Abstract
OBJECTIVE
We examined the impact of intensive versus conventional diabetes treatment upon menopause among women with type 1 diabetes in the Diabetes Control and Complications Trial (DCCT), a randomized controlled trial of intensive diabetes treatment, and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study.
RESEARCH DESIGN AND METHODS
In a secondary analysis of women in the DCCT/EDIC (n = 657), outcomes were the cumulative incidences of natural menopause and surgical menopause. Cox regression analyses were used to examine associations with treatment group, time-varying estimates of hemoglobin A1c (HbA1c), insulin dosage, BMI, and microvascular complications (retinopathy, nephropathy, and neuropathy).
RESULTS
By EDIC year 18, after an average of 28 years of follow-up, 240 (38%) women had experienced natural menopause and 115 (18%) women had experienced surgical menopause. Age at natural menopause was similar in the intensive versus conventional groups (49.9 vs. 49.0 years; P = 0.28), and age at surgical menopause was similar in the intensive versus conventional groups (40.8 vs. 42.0 years; P = 0.31). In multivariable models, treatment group, HbA1c, and microvascular complications were not associated with risk of natural or surgical menopause. Each 10 unit/day increase in insulin dosage decreased risk of natural menopause (hazard ratio [HR] 0.91, 95% CI 0.75–0.98) and each kg/m2 increase in BMI increased risk of surgical menopause (HR 1.08, 95% CI 1.00–1.16).
CONCLUSIONS
In the DCCT/EDIC, intensive versus conventional treatment group and HbA1c level were not associated with menopause risk. Greater insulin dose was associated with lower menopause risk.
Introduction
Two large cohort studies have suggested an association between type 1 diabetes with earlier age of menopause (1,2). In one report, age at menopause in women with type 1 diabetes, their healthy sisters, and unrelated controls was 41.6 versus 49.9 versus 48.0 years, respectively (1). In another report, average age at menopause in women with type 1 diabetes versus controls was 44 versus 50 years, respectively (2).
Several mechanisms may potentially explain the impact of diabetes on menopause. Women with type 1 diabetes are at increased risk of autoimmune oophoritis, which exists particularly among women who have circulating antibodies against adrenal antigens (3,4). However, adrenal disease affects less than 5% among women with type 1 diabetes, and adrenal antibodies are present in less than 1% of women with type 1 diabetes and without clinical adrenal insufficiency (5), making it unlikely that oophoritis accounts for the earlier age of menopause in women with type 1 diabetes. Accumulation of advanced glycated end products in ovarian tissue resulting from hyperglycemia may be toxic to the ovary (6). The presence of diabetes complications, including proliferative retinopathy and nephropathy, could reflect microvascular damage in the ovary (7). Finally, insulin therapy could recruit more ovarian follicles with each menstrual cycle (8) and eventually deplete follicular stores, resulting in a younger age at menopause.
Using data from the Diabetes Control and Complications Trial (DCCT) and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, we examined the impact of intensive versus conventional insulin therapy and consequent metabolic changes upon the risk of menopause.
Research Design and Methods
Population and Setting
The DCCT and EDIC studies have been described in detail (9). Briefly, the DCCT was a multicenter, randomized clinical trial designed to compare the impact of intensive and conventional diabetes treatment on the development and progression of early microvascular complications of type 1 diabetes (9). From 1983–1989, 1,441 patients were recruited at 29 centers. The intensive treatment regimen was designed to achieve glycemic control as close to the nondiabetic range as possible using ≥3 daily insulin injections or an insulin pump, with dose selection guided by frequent self-monitoring of blood glucose. Conventional treatment consisted of 1–2 daily insulin injections without stipulated target glucose levels.
The DCCT included a primary prevention cohort and a secondary intervention cohort. The primary prevention cohort consisted of 726 subjects with no retinopathy, urinary albumin excretion rate (AER) of <40 mg/24 h, and diabetes duration of 1–5 years at DCCT baseline. The secondary intervention cohort consisted of 715 subjects who had nonproliferative retinopathy, urinary AER of ≤200 mg/24 h, and diabetes duration of 1–15 years. Individuals were excluded if they had hypertension, were taking any blood pressure or lipid-lowering medications, or had a history of symptomatic ischemic heart disease or symptomatic peripheral neuropathy.
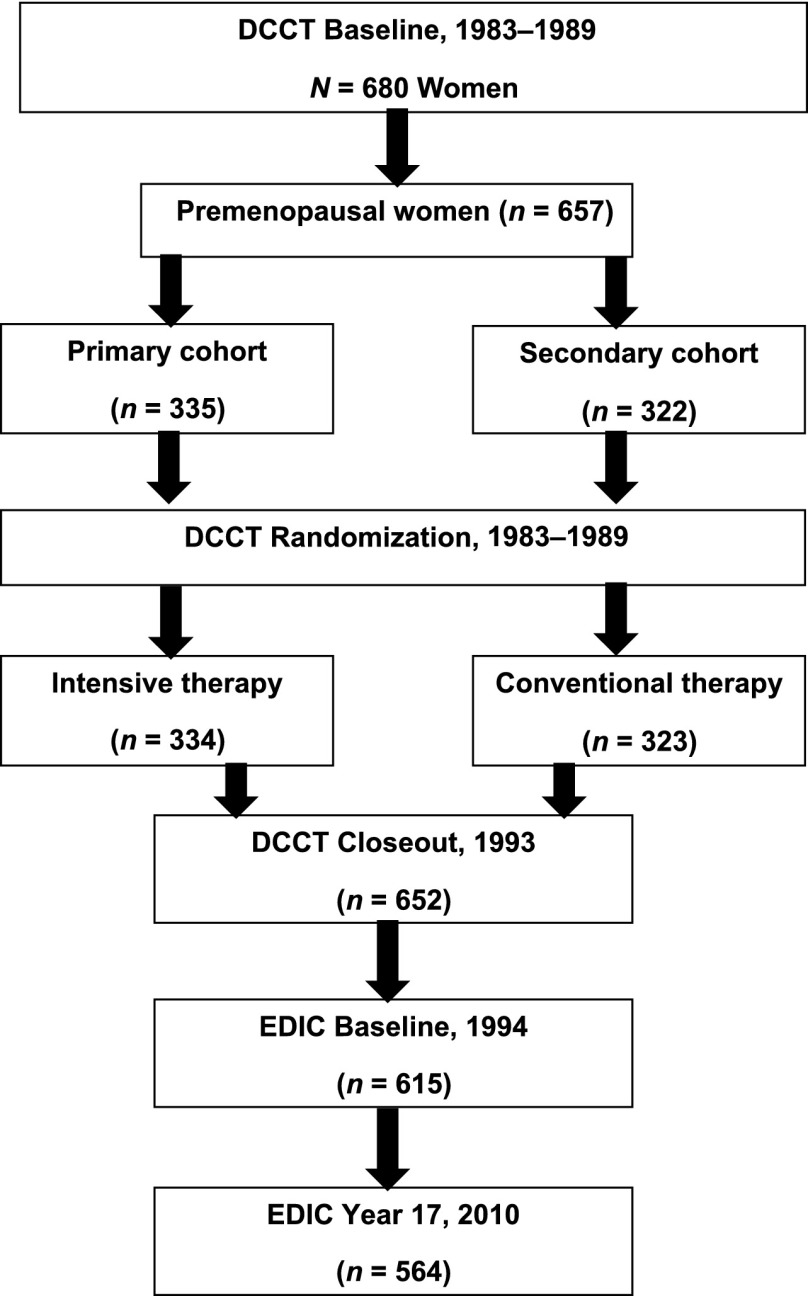
The DCCT ended in 1993 and 1,375 (96%) of the 1,428 surviving DCCT subjects enrolled in the EDIC. Of the 680 women in the original DCCT cohort, 657 were premenopausal at DCCT baseline. At DCCT baseline, 23 women were postmenopausal, and 21 of these women reported gynecologic surgery with mean age at surgery of 28.2 ± 4.5 years; these women were excluded from this analysis. Among those who were premenopausal at DCCT baseline, 334 were randomly assigned to intensive control and 323 to conventional treatment. At DCCT closeout, 652 women remained active in the trial, and 615 women completed a baseline EDIC visit. For the current analyses, we examined the cohort through the 18th year of the EDIC, when 564 women were actively participating in the EDIC (Fig. 1).
Figure 1.
Flow of female participants through the DCCT/EDIC studies.
Data Collection
During the EDIC, a standardized annual history and physical examination included a detailed interview regarding overall health status, diabetes management, occurrence of diabetes complications, development of other diseases, health behaviors such as cigarette smoking, and medication use. Women were also asked about their menstrual patterns or discontinuation of menses, gynecologic surgeries, and use of exogenous hormones. If postmenopausal, the age at menopause and whether the cause was natural or surgical was obtained. Natural menopause was defined as cessation of menses for 1 year in the absence of gynecologic surgery. Surgical menopause was defined as cessation of menses if women had undergone bilateral oophorectomy and/or hysterectomy.
BMI (kg/m2), insulin dosage (units/day), and hemoglobin A1c (HbA1c) were assessed at randomization and quarterly during the DCCT and annually in the EDIC (10). Retinopathy was assessed semiannually during the DCCT and every fourth year during the EDIC using stereoscopic fundus photography that was centrally graded using the Early Treatment Diabetic Retinopathy Study scale (9). Nephropathy was assessed annually in the DCCT and biannually in the EDIC using an AER (11). Estimated glomerular filtration rate (GFR) was calculated from serum creatinine, age, sex, and race using the Chronic Kidney Disease Epidemiology Collaboration Equation (11). Clinical peripheral neuropathy status was defined as the presence of peripheral sensorimotor neuropathy on physical examination by the study neurologist plus either abnormal nerve conduction in two different peripheral nerves or abnormal autonomic test results that were conducted at DCCT baseline, DCCT end, or EDIC year 13 (12).
Statistical Analysis
Analyses of natural menopause and surgical menopause were conducted separately. Differences in participant characteristics by randomization group were tested using the χ2 test for qualitative variables and the Wilcoxon rank sum test for quantitative variables. For natural menopause, the cumulative incidence was estimated using Gray’s method, with surgical menopause as a competing risk (13). Cox proportional hazards regression models were used to estimate the effects of intensive therapy and other covariates on the cause-specific hazard of natural menopause. While Gray’s method has a statistical test to compare the difference in cumulative incidence, this tests the difference between groups in either the outcome or the competing risk; as we were interested in testing the difference between groups in the risk of the index event, we used the proportional hazards model to calculate the cause-specific hazard and censored individuals at the time of their competing risk. Data were right-censored when no natural menopause occurred before the 18th year of the EDIC or because of loss to follow-up or because surgical menopause occurred as a competing risk. Models of surgical menopause as the outcome characterized natural menopause as a competing risk.
Separate regression models were constructed for each of the covariates of interest (total daily insulin dose, HbA1c, BMI, retinopathy, nephropathy, and neuropathy). Each measure was represented as a time-varying covariate during the DCCT/EDIC. The time-weighted arithmetic mean values for insulin dose, HbA1c, and BMI were used, representing the running means up to and including each study visit in the DCCT and EDIC, with the quarterly DCCT and annual EDIC values weighted by 3 and 12 months, respectively. Each model was adjusted for age at randomization, treatment group, primary prevention versus secondary intervention cohort, and the DCCT baseline value of the covariate. We evaluated the relationship between treatment group and unilateral oophorectomy and hysterectomy and did not find a significant association, and we also examined the relationship between treatment group and marital status, education, parity, and hormonal contraceptive use and did not observe an association (results not shown). Therefore, these factors were not included in the multivariate models. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
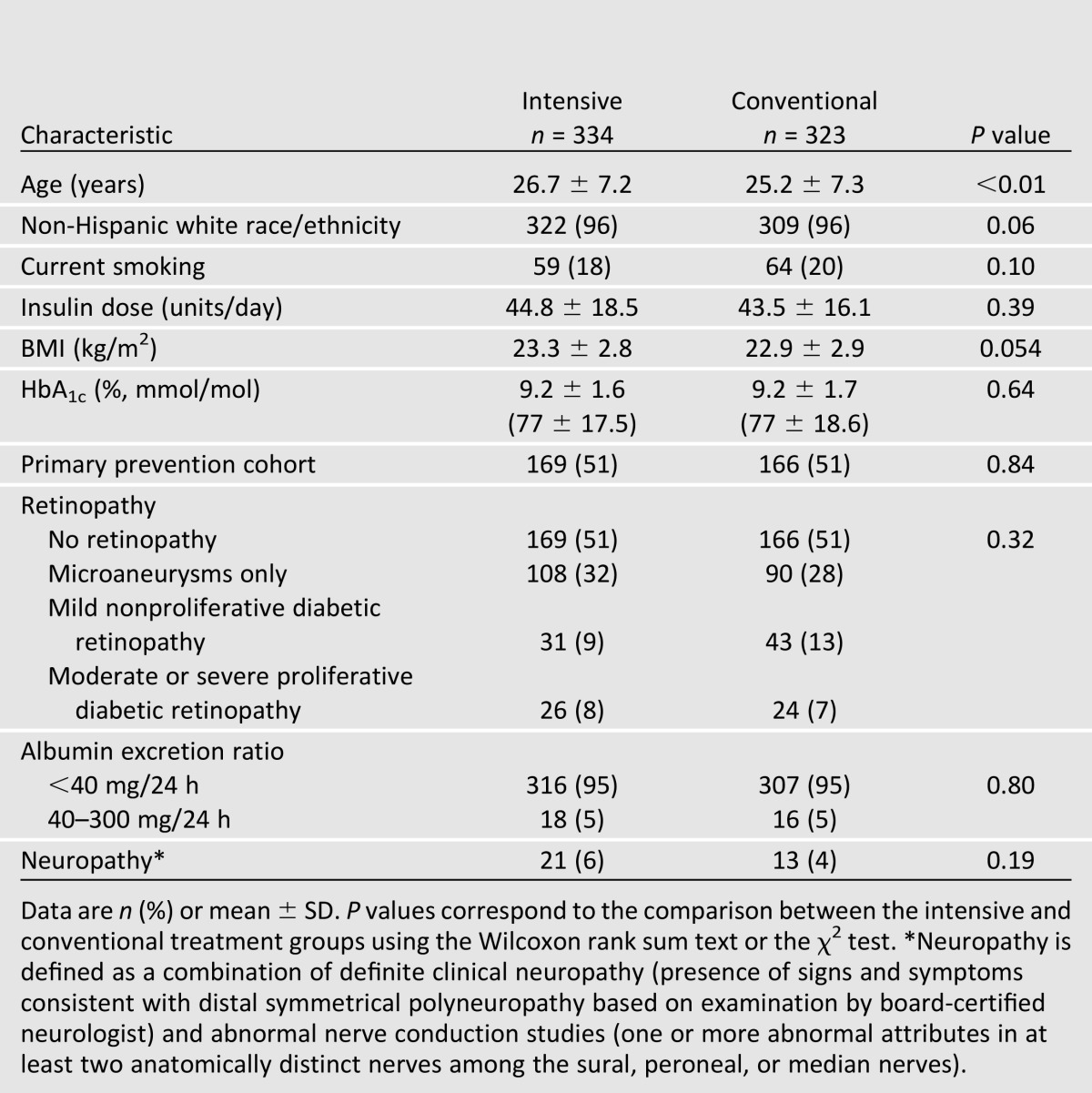
Table 1 shows participant characteristics at DCCT baseline. Women who had been randomly assigned to intensive treatment were approximately 1.5 years older than women randomized to conventional treatment. In both treatment groups, 96% of participants were white and approximately 20% were smokers. At DCCT baseline, BMI, HbA1c, and prevalence of retinopathy, nephropathy, and clinical neuropathy were similar between the two treatment groups. No participants had a GFR of <60 mL/min/1.73 m2 or an AER of >200 mg/24 h at baseline.
Table 1.
Characteristics of female participants at DCCT baseline by DCCT treatment group
After an average of 6.5 years in the DCCT, women randomly assigned to intensive therapy had significantly more weight gain than women assigned to conventional therapy (DCCT baseline to DCCT closeout difference, mean (SD) 9.2 ± 9.0 and 5.3 ± 6.9 kg; P < 0.01) and by study design had lower average HbA1c (DCCT closeout, 7.2 ± 0.8 vs. 9.0 ± 1.3%; P < 0.01). During the DCCT, a similar proportion of women had at least 1 pregnancy since randomization in the intensive versus conventional therapy arms (n = 97 [31%] vs. 91 [30%]; P = 0.94).
At EDIC year 1, women assigned to intensive therapy were 34.9 ± 7.1 years of age compared with 33.6 ± 7.2 years of age among women assigned to conventional therapy (P = 0.026), reflecting the originally older age at randomization of women assigned to intensive therapy. Among women who remained in the cohort by EDIC year 18, this age difference persisted (51.9 ± 7.1 vs. 50.2 ± 7.2 years; P = 0.006). At EDIC year 18, the time-weighted HbA1c, reflecting the average A1c values over the course of the DCCT/EDIC, was lower among women randomized to intensive therapy versus conventional therapy (7.8 ± 0.9 vs. 8.2 ± 0.9; P < 0.0001). Time-weighted BMI was higher among women randomized to intensive therapy versus conventional therapy (27.2 ± 4.4 vs. 26.2 ± 3.8; P = 0.0045).
After an average of 25 years of follow-up, 240 women had reached natural menopause (2% during the DCCT and 98% during the EDIC) and 115 women underwent surgical menopause (20% during the DCCT and 80% during the EDIC). The mean ages at natural menopause and surgical menopause were 49.6 ± 4.4 and 41.4 ± 6.7 years, respectively. Among women in the primary cohort, the average age at natural menopause was 49.3 ± 4.4 years and of surgical menopause was 41.0 ± 6.5 years. Among women in the secondary cohort, the average age at natural menopause was 49.8 ± 4.5 years and at surgical menopause was 41.9 ± 7.1 years. There were 49 women who had cessation of menses at <40 years of age and 101 women who had cessation of menses at <45 years of age. Within the intensive and conventional treatment groups, the mean ages at natural menopause were 49.9 versus 49.0 years (P = 0.28) and mean ages at surgical menopause were 40.8 versus 42.0 years (P = 0.31), respectively.
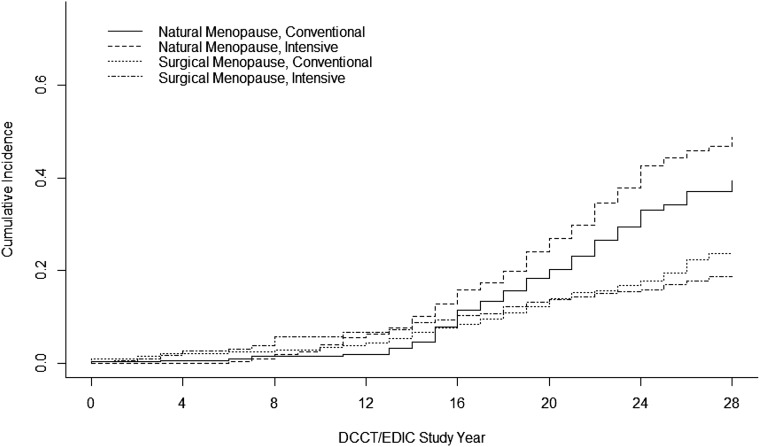
Figure 2 presents the cumulative incidence of natural and surgical menopause by treatment group allowing for natural and surgical menopause to act as competing risks for one another. At time zero (DCCT baseline), the mean age of the female cohort was 26 years (range 13–39). After an average of 25 years of follow-up, the cumulative incidence of natural menopause was 48.7 and 39.3% in the intensive and conventional groups, respectively. The cumulative incidence of surgical menopause was 18.7% in the intensive and 23.6% in the conventional groups. During the DCCT, 2 women randomized to intensive therapy and 3 women randomized to conventional therapy had experienced natural menopause, and 14 women randomized to intensive therapy and 9 women randomized to conventional therapy had experienced surgical menopause. During the EDIC, 136 women randomized to intensive therapy and 99 women randomized to conventional therapy had experienced natural menopause, and 41 women randomized to intensive therapy and 51 women randomized to conventional therapy had experienced surgical menopause.
Figure 2.
Cumulative incidence of natural menopause and surgical menopause by DCCT/EDIC treatment group. At time zero (DCCT baseline), the mean age of the female cohort was 26 years (range 13–39). After an average of 25 years of DCCT/EDIC follow-up, 240 women had reached natural menopause (2% during the DCCT and 98% during EDIC) and 115 women underwent surgical menopause (20% during the DCCT and 80% during EDIC). The mean ages at natural menopause and surgical menopause were 49.6 ± 4.4 and 41.4 ± 6.7 years, respectively.
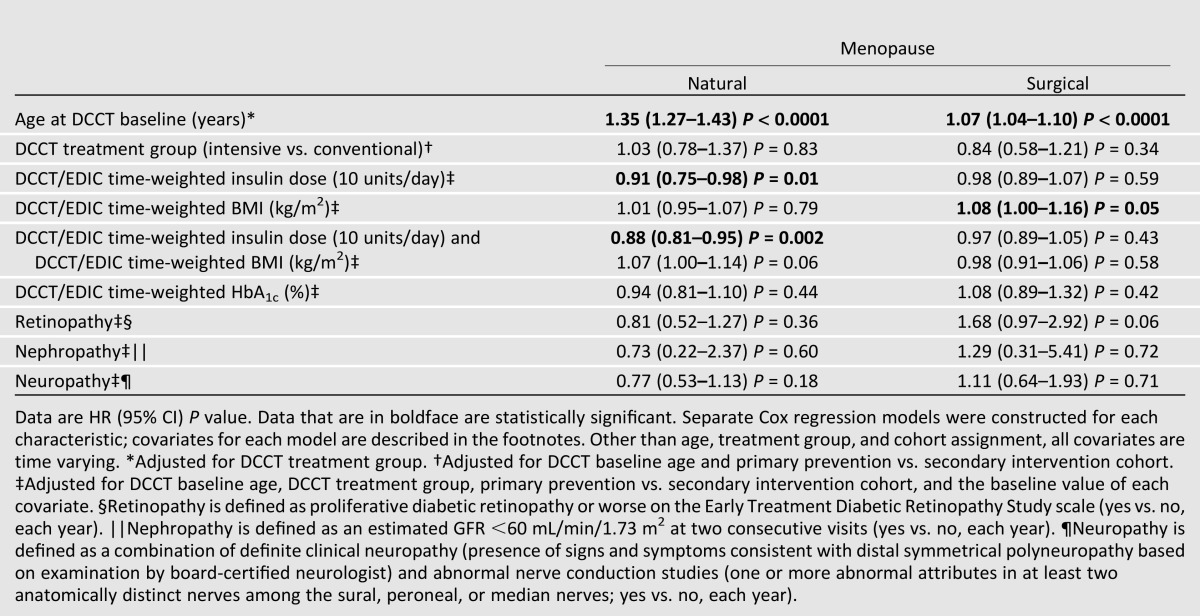
Table 2 shows the associations between treatment group and other variables with the risk of menopause in separate Cox proportional hazards regression models. Greater age at DCCT baseline was associated with an increased risk of both natural and surgical menopause, after adjusting for DCCT treatment group (P < 0.0001). In unadjusted estimates, subjects in the intensive treatment group were at a 42% increased risk of natural menopause (hazard ratio [HR] 95% CI 1.42 [1.09–1.84]). However, women randomized to intensive therapy were significantly older at baseline, and age is a significant risk factor for menopause (Table 2). After adjustment for age at randomization, treatment group was no longer associated with onset of natural menopause. We included a time-dependent indicator variable that distinguishes between the DCCT and EDIC study periods and tested for an interaction with treatment group. The interaction between DCCT/EDIC study time and treatment group was not significant, indicating that the risks of menopause by intensive versus conventional treatment group are comparable during the DCCT and EDIC periods. Neither HbA1c nor microvascular complications were associated with onset of natural or surgical menopause. HbA1c was also evaluated separately for the DCCT and EDIC periods. There were no significant associations between glycemic control in either period of the study and the risk of menopause. Greater mean time-varying insulin dosage (by 10 unit increments per day) during the DCCT and EDIC was associated with a decreased risk of natural menopause (HR [95% CI] 0.91 [0.75–0.98]). Greater mean time-varying BMI during the DCCT and EDIC was associated with a higher risk of surgical menopause (HR [95% CI] 1.08 [1.00–1.16]), although this effect was attenuated after the addition of insulin dose in the model (HR [95% CI] 0.98 [0.91–1.06]). In contrast, the association between higher insulin dose and lower risk of natural menopause persisted.
Table 2.
Relative risk of natural menopause and surgical menopause during the DCCT/EDIC study
Several additional analyses were conducted. The proportional hazards assumption for age was violated, so the risk estimates for age used a robust covariance sandwich. We also modeled age as a categorical variable, with age categories in 5-year groups with women <20 years as a reference, with no significant difference in results. Treatment group was still not associated with menopause after adjustment for age in these models, greater age was associated with increased risk of menopause, and interactions between age and treatment group were not significant. We also examined whether there were interactions between treatment arm and insulin dose to determine whether insulin dose was associated with natural menopause at all levels of dosing; interactions between insulin dose and treatment group were not significant (P = 0.47). Approximately 37% of women in the intensive treatment group and 36% in the conventional treatment group reported having any autoimmune disease by the end of EDIC year 18, primarily thyroid disease; only three women (one premenopausal and two naturally postmenopausal) reported adrenal insufficiency by the end of EDIC year 18. This variable was not associated with either type of menopause, and inclusion did not change risk estimates for other variables in the final regression models. Cigarette use (20% at baseline with declines throughout EDIC), and time-varying measures of systolic and diastolic blood pressure and lipid levels were not associated with menopause status. A composite time-varying measure for presence of all microvascular complications did not differ from the individual complications estimates, and none was associated with type of menopause. Characteristics of women who experienced menopause at less than 40 years of age were similar by DCCT treatment group to the overall cohort. When we excluded women who were <18 years of age at baseline, associations between risk factors and menopause were similar as in the overall cohort, with the exception that time-weighted BMI had reduced significance (HR 1.08; 95% CI 0.99–1.18; P = 0.099). Finally, diabetes duration was associated with age at randomization but was not associated with either type of menopause in any of these models. Thus adrenal insufficiency and cigarette use, blood pressure and lipid measures, and diabetes duration were excluded in the final regression models (results not shown).
Conclusions
Previous studies have suggested that women with type 1 diabetes had earlier age of natural menopause than women without type 1 diabetes, suggesting that hyperglycemia and/or presence of microvascular complications characterizing diabetes could influence age at menopause (1,2,7). Previous studies from the DCCT/EDIC have suggested that the benefit of intensive therapy on microvascular outcomes persists decades after randomization (11). Using data from the DCCT/EDIC cohort, a well-characterized cohort of women with type 1 diabetes, we found that former treatment group, glycemic control, and microvascular disease were not associated with age of natural or surgical menopause.
There are several possible explanations for these findings. Participants may have had better glycemic control than participants with type 1 diabetes in the other observational cohorts, and more significant elevations of glycemia may be needed to affect menopausal age. Only 10% of women were postmenopausal in one report (2), and thus the younger age of menopause (44 years) may have reflected only those who underwent earlier menopause, rather than the eventual age at menopause for the entire cohort. The other cohort was incepted in the 1950s, and it is possible that cohort-specific factors other than glycemic control contributed to earlier age at menopause (1). Along similar lines, participants in the Familial Autoimmune and Diabetes Study (1) and a Finnish registry-based study (7) had a higher prevalence of microvascular complications and included more women with significant nephropathy than observed in the DCCT/EDIC. Unlike a previous report, we did not find an association between proliferative retinopathy and end-stage renal disease with risk of menopause (7). In the DCCT/EDIC, these complications were uncommon, and it may be that a higher burden of disease is needed to affect menopausal age. Our report also did not support an autoimmune component for earlier age at menopause in women with type 1 diabetes, in that few women in the DCCT/EDIC reported clinically significant adrenal disease. In the Familial Autoimmune and Diabetes study, the DR4-DQA1*0301-DQB1*0302 haplotype was associated with age at menopause in univariate analysis, but this haplotype was not associated with age at menopause in multivariate analyses (1).
Single measures of HbA1c were not associated with menopause risk in those studies, whereas mean over time HbA1c was used in this study. It is also possible that HbA1c levels and microvascular disease do not decrease ovarian reserve to the extent that menopausal age is impacted. While previous cross-sectional studies support a negative impact of HbA1c on ovarian reserve as assessed by number of follicles or biomarkers reflecting functioning follicles (14), age at menopause reflects central regulatory mechanisms as well as ovarian follicular abnormalities.
We did find that higher insulin dosages over time, before and after adjustment for body weight, were associated with a lower risk of natural menopause. We had originally hypothesized that greater insulin dosages could lead to excess follicle recruitment and accelerated depletion of ovarian reserve. However, these results are reassuring in that increasing insulin dosages do not appear to have an adverse association with age at menopause. While mechanisms are speculative, insulin has growth-factor effects on the ovary (8) and thus may have tonic or preservatory effects upon ovarian reserve. Although women with type 1 diabetes lack pancreatic β-cell secretion of insulin, injected insulin bypasses the portal circulation, and women with type 1 diabetes may actually have higher circulating insulin levels than women without diabetes (15). The lack of an association between treatment group and risk of menopause in the presence of an association between greater insulin dose and decreased risk of menopause suggests that dosages of insulin, even at the lower ranges and even within the conventional treatment arm, are directly associated with decreased risk of menopause. It is also possible that the association between insulin and age at menopause is a chance finding, resulting from a relatively high number of comparisons.
The majority of women who underwent menopause at younger ages underwent surgical menopause. The high proportion of women who underwent surgical menopause in their early 40s is compatible with previous reports in the U.S., although there is significant variation by age, race/ethnicity, and regional factors (16). We also found that greater BMI was associated with increased hazard of surgical menopause. The DCCT/EDIC did not collect information on the reasons for hysterectomy or gynecologic surgery, so mechanisms are speculative. Among the general population of women, the risk of hysterectomy increases with increasing weight (17), often due to dysfunctional uterine bleeding. Even though weight gain was associated with intensive therapy, our results suggest that if women can maintain a lower BMI through the judicious use of physical activity diet, they can decrease their risk of surgical menopause. Of note, we did not find that estimates of surgical menopause risk influenced our estimates of natural menopause risk, an important consideration due to the high frequency of hysterectomy in the U.S. in premenopausal women (18).
Strengths of this analysis of the DCCT/EDIC cohort include use of study-adjudicated and longitudinal assessment of diabetes complications and HbA1c, the randomized trial design that reduced confounding associated with glycemic control, annual assessment of menstruation, the relatively long length of follow-up, and the high retention rate in the DCCT/EDIC. However, the DCCT/EDIC was not designed to ascertain age at menopause in women with type 1 diabetes versus unaffected women, and only approximately half of the cohort had reached menopause by EDIC year 18 or after an average follow-up of 25 years. It is possible that the impact of the factors examined may change with longer follow-up and the aging of the cohort.
It is possible that the risk associated with intensive therapy differs if women undergo premature menopause; only 49 women in the DCCT/EDIC experienced menopause at <40 years of age, and therefore we had limited power to assess this group separately. However, the characteristics of women who experienced menopause at younger ages were similar to the overall cohort at DCCT baseline and by treatment group. The age of natural menopause was slightly younger than has been reported in prospective cohort studies in the U.S. that have reported age at the final menstrual period ranging between 51 and 53 years (19), although these cohorts excluded women who had stopped menstruating prior to entry and thus do not represent all perimenopausal women. Age at menarche and pubertal history, and specifically their relationship to age at onset of type 1 diabetes, may modify the relationships between glycemic control and menopause, but such history was not assessed in the DCCT/EDIC. All women were postpubertal at enrollment. Onset of puberty may modify the relationship between insulin therapy and follicular maturation and recruitment; prepubertal girls have elevated anti-Müllerian hormone levels, a marker of ovarian reserve, but postpuberty, insulin may stimulate follicle maturation (8,15,20). A more detailed menstrual history would have also allowed the assessment of menopausal stage as an outcome, but such information was not collected. Surgical menopause was obtained by self-report, which may have decreased the precision of age at menopause, although we would not expect that misclassification would differ by treatment group or glycemic control. Our study did not include women without diabetes, although including such a population would not have yielded information regarding the impact of intensive insulin treatment, glycemic control, diabetes complications, and insulin dose.
We conclude that compared with less stringent glucose control, intensive insulin therapy aimed at lowering HbA1c to <7.0% over a period of 6 years does not have a large impact on age at natural menopause. Moreover, consequent glycemic control and microvascular complications also do not appear to be associated with age at natural menopause in women treated with modern insulin therapy. Insulin dosing may be associated with decreased risk of natural menopause, and greater BMI may be associated with increased risk of surgical menopause. Mean ages of natural menopause are significantly higher than those reported in the decades prior to strict control.
Article Information
Funding. The DCCT/EDIC has been supported by U01 Cooperative Agreement grants (1982–93, 2011–2016) and contracts (1982–2011) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grant numbers U01-DK-094176 and U01-DK-094157) and through support by the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the Genetic Clinical Research Centers Program (1993–2007), and the Clinical and Translational Science Center Program (2006–present), Bethesda, MD. Additional support for C.K. was provided by R01-DK-083297.
Duality of Interest. Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), CanAm (Atlanta, GA), Eli Lilly (Indianapolis, IN), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MI), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), OmniPod Insulin Management System (Bedford, MA), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.K. wrote the manuscript and researched data. P.A.C., C.C.C., B.H.B., R.L.D., M.E.L., P.M.G., H.B.W., D.M.N., and A.V.S. researched data and reviewed and edited the manuscript. C.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. The abstract was presented in poster form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, PA, 8–12 June 2012.
Footnotes
Clinical trial reg. nos. NCT00360815 and NCT00360893, clinicaltrials.gov.
A complete list of participants in the DCCT/EDIC Research Group can be found in ref. 6.
References
- 1.Dorman JS, Steenkiste AR, Foley TP, et al. Familial Autoimmune and Diabetes (FAD) Study Menopause in type 1 diabetic women: is it premature? Diabetes 2001;50:1857–1862 [DOI] [PubMed] [Google Scholar]
- 2.Snell-Bergeon JK, Dabelea D, Ogden LG, et al. Reproductive history and hormonal birth control use are associated with coronary calcium progression in women with type 1 diabetes mellitus. J Clin Endocrinol Metab 2008;93:2142–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakalov VK, Anasti JN, Calis KA, et al. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46,XX spontaneous premature ovarian failure. Fertil Steril 2005;84:958–965 [DOI] [PubMed] [Google Scholar]
- 4.Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev 1997;18:107–134 [DOI] [PubMed] [Google Scholar]
- 5.Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf) 1993;39:35–43 [DOI] [PubMed] [Google Scholar]
- 6.Tatone C, Carbone MC, Campanella G, et al. Female reproductive dysfunction during ageing: role of methylglyoxal in the formation of advanced glycation endproducts in ovaries of reproductively-aged mice. J Biol Regul Homeost Agents 2010;24:63–72 [PubMed] [Google Scholar]
- 7.Sjöberg L, Pitkäniemi J, Harjutsalo V, et al. Menopause in women with type 1 diabetes. Menopause 2011;18:158–163 [DOI] [PubMed] [Google Scholar]
- 8.Gaete X, Vivanco M, Eyzaguirre FC, et al. Menstrual cycle irregularities and their relationship with HbA1c and insulin dose in adolescents with type 1 diabetes mellitus. Fertil Steril 2010;94:1822–1826 [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steffes M, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem 2005;51:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer IH, Sun W, Cleary PA, et al. ; DCCT/ EDIC Research Group Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albers J, Herman W, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the DCCT on peripheral neuropathy in type 1 diabetes during the EDIC Study. Diabetes Care 2010;33:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics 1978;34:541–554 [PubMed] [Google Scholar]
- 14.Isik S, Ozcan HN, Ozuguz U, et al. Evaluation of ovarian reserve based on hormonal parameters, ovarian volume, and antral follicle count in women with type 2 diabetes mellitus. J Clin Endocrinol Metab 2012;97:261–269 [DOI] [PubMed] [Google Scholar]
- 15.Codner E, Iñiguez G, Hernández IM, et al. Elevated anti-Müllerian hormone (AMH) and inhibin B levels in prepubertal girls with type 1 diabetes mellitus. Clin Endocrinol (Oxf) 2011;74:73–78 [DOI] [PubMed] [Google Scholar]
- 16.Lepine LA, Hillis SD, Marchbanks PA, et al. Hysterectomy surveillance United States, 1980–1993. MMWR CDC Surveill Summ 1997;46:1–15 [PubMed] [Google Scholar]
- 17.Cooper R, Hardy R, Kuh D. Is adiposity across life associated with subsequent hysterectomy risk? Findings from the 1946 British birth cohort study. BJOG 2008 Jan;115(2):184–192;discussion 192 [DOI] [PubMed] [Google Scholar]
- 18.Whiteman M, Hillis S, Jamieson D, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol 2008;198:34.e1–34.e7 [DOI] [PubMed]
- 19.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–874 [DOI] [PubMed] [Google Scholar]
- 20.Soto N, Iñiguez G, López P, et al. Anti-Mullerian hormone and inhibin B levels as markers of premature ovarian aging and transition to menopause in type 1 diabetes mellitus. Hum Reprod 2009;24:2838–2844 [DOI] [PubMed] [Google Scholar]