Abstract
OBJECTIVE
We explore the effect of randomized treatment, comparing intensive to standard glucose-lowering strategies on major cardiovascular outcomes, death, and severe adverse events in older versus younger participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.
RESEARCH DESIGN AND METHODS
Participants with type 2 diabetes (n = 10,251) with a mean age of 62 years, a median duration of diabetes of 10 years, and a median A1C of 8.1% (65 mmol/mol) were randomized to treatment strategies targeting either A1C <6.0% (42 mmol/mol) or 7.0–7.9% (53–63 mmol/mol) and followed for a mean of 3.7 years. Outcomes were analyzed within subgroups defined by baseline age (<65 vs. ≥65 years).
RESULTS
Older and younger ACCORD participants achieved similar intensive-arm A1C levels and between-arm A1C differences. Within the older subgroup, similar hazards of the cardiovascular primary outcome and total mortality were observed in the two arms. While there was no intervention effect on cardiovascular mortality in the older subgroup, there was an increased risk in the intensive arm for the younger subgroup (older hazard ratio [HR] = 0.97; younger HR = 1.71; P = 0.03). Regardless of intervention arm, the older subgroup experienced higher annualized rates of severe hypoglycemia (4.45% intensive and 1.36% standard) than the younger subgroup (2.45% intensive and 0.80% standard).
CONCLUSIONS
Intensive glucose lowering increased the risk of cardiovascular disease and total mortality in younger participants, whereas it had a neutral effect in older participants. The intensive to standard relative risk of severe hypoglycemia was similar in both age subgroups, with higher absolute rates in older participants within both treatment arms.
Introduction
Diabetes prevalence in persons over 65 years old is rapidly increasing, with current estimates varying between 15 and 25% (1). During the next decade, the greatest increase in diabetes is anticipated to be among persons aged 75 and older (2). Compared with older adults without diabetes, those with diabetes have life expectancy that is reduced by 10 years and double the mortality (3,4). Reasons for increased mortality and morbidity include cardiovascular, cerebrovascular, and renal diseases and “geriatric” conditions such as cognitive impairment, physical function decline, disability, depression, incontinence, falls, and the syndrome of frailty (i.e., fatigue, weight loss, muscle weakness, and decreased overall physical function) (5,6). Despite this increase of diabetes and its complications in older adults, until recently, older adults were poorly represented in most large diabetes trials. This scenario is similar for many prevalent chronic diseases such as hypertension and cancer trials, where older adults have until recently been traditionally excluded (7,8).
The under-representation of older adults from initial trials showing the benefits of tight glycemic control led to uncertainty regarding the applicability and safety of the intervention in older persons (9). More recent glucose-lowering trials such as the Action to Control Cardiovascular Risk in Diabetes (ACCORD) (10), Action in Diabetes and Vascular Disease (ADVANCE) (11), and the Veterans Affairs Diabetes Trial (12) included older adults; however, they have not reported the effects of the glycemic interventions according to age. Although the overall benefits were modest and, in some cases, were outweighed by the harms (13), these trials can provide valuable insights into whether older adults can safely tolerate intensive therapy for diabetes. Although it has been suggested that treatment targets for older persons with long-standing type 2 diabetes may need to be different (14–16), there is little empiric evidence for such a position. Recommendations for individualization of treatment in older persons with type 2 diabetes have been based on multiple considerations, including comorbidities, polypharmacy, and patient preferences (17). However, as a group, little is known regarding the ability of older versus younger individuals to achieve glycemic targets and the effect of glycemic control on clinically important outcomes.
The purpose of the glycemic portion of the ACCORD study was to determine whether randomization to an intensive therapeutic strategy targeting normal glycated hemoglobin levels (i.e., below 6.0%, 42 mmol/mol) would reduce the rate of cardiovascular events, as compared with a standard strategy targeting glycated hemoglobin levels from 7.0 to 7.9% (53–63 mmol/mol) in people with type 2 diabetes. The ACCORD study previously reported that there were no age-related differences in the effect of the glycemia intervention on cognition (18), but that the intensive intervention reduced the risk of falls in older individuals and increased it in younger individuals (RR = 0.75 and 1.27, respectively; P interaction = 0.018) (19). The impact and tolerability of intensive management in older adults (≥65 years old; specifically, 65–89 years old; N = 3,996) versus younger adults (<65 years old; specifically, 40–64 years old; N = 6,255) on glucose control, severe hypoglycemia, severe adverse effects, the ACCORD primary/secondary outcomes, and physical function (activities of daily living and mobility) are addressed herein. Also addressed is the potentially modifying effect of age on the previously reported findings that 1) the highest risk in mortality among intensive participants occurred for participants with average postrandomization A1C >7.0% (53 mmol/mol) and 2) the increased mortality in the intensive arm was most apparent in those participants whose A1C levels fell less rapidly in the initial year of follow-up (20).
Research Design and Methods
The ACCORD design, CONSORT (Consolidated Standards of Reporting Trials) chart, and major results have been previously published (10,21–23). The ACCORD trial was a randomized, multicenter, double 2 × 2 factorial trial designed to test the effects on major cardiovascular disease (CVD) events of intensive versus standard glycemia control (plus either antihypertensive or lipid-lowering intervention components, which are not addressed in this article). Men and women (N = 10,251) with type 2 diabetes aged 40 to 79 years whose A1C was ≥7.5% (58 mmol/mol) and who had prior evidence of CVD or additional cardiovascular risk factors were recruited at 77 North American sites. The inclusion/exclusion criteria have been previously reported (24). Briefly, participants had to have type 2 diabetes with a glycated hemoglobin level of 7.5% or more, be aged 40 to 79 years with a history of prior CVD, or be aged 55 to 79 years with either anatomical evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors. Participants with frequent or recent serious hypoglycemic events, unwillingness to do home glucose monitoring or inject insulin, a BMI of more than 45 kg/m2, a serum creatinine level of more than 1.5 mg/dL (133 mmol/L), or other serious illness were excluded. Participants were initially recruited into a Vanguard phase of ACCORD (N = 1,184 from January to June 2001), with the subsequent randomization of 9,067 participants taking place from February 2003 to October 2005. In February 2008, the glycemia trial was terminated early due to higher mortality in the intensive compared with the standard glycemia strategies (10), while the antihypertensive and lipid-lowering components (not discussed here) were continued until Spring 2009.
Clinical Measurements
The primary ACCORD outcome was a composite representing the first occurrence of either nonfatal myocardial infarction (MI) or nonfatal stroke or cardiovascular death. This outcome, as well as secondary outcomes, including all cause mortality, an expanded composite comprising the primary outcome plus revascularization or hospitalization for heart failure (congestive heart failure [CHF]), total (i.e., fatal or nonfatal) MI, total stroke, and fatal or nonfatal CHF (21), were included in the current analysis. Also included were adverse events such as severe hypoglycemia (i.e., requiring assistance) or an adverse experience that was life threatening and/or resulted in death, permanent disability, hospitalization, or prolongation of hospitalization.
Two physical function limitations were assessed based on responses to the Health Utilities Index Questionnaire (25), which contains a question that addresses mobility limitations (walking) and another that addresses limitations in the ability to perform basic activities of daily living. These data were obtained at baseline and 12, 36, and 48 months of follow-up and coded in terms of having no difficulty versus any difficulty. A1C was collected in each arm at visits scheduled at 4-month intervals, and the updated average A1C was computed by calculating a weighted average of the cumulative A1C values, with weights defined by the length of intervals between blood draws (20).
Statistical Analysis
Means and percentages were used to compare baseline characteristics for those <65 versus ≥65 years old at enrollment. Median A1C was calculated by follow-up month within glycemia and age subgroup. The percentage of participants experiencing hypoglycemia and other serious adverse events was calculated by glycemia intervention arm. Events per 100 person-years were calculated by dividing the total number of initial events by the total person-years accrued until either the time of the event or censoring.
Time until the initial occurrence of each of the clinical outcomes was analyzed using Cox proportional hazard (PH) regression analyses according to the principle of intention to treat. Hazard ratios (HRs) and 95% CIs were derived from these models within subgroups defined by <65 versus ≥65 years old at enrollment. Analyses were performed for events occurring from randomization until the date of transition (glycemia substudy: 5 February 2008). Cox PH regression models contained a term representing glycemia arm allocation plus the following terms accounting for subgroups of participants that were prespecified in the protocol for analysis of the primary outcome: 1) additional assignment to the blood pressure (BP) or lipid trial, 2) randomization to the intensive BP intervention within the blood pressure trial, 3) randomization to fibrate within the lipid trial, and 4) participants with prior evidence of CVD versus those with no prior CVD. In addition, as done for the ACCORD primary analysis, a term representing the Clinical Center Network was included for analysis of the primary outcome. For each outcome, the consistency of the intervention effects within those <65 versus ≥65 years old was assessed within the Cox models using statistical tests of interactions between the variables representing age and the glycemia intervention. Within those <65 years old and those ≥65 years old, previously described Cox regression models (using penalized B-splines) and Poisson regression models (20), were used to explore the relationship between updated average A1C and mortality risk and to estimate mortality rates in relation to the magnitude of the 1-year change of A1C within each treatment arm, respectively. For both models, likelihood ratio tests were used to test for between-subgroup heterogeneity in both the location and shape of lines fitted within glycemia arms.
A comparison between glycemia arms on the proportion of participants that reported any difficulty in the two functional activities at each follow-up time was performed using logistic regression and generalized estimating equations, controlling for baseline difficulty, assignment to the BP or lipid trial, randomization to the intensive BP intervention within the BP trial, and randomization to fibrate within the lipid trial.
Results
Baseline Characteristics
At the time of randomization, the younger subgroup comprised 6,776 participants and the older subgroup comprised 3,475 participants (1,888 aged 65–69 years, 1,054 aged 70–74 years, 486 aged 75–79 years, and 47 aged 80+ years). As noted in Supplementary Table 1, compared with the younger subgroup, the older subgroup had a longer duration of diabetes (median of 10 vs. 9 years); fewer women (25 vs. 40%) and minorities (34 vs. 39%); and lower body weight, BMI, waist circumference, A1C, diastolic BP, fasting glucose, LDL, total cholesterol, and triglycerides. Baseline mean systolic BP levels were somewhat higher in the older subgroup, and mean levels of HDL and serum creatinine were also higher in this subgroup.
Control of Glycemia
Supplementary Fig. 1 shows the median A1C levels achieved over follow-up through month 80 in the older and younger intensive and standard therapy glycemia arms. The levels achieved were equivalent for intensive therapy for the older and younger subgroups (median ≈6.4 from months 12–48), but for standard therapy, the median A1C was modestly lower in the older subgroup (median ≈7.5 from months 12–48) compared with the younger subgroup (median ≈7.6 from months 12–48).
Medication Use
An age subgroup comparison of medication used to achieve these levels prior to the transition of intensive participants to standard therapy identified that a lower percentage of older versus younger intensive glycemia participants were prescribed metformin (66.6% older, 79.3% younger), any secretagogue (57.7% older, 63.9% younger), and any thiazolidinedione (49% older, 57% younger). Similar percentages of older and younger intensive glycemia participants were placed on any insulin and prandial insulin. The total dose of insulin was slightly lower in the older versus younger intensive glycemia participants (mean = 0.63 vs. 0.74 units/kg, respectively) and 18.1% of older versus 28.2% of younger intensive glycemia participants were on three or more classes of medication plus insulin (see Supplementary Table 2). Of final note, older adults had higher rates than younger adults for discontinuation of active management of glycemia medication regimens by ACCORD physicians at some point during follow-up (11.3 vs. 8.9%, respectively; P = 0.0001). Among older participants, 12.4% in intensive glycemia versus 10.1% in standard glycemia discontinued ACCORD medication management at least once during follow-up (9.6 vs. 8.2%, respectively, in the younger group).
Adverse Events
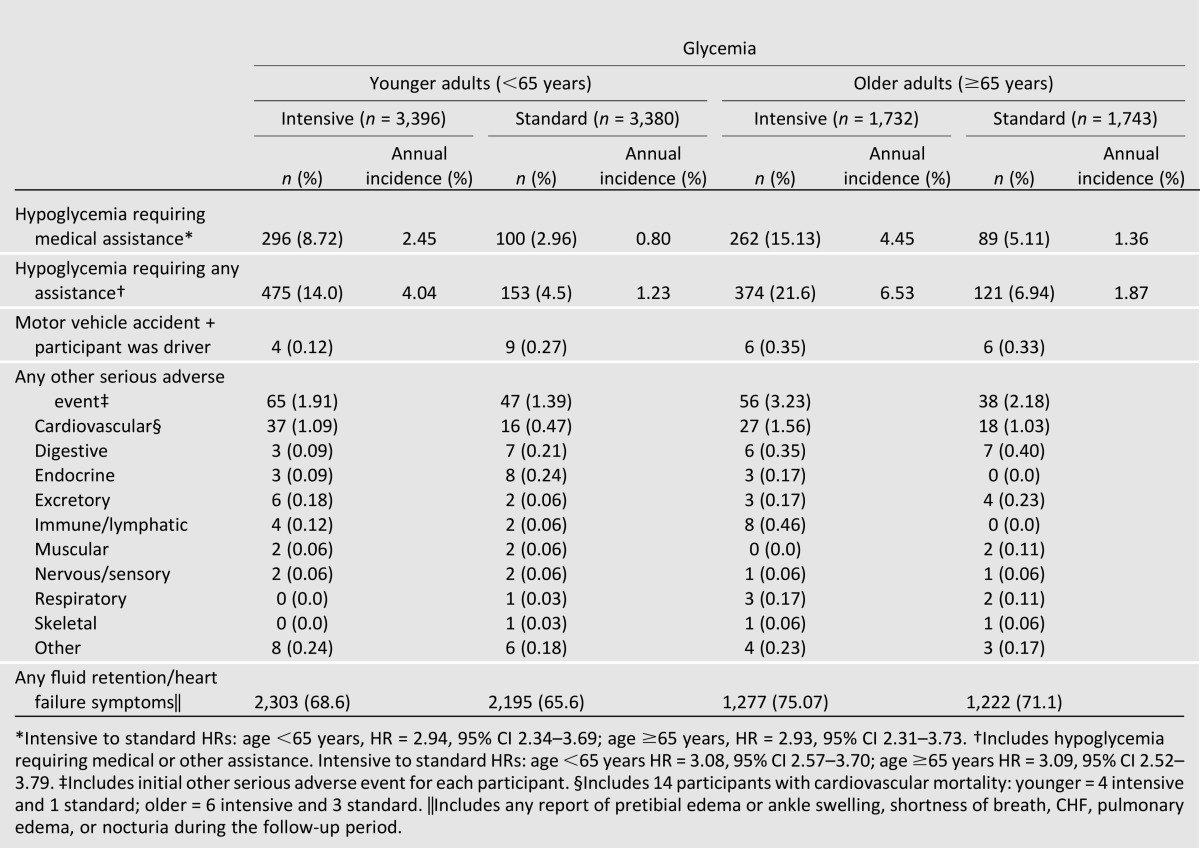
As noted in Table 1, participants allocated to intensive glycemic therapy had approximately three times the rate of severe hypoglycemia as those allocated to standard therapy. This increased risk of hypoglycemia attributable to intensive therapy was similar for the two age subgroups. However, the absolute annual incidence of severe hypoglycemia was greater for older individuals allocated to both treatment arms (4.45 and 1.36% in intensive and standard glycemia, respectively) than in younger individuals (2.45 and 0.80% for intensive and standard glycemia, respectively). Over a mean follow-up of 3.7 years, these rates translated into 15.1 and 8.7% of intensively treated older and younger participants, respectively, reporting severe hypoglycemia (5.1 versus 3.0% of older and younger standard glycemia participants, respectively).
Table 1.
Adverse events by glycemia and age subgroup for events occurring before the transition (5 February 2008)
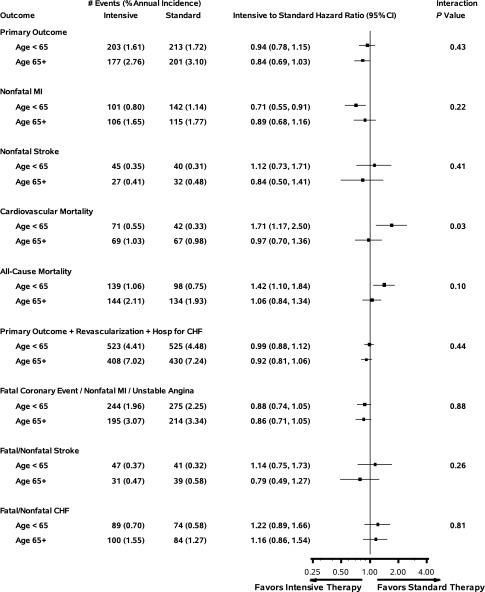
Primary and Secondary Outcomes
As presented in Fig. 1, the effect of the intervention on the primary and all but one of the secondary outcomes was similar across age subgroups. There was no intervention effect on cardiovascular mortality in the older subgroup (HR = 0.97; 95% CI 0.70–1.36), but an increased risk in the intensive arm for younger (HR = 1.71; 95% CI 1.17–2.50) versus older participants (P interaction = 0.03). As expected, the older subgroup had higher absolute event rates for all outcomes considered within both treatment arms. The percentage of participants who were lost to follow-up for outcomes was 3.7% for older adults and 4.1% for younger adults (P = 0.30).
Figure 1.
Horizontal bars represent the 95% CI on the HR. Hosp, hospitalization.
Functional Limitations as an Outcome
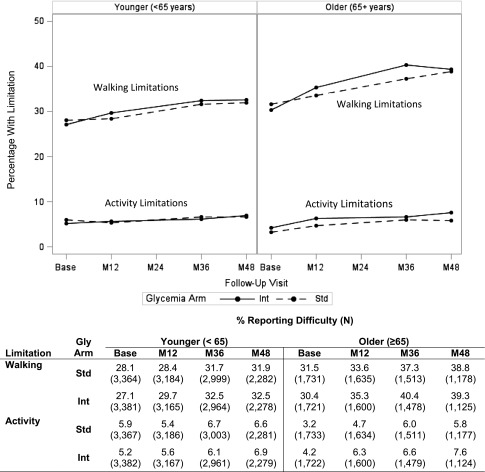
For self-reported difficulty with walking, the percentage of participants reporting difficulty ranged from 30–40% for the older subgroup and from 27–33% for the younger subgroup, and for difficulty with activity, the percentages ranged from 3.2–7.6% and 5.9-6.9% within the same age groups, respectively (Fig. 2). The differences between glycemia arms (for either age group) in percentage reporting either difficulty (walking or activity) were clinically minimal (<2% on an absolute scale).
Figure 2.
Plots represent the proportion of participants reporting difficulty with either walking or activities at each follow-up visit. In the table below the figure, the number of participants providing data at each time point is presented in parentheses, after the percentage of participants reporting difficulty. M12, month 12; M24, month 24; M36, month 36; M48, month 48; Int, intensive; Std, standard; Gly, glycemia.
For the walking outcome, we found greater limitations in the intensive arm averaged across time points (P = 0.024) and at the 36-month visit (P = 0.014) for older participants and no differences at 12 months (P = 0.09) or 48 months (P = 0.33). Among younger participants, there were no differences between the glycemia arms across time points (P = 0.06) or at any visit (P = 0.06 for month 12; P = 0.38 at month 36; P = 0.13 at month 48).
For activity limitations, slightly higher levels of limitations existed in the intensive arm within the older subgroup across time points (P = 0.012) and at the 12-month (P = 0.027) and 48-month (P = 0.042) visits, and no differences were found in activity for younger participants (P = 0.72 overall; P = 0.61 at month 12; P = 0.60 at month 36; P = 0.47 at month 48).
Epidemiological Analysis of Mortality and A1C Levels
Figure 3 contains plots of the relationships between all-cause mortality and both the updated average A1C (Fig. 3A and B) and the initial 12-month fall in A1C (Fig. 3C and D) by age subgroup and intervention arm. Comparing the relationships between Fig. 3A and B, we note that there is no difference between age subgroups in the shape (slope of lines at each updated, average A1C value) for the intensive (P = 0.38) or standard (P = 0.52) arms; however, the vertical positioning of the lines for the older subgroup are significantly higher within both intensive (P = 0.01) and standard (P < 0.01) arms. There is a significant difference between the shapes of the intensive and standard lines within Fig. 3A (P = 0.005) but not Fig. 3B (P = 0.06). For both Fig. 3A and B, the confidence intervals for the standard lines for A1C <6.8 completely cover the intensive line, thus limiting conclusions about differences in this tail of the distribution. The increase in mortality risk within intensively treated participants was primarily among those with updated average A1C >7.0 (53 mmol/mol), regardless of age subgroup.
Figure 3.
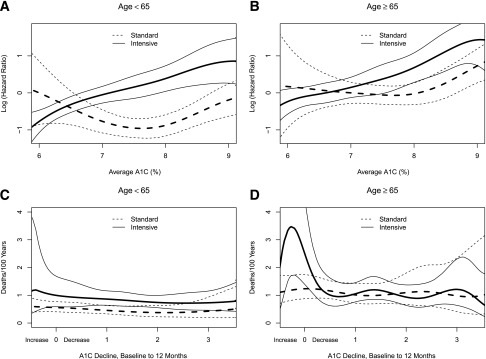
Spline curves of the risk of all-cause mortality with the two treatment strategies. A and B: The linear part of the PH model for average A1C from 6.0 to 9.0% (42 to 75 mmol/mol) for participants aged <65 and ≥65 years at randomization, respectively. For clarity, the figure omits values <6 (42 mmol/mol) and >9% (75 mmol/mol); approximately 5% of deaths are excluded from the plot at the lower and also at the higher end of the A1C range, but these data are included in the models. The plotted values are relative to a standard participant, aged <65 years at randomization and at an A1C of 6%. The dashed lines represent estimates and CIs for standard participants, solid lines are for intensive participants. C and D: The results from a Poisson regression model of all-cause mortality rates by treatment and age subgroup for the whole period of follow-up, over a range of decreases in A1C from baseline in the first year of treatment (as %A1C). The figures omit values beyond the 5th and 95th percentiles of A1C changes. The full range of values was from 6.8% (51 mmol/mol; an increase) to 7.4% (57 mmol/mol; a decrease) from baseline. The calculations used a Poisson regression model with data from model 3 of Riddle et al. (20).
When relating mortality risk to the initial 12-month fall in A1C (Fig. 3C and D), we could not conclude that the shape of the lines between the two age subgroups were different within intensive (P = 0.24) or standard (P = 0.23) arms; however, the location of the lines were higher for older participants in both intensive (P = 0.03) and standard (P < 0.01) arms. Within age groups, the shapes of intensive and standard curves were not different within Fig. 3C (P = 0.79) but were different within Fig. 3D (P = 0.003). While the older subgroup that was treated intensively displays a large elevation in the mortality rate for those with little reduction in A1C levels (Fig. 3D), this elevation must be considered within the context of the variability in estimates; only 6 deaths and 219 person-years of follow-up occurred among intensive participants with an increase in A1C during the initial 12 months.
Conclusions
Overall, our analysis of the impact of baseline age on the effect of more intensive blood glucose lowering in the ACCORD trial indicates that, relative to standard glycemic treatment, intensive treatment resulted in similar metabolic (e.g., attained A1C targets) and primary and secondary end point effects in older and younger participants. These results illustrate that age is not a primary factor in success of achieving glycemia treatment targets within the age range included in ACCORD. Similarly, the ADVANCE study found that the effect of intensive glycemic control on the primary outcome of major cardiovascular events was comparable within younger and older age subgroups (11). These results are analogous to findings such as those of the SHEP (Systolic Hypertension in the Elderly Program) study that contradicted commonly held beliefs that older adults would not tolerate targeted BP reduction as well as younger patients with hypertension (26).
Compared with standard therapy, ACCORD’s intensive glycemia strategy resulted in a higher incidence of cardiovascular mortality in the younger participants but not in older participants (P = 0.03 for interaction). A similar trend between younger and older participants was seen for total mortality (P = 0.10). As might be expected, the absolute incidence of outcome and adverse events was generally greater in the older compared with the younger subgroup.
It has been previously suggested that older adults may be more susceptible to hypoglycemic episodes (27–29). ACCORD investigators and others have shown that that there are definable subgroups of older adults, such as those persons with evidence of early cognitive impairment (30) or dementia (31), who are at greatest risk for serious treatment side effects such as hypoglycemia. Our results now amplify prior findings in older patients. In ACCORD, older adults were at higher risk of hypoglycemia than younger adults, and intensive glycemic therapy appeared to triple the risk in both older (4.45% intensive and 1.36% standard annualized risk) and younger (2.45% intensive and 0.80% standard annualized risk) adults. In the older group, over a mean follow-up of 3.7 years, these rates translated into a total of 15.1% intensive and 5.1% standard arm participants reporting severe hypoglycemia. The trend for increased hypoglycemia risk among older participants was identified early during ACCORD follow-up in those ≥80 years old at randomization, where early monitoring of hypoglycemia rates by the external data and safety monitoring board (DSMB) identified elevated rates of hypoglycemia in participants who were ≥80 years old at randomization. The ACCORD DSMB recommended at their May 2003 meeting that no additional participants over 80 years old be recruited into the main ACCORD trial, and this recommendation was quickly implemented. At that time, among the 1,184 Vanguard participants followed for an average of 1.8 years (1,150 were <80 years old, 34 were 80+ years old), 20.5% of those ≥80 years old and 4.7% of those <80 years old had reported hypoglycemia requiring emergency medical assistance.
Two hypotheses set forth for the increased mortality in participants treated intensively for glycemia have involved the speed of decline in A1C (20) and the increase in hypoglycemia rates (32). In epidemiologic analyses, Riddle et al. (20) have shown that the highest rates of mortality in the intensive arm was in the subgroup of participants with the least rapid drop in A1C levels during the initial 12-months of follow-up. The elevated mortality risk associated with less A1C decline in the initial 12 months was most prominent in those in the older subgroup (see Fig. 3). However, any inference regarding glycemia treatment differences in these figures should be severely restricted due to the variability in estimates at the tails of the distributions. Regarding hypoglycemia, Bonds et al. (32) were unable to directly link higher rates of severe hypoglycemia in the intensive arm to the overall increased risk of mortality in intensively treated participants. Notably, our analyses identified no increase in total mortality risk associated with intensive therapy compared with standard therapy among older participants (HR = 1.06) but a similar relative increase in risk of hypoglycemia for intensive versus standard treated participants for older and younger participants (approximately 3.0 in both subgroups). In addition, the neutral effect of allocation to the intensive treatment on mortality in older participants did not appear to be due to the slightly higher rate of discontinuation of ACCORD medication management in this group (unreported analyses). Finally, because a wide range of glycemic approaches were used and individually tailored to participant characteristics, epidemiologic analyses of ACCORD medications data may be unable to detect small but important relationships within subgroups.
These results should be viewed as hypothesis generating and interpreted with caution since they are tertiary analyses for the ACCORD trial involving subgroups, some of which may be quite small (33). Specifically, the demographic differences between the two age groups relative to gender, racial composition, and other characteristics should also be noted relative to the patient populations for whom these subgroup analyses apply. Finally, ACCORD was designed to include only community-dwelling ambulatory participants; thus these results cannot be applied to more frail and disabled or institutionalized groups of older adults.
In summary, while we have shown that similar glycemic levels can be reached in ambulatory, community-dwelling older and younger adults, the frequency of serious adverse events associated with intensive targets was consistently higher within the older subgroup. The increased risk of hypoglycemia in older versus younger adults, regardless of whether they were in intensive or standard therapy, also suggests the need to individualize therapy in older adults with type 2 diabetes, as suggested by others (15,34,35). The recent 2012 American Diabetes Association Consensus Report emphasizes a need to stratify targets based on comorbid illness and functional status, among other factors, rather than on age alone (17). Where the ACCORD results do not indicate that significant excess mortality occurred among intensively versus standard treatment older adults, there is little evidence to suggest that older adults received a CVD benefit. Importantly, the ACCORD glycemia experience supports the concept that older adults should be included in clinical trials along with careful monitoring of adverse effects. Exploratory analyses of the type we have performed can help to inform the design, implementation, and monitoring of future clinical trials that include older patients with type 2 diabetes and other chronic diseases.
Supplementary Material
Article Information
Acknowledgments. Members of the ACCORD DSMB included Antonio M. Gotto Jr. (chair), Weill Medical College of Cornell University; Kent Bailey, Section on Biostatistics, Mayo Clinic; Dorothy Gohdes, Consultant; Steven Haffner, Department of Medicine, The University of Texas Health Science Center at San Antonio; Roland Hiss, Department of Medical Education, University of Michigan; Kenneth Jamerson, Department of Internal Medicine/Hypertension, University of Michigan; Kerry Lee, Duke Clinical Research Institute; David Nathan, Diabetes Center, Massachusetts General Hospital; James Sowers, Endocrine Division, SUNY Downstate; and LeRoy Walters, Kennedy Institute of Ethics, Georgetown University.
Funding. This work was supported by National Heart, Lung, and Blood Institute contracts N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA #Y1-HC-9035, and IAA#Y1-HC-1010. Other components of the National Institutes of Health, including the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Eye Institute, contributed funding. The Centers for Disease Control and Prevention funded substudies within ACCORD on cost-effectiveness and health-related quality of life. General Clinical Research Centers provide support at many sites. The work of M.E.M., J.D.W., and W.B.A. was partially supported by the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332).
Duality of Interest. H.C.G. reports consulting fees from Sanofi, Novo Nordisk, Lilly, Bristol-Myers Squibb, Roche, AstraZeneca, Novartis, GlaxoSmithKline, and Bayer; lecture fees from Sanofi and Bayer; and support for research or continuing education through his institution from Sanofi, Lilly, Merck, Novo Nordisk, Boehringer Ingelheim, Bristol-Myers Squibb, and AstraZeneca. R.P.B. reports receiving consulting fees from Lilly. W.C.C. reports consulting fees from Takeda, AstraZeneca, Merck, Omron, Daiichi-Sankyo, and Novartis and grants from Merck. H.N.G. reports consulting fees from Merck, Sanofi, Bristol-Myers Squibb, AstraZeneca, Pfizer, and Novartis and grant support from Merck and Sanofi. The following companies provided study medications, equipment, or supplies: Abbott Laboratories (Abbott Park, IL), Amylin Pharmaceuticals (San Diego, CA), AstraZeneca Pharmaceuticals LP (Wilmington, DE), Bayer HealthCare LLC (Tarrytown, NY), Closer Healthcare Inc. (Tequesta, FL), GlaxoSmithKline Pharmaceuticals (Philadelphia, PA), King Pharmaceuticals Inc. (Bristol, TN), Merck & Co. Inc. (Whitehouse Station, NJ), Novartis Pharmaceuticals Inc. (East Hanover, NJ), Novo Nordisk Inc. (Princeton, NJ), Omron Healthcare Inc. (Schaumburg, IL), Sanofi U.S. (Bridgewater, NJ), and Schering-Plough Corporation (Kenilworth, NJ). No other potential conflicts of interest relevant to this article were reported.
The donors of medications and devices had no role in the study design, data accrual and analysis, or manuscript preparation.
Author Contributions. M.E.M. and L.L. researched data and wrote the manuscript. J.D.W., H.C.G., R.P.B., W.C.C., and H.N.G. reviewed and edited the manuscript. W.T.A. researched the data. W.B.A. wrote the manuscript. M.E.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00000620, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-1545/-/DC1.
The entire list of ACCORD Investigators can be found in an appendix to ref. 10. Protocol available at www.accordtrial.org.
References
- 1.Department of Health and Human Services CfDCaP National diabetes fact sheet; national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 2.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–1890 [DOI] [PubMed] [Google Scholar]
- 3.Panzram G. Mortality and survival in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1987;30:123–131 [DOI] [PubMed] [Google Scholar]
- 4.Stengård JH, Tuomilehto J, Pekkanen J, et al. Diabetes mellitus, impaired glucose tolerance and mortality among elderly men: the Finnish cohorts of the Seven Countries Study. Diabetologia 1992;35:760–765 [DOI] [PubMed] [Google Scholar]
- 5.Katakura M, Naka M, Kondo T, et al. Prospective analysis of mortality, morbidity, and risk factors in elderly diabetic subjects: Nagano study. Diabetes Care 2003;26:638–644 [DOI] [PubMed] [Google Scholar]
- 6.Brown AF, Mangione CM, Saliba D, Sarkisian CA, California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003;51(Suppl. Guidelines):S265–S280 [DOI] [PubMed] [Google Scholar]
- 7.Applegate WB, Curb JD. Designing and executing randomized clinical trials involving elderly persons. J Am Geriatr Soc 1990;38:943–950 [DOI] [PubMed] [Google Scholar]
- 8.Cerreta F, Eichler HG, Rasi G. Drug policy for an aging population—the European Medicines Agency’s geriatric medicines strategy. N Engl J Med 2012;367:1972–1974 [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 10.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 12.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 13.Skyler JS, Bergenstal R, Bonow RO, et al. American Diabetes Association. American College of Cardiology Foundation. American Heart Association Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the u.s. Diabetes Care 2006;29:2415–2419 [DOI] [PubMed] [Google Scholar]
- 15.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011;154:554–559 [DOI] [PubMed] [Google Scholar]
- 16.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Launer LJ, Miller ME, Williamson JD, et al. ACCORD MIND investigators Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz AV, Margolis KL, Sellmeyer DE, et al. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care 2012;35:1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddle MC, Ambrosius WT, Brillon DJ, et al. Action to Control Cardiovascular Risk in Diabetes Investigators Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010;33:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerstein HC, Riddle MC, Kendall DM, et al. ACCORD Study Group Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99(12A):34i–43i [DOI] [PubMed] [Google Scholar]
- 22.Ginsberg HN, Bonds DE, Lovato LC, et al. ACCORD Study Group Evolution of the lipid trial protocol of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99(12A):56i–67i [DOI] [PubMed] [Google Scholar]
- 23.Cushman WC, Grimm RH, Jr, Cutler JA, et al. ACCORD Study Group Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99(12A):44i–55i [DOI] [PubMed] [Google Scholar]
- 24.Buse JB, Bigger JT, Byington RP, et al. ACCORD Study Group Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99(Suppl.):21i–33i [DOI] [PubMed] [Google Scholar]
- 25.Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med 2001;33:375–384 [DOI] [PubMed] [Google Scholar]
- 26.SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255–3264 [PubMed] [Google Scholar]
- 27.Schade DS, Burge MR. Brittle diabetes: etiology and treatment. Adv Endocrinol Metab 1995;6:289–319 [PubMed] [Google Scholar]
- 28.Tattersall R, Gregory R, Selby C, Kerr D, Heller S. Course of brittle diabetes: 12 year follow up. BMJ 1991;302:1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SJ, Boscardin WJ, Stijacic Cenzer I, Huang ES, Rice-Trumble K, Eng C. The risks and benefits of implementing glycemic control guidelines in frail older adults with diabetes mellitus. J Am Geriatr Soc 2011;59:666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Punthakee Z, Miller ME, Launer LJ, et al. ACCORD Group of Investigators. ACCORD-MIND Investigators Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaffe K, Falvey CM, Hamilton N, et al. Health ABC Study Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med 2013;173:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials 2010;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genuth S, Ismail-Beigi F, Ioannidis JP, Altman DG, Hayward RA. Clinical implications of the ACCORD trial. J Clin Endocrinol Metab 2012;97:41–48 [DOI] [PubMed] [Google Scholar]
- 35.Del Prato S, LaSalle J, Matthaei S, Bailey CJ, Global Partnership for Effective Diabetes Management Tailoring treatment to the individual in type 2 diabetes practical guidance from the Global Partnership for Effective Diabetes Management. Int J Clin Pract 2010;64:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.