-lowering medications was 38 (GeneSTAR), 47 (NHANES), and 75% (MESA). Levels of cardiovascular risk factors and treatments are shown by sex and diabetes status in Supplementary Table 1.
Table 1.
Baseline demographic and selected clinical characteristics of participants by sex and cohort
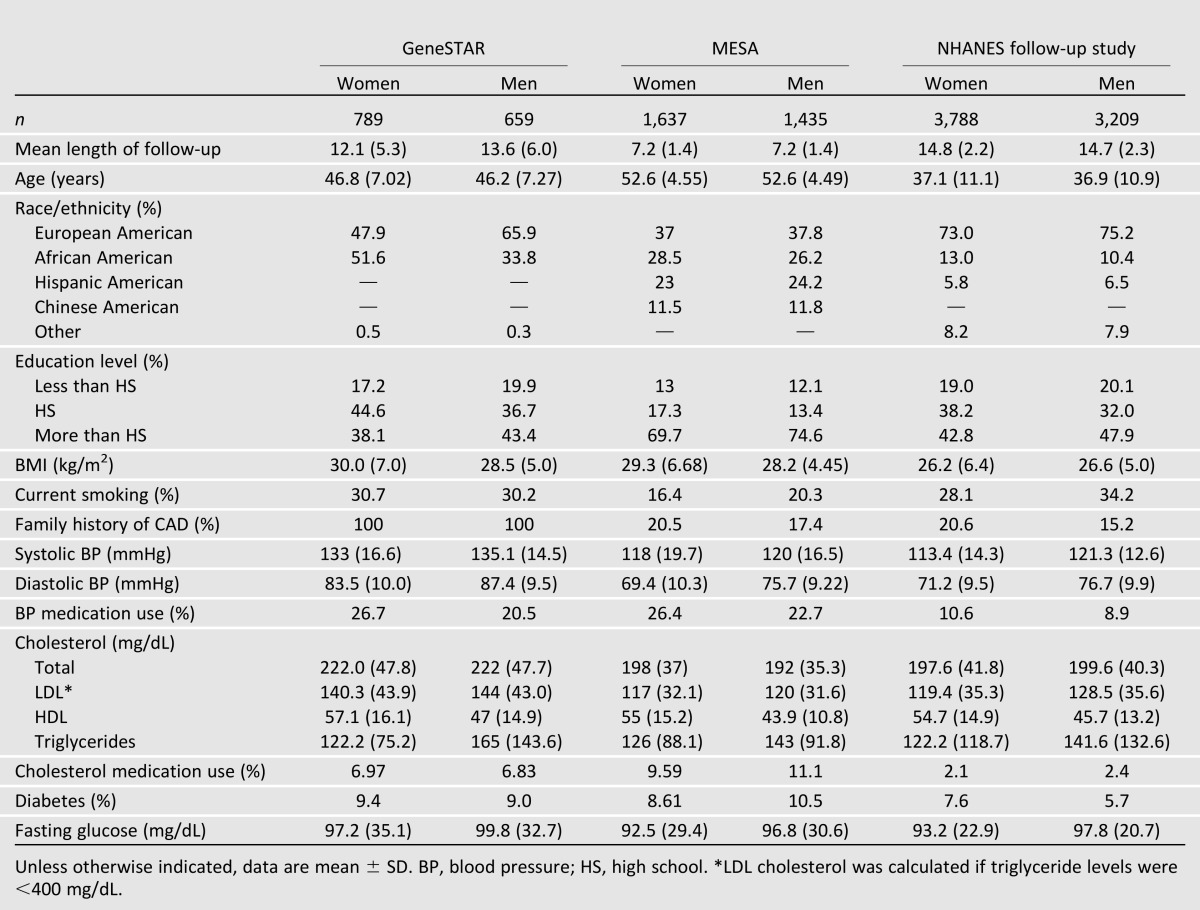
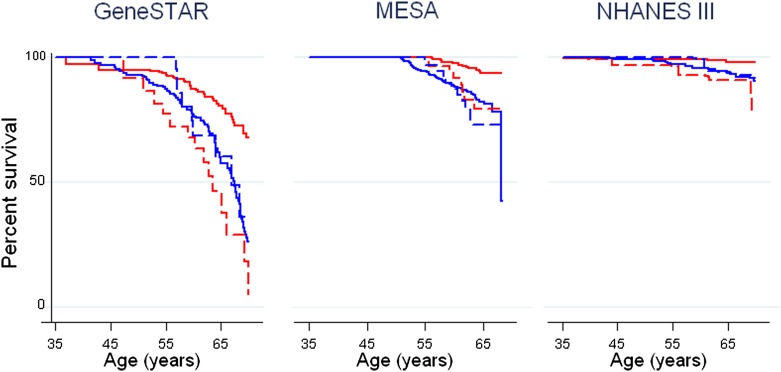
Figure 1 displays Kaplan-Meier estimates for cumulative incident and fatal CAD events by sex and cohort. Women with diabetes experienced significantly higher CAD events than women without diabetes (log-rank P < 0.01 in each cohort). In contrast, men with versus without diabetes had similar CAD rates (log-rank P > 0.05 in each cohort). Overall, women versus men without diabetes experienced a significantly lower rate of CAD events (log-rank P < 0.001 for GeneSTAR and MESA; P = 0.07 for NHANES). Also, CAD events were similar in women versus men with diabetes (log-rank P > 0.05 in each cohort). CAD risk was highest among GeneSTAR versus MESA versus NHANES participants overall and by sex and diabetes status, with absolute event rates per 1,000 person-years included in Table 2.
Figure 1.
Kaplan-Meier curves for CAD event-free survival in men and women with and without diabetes, <60 years old at baseline. Time-to-event analyses for incident CAD events in men without diabetes (blue solid line), men with diabetes (blue dashed line), women without diabetes (red solid line), and women with diabetes (red dashed line) are displayed by cohort. In all three cohorts, CAD event-free survival is significantly lower in young and middle-aged women with versus without diabetes (log-rank P < 0.001 for GeneSTAR; P = 0.002 in MESA; P = 0.007 in NHANES), but similar between men with versus without diabetes (all log-rank P > 0.05). Interestingly, among those without diabetes, women had significantly better survival than men in all cohorts (log-rank P < 0.001 for GeneSTAR; P < 0.001 for MESA; P = 0.07 for NHANES). However, the presence of diabetes equalized CAD-event free survival between men versus women (log-rank P > 0.05 in all cohorts). Only fatal CAD events were ascertained in NHANES. (A high-quality color representation of this figure is available in the online issue.)
Table 2.
Cox regression models demonstrating relative risk (HRs and 95% CIs) of incident CAD events associated with diabetes status by sex in adults aged <60 years at baseline
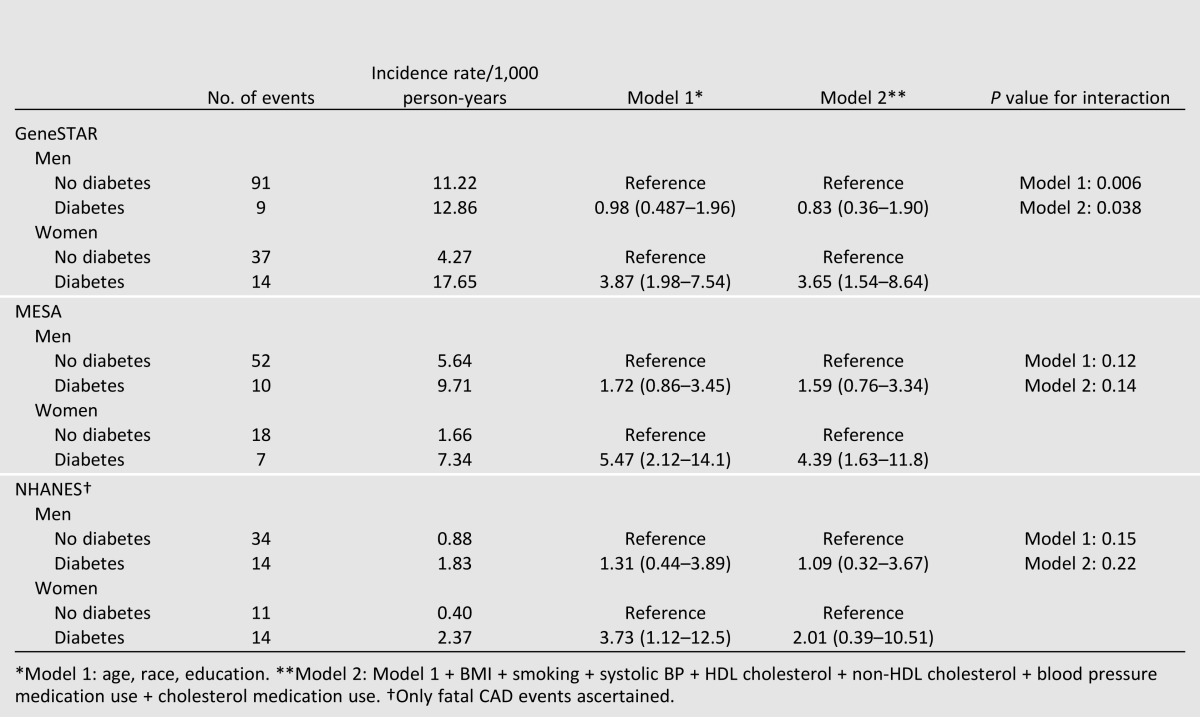
Table 2 displays the crude and multivariable-adjusted association of sex with incident and fatal CAD by cohort. In all, 210 CAD events were observed in men and 101 in women. In models adjusted for age, race, and education (model 1), diabetes was associated with an adjusted hazard ratio (HR) of 3.87 (95% CI 1.98–7.54) in GeneSTAR women and adjusted HR of 0.98 (0.49–1.96) in GeneSTAR men of CAD. After further adjustment for traditional cardiovascular risk factors in model 2, the risk of CAD was largely unchanged for GeneSTAR women comparing diabetes versus no diabetes (HR 3.65 [95% CI 1.54–8.64]) and GeneSTAR men comparing diabetes versus no diabetes (HR 0.83 [0.36–1.90]). The interaction of diabetes and sex was significant in fully adjusted models (P = 0.04).
Similarly, MESA women with versus without diabetes had an increased risk of CAD (HR 5.47 [2.12–14.1]) in model 1 (Table 2). MESA men with versus without diabetes also had higher risk for CAD, but this was not statistically significant (HR 1.72 [0.86–3.45]). Further adjustment in model 2 did not change estimates, with significantly increased HR still observed in women (4.39 [1.63–11.8]) and nonsignificantly increased HR in men (1.59 [0.76–3.34]) conveyed by diabetes. Though the patterns were similar to GeneSTAR, the interaction of sex and diabetes was not found for MESA in fully adjusted models (P = 0.14).
Similarly, NHANES women with versus without diabetes had significantly increased CAD mortality risk (HR 3.73 [1.12–12.5]) but not NHANES men (HR 1.31 [0.44–3.89]) in model 1 (Table 2). After further adjustment for covariates in model 2, the HR for women was no longer significant (HR 2.01 [0.39–10.5]). Though patterns were similar to GeneSTAR, interaction by sex was not present in fully adjusted models for NHANES (P = 0.22).
The use of HRT in women at the time of study entry ranged from 6% in NHANES and 15% in GeneSTAR to 38% in MESA. Sensitivity analysis with additional adjustment for HRT did not significantly affect HR estimates in any cohort.
Further, all of the HR findings remained qualitatively unchanged in sensitivity analyses that stratified by birth cohort, including the sex*diabetes interaction, which remained statistically significant in GeneSTAR.
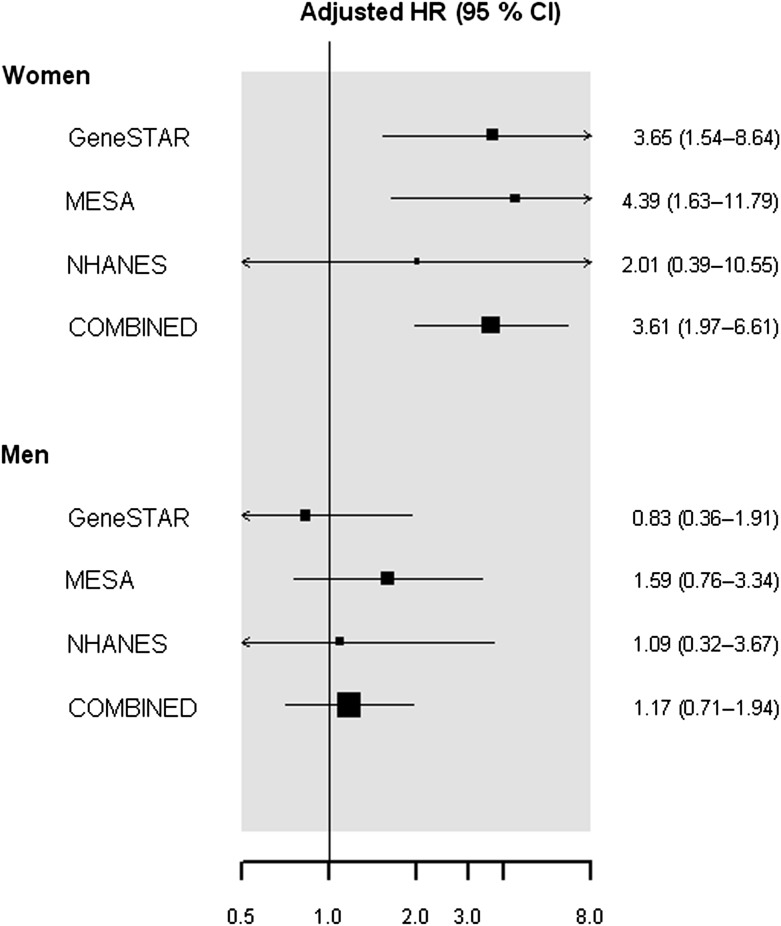
In meta-analysis of three cohorts, women with versus without diabetes had an approximately fourfold higher likelihood of CAD events during follow-up (adjusted HR 4.23 [2.57–6.95]) after accounting for age, race, and education in model 1. In contrast, men with versus without diabetes did not have significantly increased CAD events (HR = 1.30, 0.83–2.03). After further adjustment for traditional cardiovascular risk factors in model 2 (Fig. 2), the adjusted HR for women remained significant (HR = 3.61, 1.97–6.61) and nonsignificant in men (HR = 1.17, 0.71–1.94; P value for interaction by gender = 0.005). In sensitivity analyses pooling only MESA and GeneSTAR, the fully adjusted HR of incident CAD was significantly higher in women (HR 3.95 [2.06–7.57]) but not men (HR 1.19 [0.69–2.07]) with versus without diabetes (P value for interaction = 0.006).
Figure 2.
Meta-analysis of three cohort studies demonstrating HRs of incident CAD, adjusted for traditional cardiovascular risk factors, in persons with versus without diabetes by sex. In meta-analyses, after accounting for age, race, education, BMI, smoking, hypertension, HDL and non-HDL cholesterol, and antihypertensive and cholesterol-lowering medication, the fully adjusted HR for incident CAD in those with versus without diabetes was significant in women (HR 3.61 [1.97–6.61]) and nonsignificant in men (HR 1.17 [0.71–1.94]). The interaction by sex was significant (P = 0.005). Only fatal CAD events were ascertained in NHANES.
In regression analyses comparing the relative risk of incident and fatal CAD by gender (Supplementary Table 2), men versus women without diabetes had higher rates of CAD events; the results were significant for GeneSTAR (HR 2.33 [1.54–3.57]) and MESA (HR 3.23 [1.82–5.88]) but not NHANES (HR 1.20 [0.42–3.45]) in fully adjusted models. However, men and women with diabetes had similar rates of CAD events in all cohorts (reciprocal interactions to Table 2). In meta-analyses comparing men versus women, the HR for CAD events was significantly different in participants without diabetes (HR 2.92 [2.19–3.88]) but similar in those with diabetes (HR 0.85 [0.46–1.57]) in model 1. In fully adjusted models (Supplementary Fig. 1), these results were largely unchanged (HR 2.43 [1.76–3.35] with diabetes and HR 0.89 [0.43–1.83] without diabetes; P value for interaction by diabetes status = 0.013).
Conclusions
We found that diabetes dramatically increased the risk of incident and fatal CAD in young and middle-aged women with diabetes by four- to fivefold, with a much reduced effect in men, who had a higher baseline rate of CAD even without diabetes. In other words, the presence of diabetes equalized rates of CAD by sex. Remarkably, this finding was consistent across three cohorts with different underlying risks for heart disease. As might be expected, absolute CAD event rates were highest in the high-risk GeneSTAR cohort based on their ascertainment from an early-onset CAD proband and lowest in the population-based NHANES cohort. Interestingly, the contribution of traditional cardiovascular risk factors to these relationships varied by cohort. GeneSTAR had a worse cardiovascular risk factor profile while NHANES was, in general, healthier and MESA participants had a mixed profile.
Our study adds to growing evidence that gender differences exist in the risk of CAD conferred by diabetes. However, this is in contrast to the meta-analysis by Huxley et al. (7), which demonstrated that the relative risk of fatal coronary heart disease associated with diabetes is 50% higher in women than men. This study examined international cohorts of both elderly and nonelderly participants. In contrast, our study more specifically demonstrates that sex differences in the risk of both fatal and nonfatal CAD exist in otherwise healthy young- and middle-aged U.S. adults with diabetes, which is a novel finding. Further, we also report a greater magnitude of risk in women than observed in prior studies (4,5,7,24–26). This difference may be due to the exclusion of older adults in our study. We focused on younger and middle-aged adults since these individuals might accrue the greatest opportunity for prevention. Although in young and middle-aged adults, incident and fatal CAD rates are lower among women, the presence of diabetes narrows this purported sex gap. Indeed, we find that the relative risk conveyed by diabetes in women is greater than in men. We report consistent findings across three cohorts of diverse, relatively healthy younger adults, with different underlying risks for heart disease and a particularly large number of participants. In our study, we found no difference in CAD risk between men with and without diabetes. These findings may relate to previous observations of an earlier incidence of CAD in men compared with women in the general population (17,19). In other words, male sex may represent an early CAD risk factor that “borrows” from the future, potentially comparable to the CAD risk observed for women later in life. Thus, the additional presence of diabetes may not have as great an impact on increasing CAD risk in young and middle-aged men compared with women.
The higher relative risk of CAD conferred by diabetes in women has several possible explanations. There may be risks for CAD that are unique by sex. The vasodilatory effects of estrogen in women may be protective (27), though studies examining HRT use have found inconsistent benefits (28,29), and our results did not change after accounting for HRT use. Androgen exposure in early life may further have a role in coronary atherosclerosis development in men (16). Inflammatory factors may also have a greater role in perturbing insulin action in women (30). Genes that may impact the effect of cardiovascular risk factors differentially by sex are still being investigated (27). Additionally, adherence to heart-healthy lifestyle behaviors (e.g., vegetable intake) is ∼50% higher in women (31), but benefits may be offset by diabetes. Women with diabetes may be less adherent to oral hypoglycemics and standard cardiovascular prevention treatments (32). It is possible that there exists a treatment bias in favor of men with diabetes who, overall, may receive better therapies and more comprehensive care (33,34). In our study, though women used diabetes medications at similar levels or more to men in GeneSTAR (40% women vs. 34% men) and MESA (77% women vs. 73% men), interestingly, women in NHANES were less likely to use glucose-lowering agents compared with men (41% vs. 55%). Differences in compliance and treatment intensity between sexes should be investigated in future studies. Further, a consistent effect on coronary risk is the duration of diabetes in past studies (35) and poor glycemic control (30). Interestingly, women may have worse control of type 2 diabetes (36).
Strengths of our study are the inclusion of three large and diverse cohorts, all with different underlying risks for heart disease and with well-validated and standardized protocols that are extensively documented. We included a multiethnic cohort (MESA) to test consistency of observed associations and found no interactions by ethnicity (data not shown). Our findings were similar among participants with family history of cardiovascular disease, particularly in the high-risk GeneSTAR cohort. The inclusion of a nationally representative cohort (NHANES) more directly allows for generalizability of our findings. CAD outcomes were adjudicated, limiting misclassification bias. We were able to examine prospective associations to infer temporality. Also, most previous studies occurred a decade ago and were based on older, more conservative diagnostic criteria for diabetes (2,7,11). Our study used the most up-to-date criteria and may be more directly extrapolated to the current diabetes population. Lastly, by pooling estimates in meta-analyses and the use of individual participant data, we were able to obtain greater statistical power and better explore the effects of sex and diabetes status on CAD event rate in healthy young- and middle-aged adults.
Limitations of our study include the inability to examine nonfatal CAD events in NHANES. Patients who had CAD could have been classified with another cause of death, and thus, event rates are underestimated for NHANES. However, if this was the case, we would not have anticipated the similar pattern of findings we observed in NHANES. Though the number of events in healthy young adults with diabetes was relatively small, we still discerned significant results. We did not have information regarding postchallenge glucose levels from an oral glucose tolerance test, which may have led to persons with undiagnosed diabetes in our study and potentially influenced the CAD event rates reported for both nondiabetes and diabetes groups. Differences in diabetes medication use, particularly among women, in the three cohorts may have contributed to variations in the magnitude of risk-ratios observed. We examined CAD event rates according to diabetes medication use in each cohort and found that women with diabetes who used glucose-lowering medications had higher event rates compared with women who were nonusers of these medications in each cohort (data not shown). Greater disease severity may be potentially related to use of glucose-lowering medications and higher event rates, but requires further examination. We were unable to account for underlying diabetes characteristics that may differ by sex. The lack of information about hemoglobin A1c, diabetes duration, and other characteristics such as specific hypoglycemic therapy may limit the interpretation of our results. The presence of comorbid conditions may have influenced the risk of CAD observed for participants in our study. However, comorbidities such as renal disease, stroke, and pulmonary disease were either excluded for at baseline or low in the three cohorts examined, given that participants were relatively young and otherwise healthy, and unlikely to have significantly impacted results. Lastly, we were unable to distinguish whether women truly have a relative disadvantage, or men have an advantage, regarding the risk of CAD conferred by diabetes since men have higher rates of disease at earlier ages. Future studies are needed to better understand underlying mechanisms that can explain these sex differences.
To our knowledge, we are the first to demonstrate that diabetes equalizes the risk of CAD by sex, specifically among healthy young- and middle-aged adults with different underlying risks for heart disease. Our findings have important implications and suggest that aggressive preventive strategies may be as important for younger women with diabetes as they are for men. Clinical guidelines that favor earlier initiation of primary cardiovascular prevention strategies in men versus women with diabetes may warrant reconsideration given that rates of CAD are similar in young and middle-aged adults with diabetes irrespective of sex. However, our study does not diminish the importance of cardiovascular disease prevention in men. Results from studies such as ours can ultimately inform evidence-based guidelines for prevention of CAD in both men and women with diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the other investigators, the staff, and the participants of the MESA study for valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grants K23-DK-093583, K24-DK-062222, and P60-DK-079637). The GeneSTAR study was supported through various grants (U01-HL-72518-05, R01-HL-071025, R18-HL-58625, R01-NR-08153, R01-HL-49762, R01-HL-59684, R01-NR-02241, and M01-RR-00052). The MESA study was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and grants UL1-RR-024156 and UL1-RR-025005 from the National Center for Research Resources.
Duality of Interest. D.V. is a consultant for MyBodyCount8. No other potential conflicts of interest relevant to this article were reported.
Author Contributions.R.R.K. was responsible for study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. M.L., D.B., and D.V. were responsible for study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. P.O., E.T., and F.B. were responsible for study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. K.C. was responsible for study concept and design and critical revision of the manuscript for important intellectual content. M.L. and D.V. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral abstract form at the 2012 Scientific Sessions of the American Heart Association, Los Angeles, CA, 3–7 November 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-1755/-/DC1.
References
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421 [PubMed] [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation 1979;59:8–13 [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 4.Howard BV, Cowan LD, Go O, Welty TK, Robbins DC, Lee ET. Adverse effects of diabetes on multiple cardiovascular disease risk factors in women. The Strong Heart Study. Diabetes Care 1998;21:1258–1265 [DOI] [PubMed] [Google Scholar]
- 5.Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care 2000;23:962–968 [DOI] [PubMed] [Google Scholar]
- 6.Kappert K, Böhm M, Schmieder R, et al. ONTARGET/TRANSCEND Investigators Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET). Circulation 2012;126:934–941 [DOI] [PubMed] [Google Scholar]
- 7.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natarajan S, Liao Y, Cao G, Lipsitz SR, McGee DL. Sex differences in risk for coronary heart disease mortality associated with diabetes and established coronary heart disease. Arch Intern Med 2003;163:1735–1740 [DOI] [PubMed] [Google Scholar]
- 9.Becker A, Bos G, de Vegt F, et al. Cardiovascular events in type 2 diabetes: comparison with nondiabetic individuals without and with prior cardiovascular disease. 10-year follow-up of the Hoorn Study. Eur Heart J 2003;24:1406–1413 [DOI] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Papacosta O, Lawlor DA, et al. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012;55:80–87 [DOI] [PubMed] [Google Scholar]
- 11.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2002;162:1737–1745 [DOI] [PubMed] [Google Scholar]
- 12.Mosca L, Benjamin EJ, Berra K, et al. American Heart Association Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation 2011;124:2145–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pignone M, Alberts MJ, Colwell JA, et al. American Diabetes Association. American Heart Association. American College of Cardiology Foundation Aspirin for primary prevention of cardiovascular events in people with diabetes. J Am Coll Cardiol 2010;55:2878–2886 [DOI] [PubMed] [Google Scholar]
- 15.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 2003;24:313–340 [DOI] [PubMed] [Google Scholar]
- 16.Tunstall-Pedoe H, Kuulasmaa K, Mähönen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet 1999;353:1547–1557 [DOI] [PubMed] [Google Scholar]
- 17.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA 2006;295:306–313 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaidya D, Yanek LR, Moy TF, Pearson TA, Becker LC, Becker DM. Incidence of coronary artery disease in siblings of patients with premature coronary artery disease: 10 years of follow-up. Am J Cardiol 2007;100:1410–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881 [DOI] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Washington, D.C., Department of Health and Human Services, 1994 (publication no. 94-1308, Vital and health statistics, Series 1. No. 32)
- 22.NHANES III Mortality Follow-Up. National Center for Health Statistics Center for Disease Control and Prevention [Internet], 2010. Available from www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm Accessed 30 October 2013
- 23.Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Environmental Health, National Center for Health Statistics Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988––1994. Washington, DC, Department of Health and Human Services, 1996 [Google Scholar]
- 24.Hyvärinen M, Tuomilehto J, Laatikainen T, et al. The impact of diabetes on coronary heart disease differs from that on ischaemic stroke with regard to the gender. Cardiovasc Diabetol 2009;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 1979;2:120–126 [DOI] [PubMed] [Google Scholar]
- 26.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 1991;265:627–631 [PubMed] [Google Scholar]
- 27.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation 1997;95:252–264 [DOI] [PubMed] [Google Scholar]
- 28.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605–613 [DOI] [PubMed] [Google Scholar]
- 29.Vaidya D, Becker DM, Bittner V, Mathias RA, Ouyang P. Ageing, menopause, and ischaemic heart disease mortality in England, Wales, and the United States: modelling study of national mortality data. BMJ 2011;343:d5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 2004;27:2898–2904 [DOI] [PubMed] [Google Scholar]
- 31.Miller RR, Sales AE, Kopjar B, Fihn SD, Bryson CL. Adherence to heart-healthy behaviors in a sample of the U.S. population. Prev Chronic Dis 2005;2:A18. [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Thumula V, Pace PF, Banahan BF, 3rd, Wilkin NE, Lobb WB. Predictors of medication nonadherence among patients with diabetes in Medicare Part D programs: a retrospective cohort study. Clin Ther 2009;31:2178–2188; discussion 2150–2151 [DOI] [PubMed] [Google Scholar]
- 33.Kramer H, Raum E, Ruter G, et al. Gender disparities in diabetes and coronary artery disease medication among patients with type 2 diabetes: results from the DIANA study. Circulation 2012;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brännström J, Hamberg K, Molander L, Lövheim H, Gustafson Y. Gender disparities in the pharmacological treatment of cardiovascular disease and diabetes mellitus in the very old: an epidemiological, cross-sectional survey. Drugs Aging 2011;28:993–1005 [DOI] [PubMed] [Google Scholar]
- 35.Cho E, Rimm EB, Stampfer MJ, Willett WC, Hu FB. The impact of diabetes mellitus and prior myocardial infarction on mortality from all causes and from coronary heart disease in men. J Am Coll Cardiol 2002;40:954–960 [DOI] [PubMed] [Google Scholar]
- 36.Franzini L, Ardigò D, Cavalot F, et al. IT Study Group of the Italian Society of Diabetology Women show worse control of type 2 diabetes and cardiovascular disease risk factors than men: results from the MIND. Nutr Metab Cardiovasc Dis 2013;23:235–241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.