Abstract
OBJECTIVE
Cross-sectional and longitudinal associations among regimen distress (RD), self-management, and glycemic control were undertaken to explore mechanisms of operation among these variables.
RESEARCH DESIGN AND METHODS
In a behavioral randomized control trial (RCT) to reduce RD, 392 adults with type 2 diabetes were assessed for RD, diet, exercise, medication adherence, and HbA1c at baseline and at 4 and 12 months. Associations among RD, self-management, and HbA1c were examined in cross-sectional analyses at baseline, in prospective analyses using baseline values to predict change over time, and in time-varying analyses.
RESULTS
At baseline, greater RD and poorer medication adherence were independently associated with higher HbA1c (P = 0.05 and P < 0.001, respectively), and greater RD was associated with poorer medication adherence (P = 0.03). No consistent pattern of significant prospective associations was found. Significant time-varying findings showed that decreases in RD were associated with improvements in medication adherence (P < 0.01), physical activity (P < 0.001), and HbA1c (P = 0.02) over time following intervention. Changes in self-management were not associated with changes in HbA1c over time.
CONCLUSIONS
In the context of an RCT to reduce distress, RD, self-management, and HbA1c were interrelated in cross-sectional and time-varying analyses. Decreases in RD were associated with improvements in both self-management and HbA1c over 12 months. Findings point to the complex and likely multifaceted pathways of association among these key constructs, with results indicating significant linkages between RD and both self-management and glycemic control over time.
Introduction
Diabetes distress refers to the often hidden emotional burdens, stresses, and worries that are part of managing diabetes (1). Regimen distress (RD) is a critical area of diabetes distress that focuses on daily disease management and is significantly associated with poorer diabetes outcomes, even after controlling for clinical depression (2–4). Although RD, self-management behavior, and glycemic control are core constructs central to the treatment of type 2 diabetes, the mechanisms underlying their interrelationships over time are not well understood. Cross-sectional studies have shown that high diabetes distress is associated with poor glycemic control and poor behavioral self-management (e.g., healthy diet, physical activity, medication adherence) (2–6), and to a lesser extent, poor self-management is associated with poor glycemic control (7,8). However, although critically important (9), little longitudinal research is available, severely restricting the identification of the causal and interactive mechanisms to explain these findings. Thus, how these constructs are causatively linked or how a potential third variable might be involved remain unclear. For example, it may be that RD interferes with behavioral management, which in turn affects glycemic control (10); that RD affects glycemic control directly through physiological mechanisms (11,12); or that poor glycemic control leads to low motivation for making behavioral lifestyle changes, which in turn increases RD. There is also some evidence that these constructs may influence one another bidirectionally and iteratively over time (3,13,14).
Randomized controlled trials (RCTs) provide a useful setting in which to study potential mechanisms among or between key constructs over time because the interventions themselves are developed to enhance change and to make the interactive patterns of change among the variables more visible. This approach stands in contrast to static, cross-sectional comparisons. Furthermore, RCTs enable an examination of individual subject change over time rather than focus exclusively on average differences between groups of patients over time.
Within the context of a 12-month RCT aimed at reducing diabetes distress, the current article explores potential linkages among RD, disease management, and glycemic control, making use of the following group- and patient-level changes over time: traditional group-level baseline cross-sectional comparisons, group-level prospective analyses in which baseline levels of one construct are used to predict change in another over time; and patient-level time-varying analyses in which change in one construct over time is associated with change in another over time. To explore potential mechanisms of interaction among the variables of interest, we hypothesized that 1) concurrent associations will occur among high RD, high HbA1c, and poor behavioral management; 2) low distress at baseline will predict greater decreases in HbA1c and increases in behavioral management over time; and 3) decreases in RD will be associated with decreases in HbA1c and increases in behavioral management over time. Addressing these questions will further our understanding of potential mechanisms of action among RD, self-management behavior, and glycemic control.
Research Design and Methods
Subjects
Patients with type 2 diabetes were recruited from the patient registries of several community medical groups and diabetes education centers. The primary inclusion criterion was a mean score of ≥1.5 on the two-item Diabetes Distress Screener (15) (confirmed later by the full scale). Response options for the two items ranged from 1 (not a problem) to 6 (very serious problem), with a response of 2 defined as a little problem, thus including individuals with at least a modest level of diabetes distress (16). Additional inclusion criteria were a registry-recorded diagnosis of type 2 diabetes ≥12 months, age ≥21 years, ability to read and speak English, at least moderate computer use facility, easy availability of a computer with Internet access, and self-reported problems with adherence to diabetes management (healthy eating or exercise plan not followed for 3 of 4 days during the previous week or medications not taken ≥2 days during the previous week) based on the Summary of Diabetes Self-Care Activities (17). Exclusion criteria included clinical depression [Patient Health Questionnaire 8 score ≥15 (18)] and severe diabetes complications or functional deficits (e.g., dialysis, blindness). Thus, the sample included patients who had at least modest RD and some behavioral management difficulties so that change in one or more of these variables could be observed over time.
Procedures
A description of the study protocol and the intervention program have been previously published (19). Patients received a letter from their health-care facility informing them of the Reducing Distress and Enhancing Effective Management (REDEEM) study. During a subsequent call, the project was explained and patients were screened, and eligible patients were invited to a meeting where eligibility requirements were confirmed, informed consent was obtained, and a 1.5-h baseline assessment was completed. The assessment included height and weight, questionnaires, a brief interview, and visit to a community laboratory for collection of biological data. Patients were then randomized to one of the three interventions by a computer-generated algorithm, and an intervention visit was scheduled. In keeping with a pragmatic design and comparative effectiveness research (20), no usual care condition was included because of concerns about maintaining distressed patients in a noninterventional study arm. The interventions were 1) Computer-Assisted Self-Management (CASM), which featured a 40-min, previously validated, web-based, diabetes self-management improvement program (21,22); 2) CASM plus problem-solving therapy (CAPS) (23,24); and 3) a minimal intervention that featured a 20-min, computer-delivered health risk appraisal and diabetes information (Leap Ahead) (25). In all conditions, patients received a live supplemental booster session at month 5, which included a repeated health risk appraisal for Leap Ahead patients and an automated program to reduce negative behavioral practices for patients in CASM and CAPS. Patients in all conditions also received the same sequence of eight 15-min live phone calls (at weeks 2, 4, 7, 12, 24, 28, 36, and 44) to check progress and provide encouragement. Assessments were repeated at 4 and 12 months postintervention. The University of California, San Francisco, Institutional Review Board and the committees of collaborating institutions approved this study. Data were collected between 2008 and 2011 and analyzed in 2012.
Measures
Patient demographic variables included age, sex, ethnicity (dichotomized as white and nonwhite), and education (trichotomized as less than college, technical school, and college). Diabetes status included use of insulin (yes/no), years since diagnosis, and total number of comorbidities (e.g., asthma, rheumatoid arthritis) and complications (e.g., kidney problems, stroke).
RD was assessed by the five-item RD subscale (α = 0.90) (16) from the Diabetes Distress Scale (16). RD was selected because it has the highest prevalence (19) and was directly targeted by the interventions. RD items were rated on a 6-point scale from 1 (not a problem) to 6 (very serious problem). Physical activity was assessed by the CHAMPS (Community Healthy Activities Model Program for Seniors) instrument (26,27), which measures weekly caloric expenditure of light, moderate, and heavy physical activity by assessing the frequency of engaging in various forms of physical activity. Only the light physical activity composite scale (eight items, e.g., walking, gardening, housework) was used in the present analyses because of its appropriateness for a primarily older age-group and because light physical activity was the area of focus targeted by the interventions. Healthy eating was assessed by the National Cancer Institute Percent Energy From Fat Screener (28), a validated instrument that estimates percent energy (calories) from fat based on the consumption of 14 foods (e.g., margarine or butter, rice, cheese) that is sensitive to change. Medication nonadherence was assessed by the eight-item Hill-Bone Compliance Scale (α = 0.80) (29), which assesses how often and why respondents miss taking medications as rated on a 4-point scale from none of the time to all of the time. This scale yields a single nonadherence index score. Glycemic control was assessed by HbA1c, which was analyzed in a central laboratory for all participants.
Data Analysis
Descriptive statistics were computed to review score distributions. Missing data were imputed with multiple imputation procedures using NORM version 2 software (30). NORM imputes data through the expectation-maximization algorithm, which provides an efficient estimation of means, variances, and covariances, and uses a data augmentation procedure that generates multiple imputations of missing values.
One-way ANOVA and χ2 tests, as appropriate, were conducted to test for baseline differences across the three treatment conditions and to examine potential differences in outcomes between dropouts and continuing participants. For all cross-sectional, prospective, and time-varying analyses, sex, age, insulin use, ethnicity (white vs. other), education (three levels), years since diabetes diagnosis, and number of comorbidities and complications were included as covariates. Intervention group was also included as a covariate in all analyses. Continuous variables were grand mean centered to facilitate interpretation of results.
Baseline Cross-sectional Models
A multivariable linear regression model was specified for each outcome: HbA1c, RD, medication nonadherence, diet, and physical activity. Predictors included intervention group, sociodemographic variables, and baseline values of the other four outcomes. Additional models were conducted to examine whether results differed when each outcome was predicted by baseline level of only one other outcome at a time.
Prospective Models
These analyses examined whether change over time was associated with baseline values of key variables. The outcome variables were change from baseline within each individual using five random-intercept general linear mixed models, one for each outcome. Predictors included time code (0 at baseline, 0.33 at 4 months, and 1.00 at 12 months), each individual’s personal mean across three time points to adjust for between-person differences in the outcome, intervention group, sociodemographic variables, and baseline values of the other four outcomes. Additional random-intercept mixed models examined whether results differed when each change-scored outcome was predicted by the baseline level of only one other outcome at a time.
Time-Varying Covariate Models
Five random-intercept linear mixed models were specified to determine whether change from baseline in each outcome was associated with change from baseline among the other outcomes, using approaches that completely partition between- and within-participant effects (31,32). Additional analyses examined whether results differed when each outcome was predicted by change in only one other outcome at a time. Statistical analyses were performed with SPSS version 19.0 (IBM Corporation, Chicago, IL).
Results
Preliminary Analyses
Of 2,606 patients identified from registries who could be contacted, 658 were eligible, and 436 agreed to participate (66.6%). Problems with time and conflicting life demands were the most frequent reasons for nonparticipation. Of these, 392 (89.5%) completed baseline assessment and intervention, with 150 randomized to CASM, 146 to CAPS, and 96 to Leap Ahead. The smaller Leap Ahead sample was built into the randomization allocation algorithm to provide more power to compare the two active arms. According to telephone screening data, there were no significant differences between those who participated and those who refused on the basis of sex, age, ethnicity, education level, and years since diagnosis.
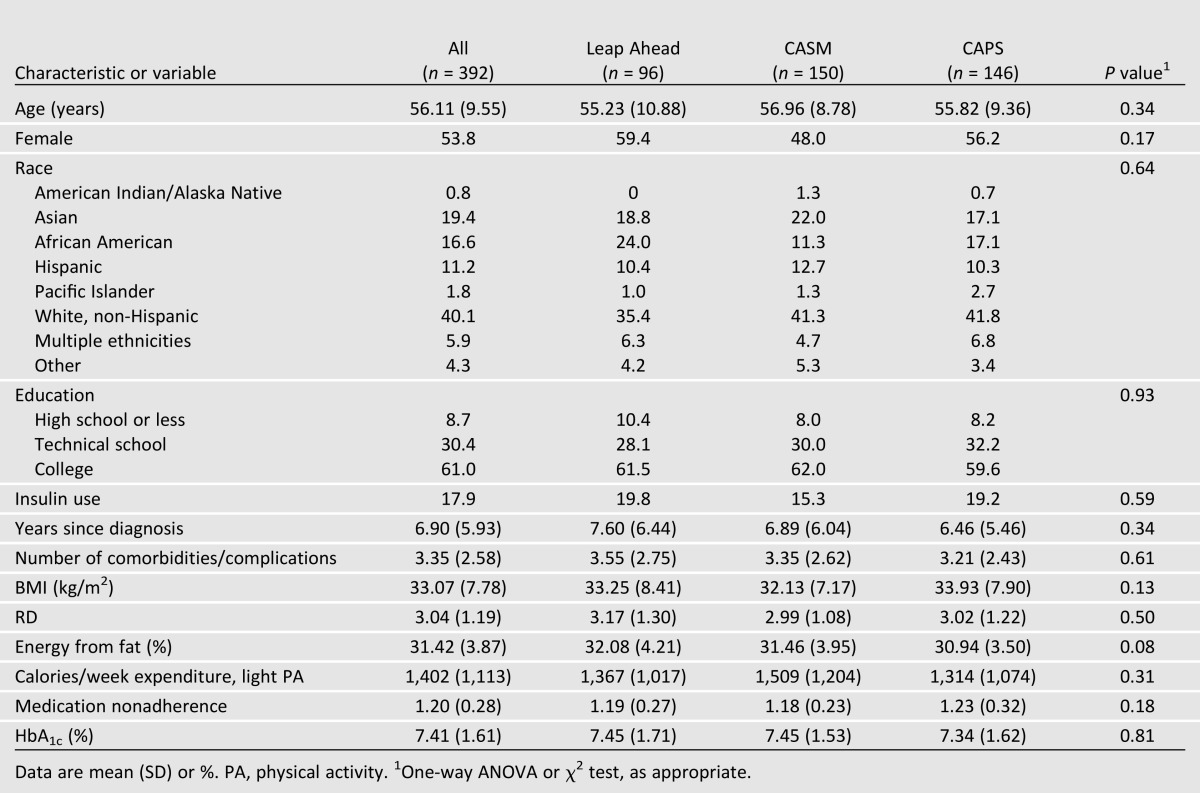
Attrition was 13.8% from baseline to 4 months, 5.7% from 4 to 12 months, and 18.7% from baseline to 12 months. Only 8.4% of patients missed both 4- and 12-month assessments. There were no significant between-group differences in attrition across either time period on any key study variable. There were no significant baseline differences among the three study groups on any key demographic or diabetes status variable (Table 1) or differences on key variables based on recruitment source (community group vs. diabetes education center). The diverse sample had a mean age of 56 (SD 9.6) years, 53.8% of the sample was female, 8.7% of patients had ≤ 12 years of education, and mean baseline HbA1c was 7.4% (1.6%) [57.0 (17.6) mmol/mol].
Table 1.
Baseline characteristics of participants by intervention group
Cross-sectional Analyses
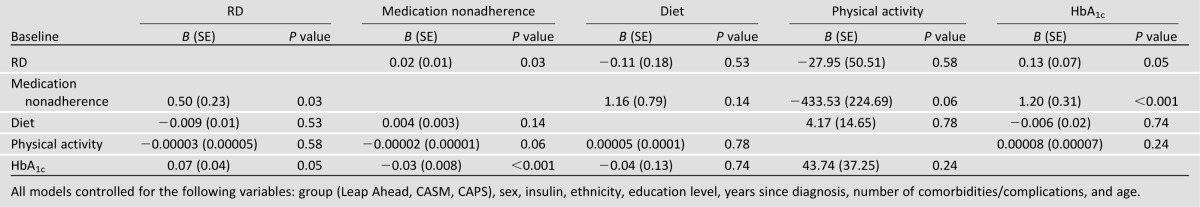
In models that included all the primary predictors at baseline, both higher RD and poorer medication adherence were associated with higher HbA1c, and higher RD was associated with poorer medication adherence (Table 2). Age and comorbidities/complications were the only background characteristics consistently related to the outcomes. Younger patients reported higher RD, HbA1c, and medication nonadherence. Patients with more comorbidities/complications had higher RD, higher medication nonadherence, and higher fat diets. These patients also reported greater physical activity. The same pattern was found when each of the four key predictors was entered in separate models, indicating that these associations were independent of one another. Thus, we found significant baseline cross-sectional associations among RD, medication nonadherence, and HbA1c.
Table 2.
Baseline cross-sectional associations
Prospective Analyses
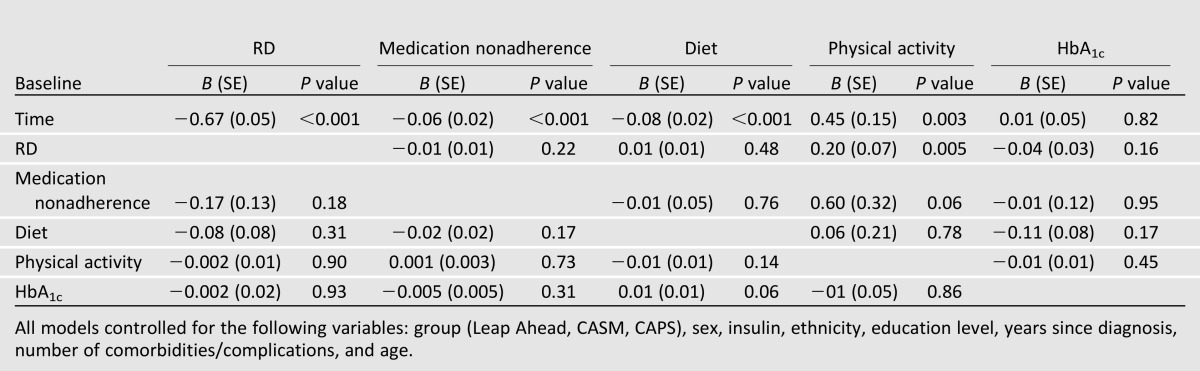
Significant improvements over time were found among RD, medication nonadherence, physical activity, and dietary fat intake. Although the average change in HbA1c over time was not significantly different from zero for the sample as a whole, there was significant variation in change in HbA1c over time, with some patients showing significant increases and others significant decreases. There were only two models in which the baseline value of a construct predicted change in an outcome over time: Higher baseline RD and higher medication nonadherence significantly predicted greater improvement in physical activity over time (Table 3). Male patients, those with fewer comorbidities/complications, and those with less education had larger decreases in HbA1c over time. Men showed less improvement in physical activity, and younger patients demonstrated greater decreases in medication nonadherence but less improvement in dietary fat intake. The same results were observed in models that included only one of the primary predictors at a time. Thus, few significant prospective associations occurred in which the baseline level of one core construct predicted change over time in another.
Table 3.
Baseline predictors of change in outcomes over time
Time-Varying Analyses
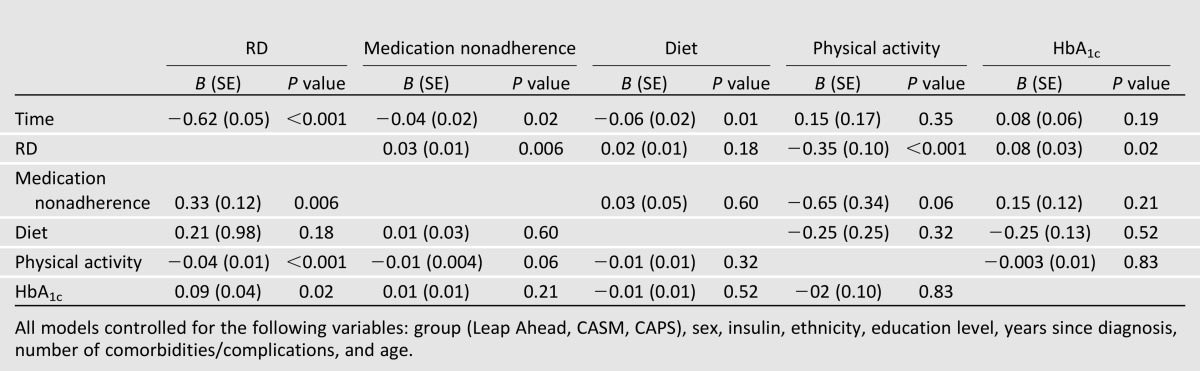
Decreases in RD over time were significantly associated with decreases in HbA1c (Table 4). None of the changes among the three self-management variables were associated with changes in HbA1c over time. Reductions in RD over time were significantly related to decreases in medication nonadherence, and improvements in physical activity were marginally associated with decreases in medication nonadherence. Likewise, reductions in RD were significantly associated with increases in physical activity, and there was a marginal association between decreases in medication nonadherence and improvements in physical activity. None of the time-varying variables were associated with change in fat intake over time. The same pattern of results was found in models that included only one time-varying predictor at a time. Thus, two sets of significant time-varying associations were found: Improvement in RD was associated with improvement in self-management over time (i.e., medication adherence, physical activity), and improvement in RD but not self-management was associated with decreases in HbA1c.
Table 4.
Time-varying predictors of change in outcomes over time
Conclusions
We examined cross-sectional, prospective, and time-varying associations among RD, self-management behavior, and glycemic control in a sample of adults with type 2 diabetes within the context of an RCT aimed at reducing distress. In line with previous reports (5,6,8), we found significant cross-sectional associations among RD, medication nonadherence, and HbA1c: Higher RD was associated with higher medication nonadherence and higher HbA1c. We found no consistent support for prospective longitudinal relationships among any of the key constructs: Baseline levels of RD, self-management behaviors, and HbA1c did not predict change among the key constructs over time. Finally, in line with previous findings (3,13,14), consistent and significant time-varying associations were found: After controlling for demographics, disease status, and intervention group, decreases in RD over time were significantly associated with improvements in self-management (physical activity, medication adherence) and reductions in HbA1c. Changes in self-management, however, were not significantly associated with changes in HbA1c. Thus, not only was the intervention successful in reducing distress but also the reductions in distress were significantly associated with improved disease management and improved glycemic control.
The significant time-varying relationships between RD with disease management and glycemic control within a sample of adults with type 2 diabetes exposed to a distress intervention suggests that disease-related distress may have important consequences for management in clinical settings. Distress appears to be malleable, even with modest intervention, if it is addressed regularly and directly (19). Thus, including an affective component within diabetes education and regular clinical assessment can lead to improved clinical outcomes (1). Furthermore, the relationships reported here were found in a sample of patients without clinical depression, which may help patients and clinicians to view distress as part of the spectrum of diabetes, distinct from a diagnosed mood disorder.
The absence of significant time-varying associations between change in self-management behaviors and change in HbA1c is worth noting. Two explanations may be relevant. First, a link between co-occurring changes in behavior and HbA1c may have occurred if the sample had contained a wider range of initial HbA1c values. Mean baseline HbA1c was only 7.4%, making significant reductions as a function of an intervention difficult to achieve. Second, the assessment of diet, physical activity, and medication nonadherence may not have been sufficiently comprehensive or sensitive to document relevant change.
The multiple linkages found among RD, disease management behavior, and glycemic control over time as a result of intervention suggest that a search for a single mechanism or causative pathway that explains all the observed dynamic relationships may not be realistic. It is most likely the case that multiple factors and pathways link these variables to one another. These may include biological (e.g., hypothalamic-pituitary-adrenal axis), behavioral (e.g., medication nonadherence), and affective (e.g., burden of management) mechanisms (12). Moreover, other findings suggest that the impact of change in one variable on another over time is complex and most likely reciprocal and iterative. This process is most likely not uniform across individuals and may vary as a function of both stage in the disease process (33,34) and other contextual factors (e.g., age, sex, disease burden, comorbidities) (35). Thus, it may make little sense to search for a simple, causative magic bullet that accounts for all these complex relationships; instead, consistent with precision medicine approaches, it may make better sense to seek multiple causative pathways that operate differently among different patient groups. This strategy may increase complexity but have a greater chance of identifying causal mechanisms.
Several options should be considered. These include the use of more-frequent longitudinal assessments (e.g., daily or weekly assessment) to tease out the direction and pace of influence for different patient groups. For example, in a 21-day diary study, negative affect was associated with next-day fasting glucose, whereas fasting glucose failed to predict next-day negative affect, suggesting a unidirectional linkage (35). Another option is to stimulate change in one variable and observe changes in the others. For example, studies have shown that the emotional distress that frequently accompanies class III obesity subsides dramatically after bariatric surgery, even before significant weight loss and HbA1c change occur (36). This differential timing and rate of change among the relevant variables over time suggest the operation of a potential third variable. Similarly, it may be helpful to observe whether changes in biological variables (e.g., hypothalamic-pituitary-adrenal axis) occur at the same rate and within the same time period as changes in affect or behavior. Finally, variations in personal behavioral styles and traits may moderate many of these processes. For example, considerable data on conscientiousness, neuroticism, positive affect, and other trait-like variables have been shown to influence disease management, distress, and health outcomes (37,38) and may attenuate or intensify hypothesized relationships among these variables over time. Thus, when exploring potential causal mechanisms, it may be helpful to explore alternative methods that stimulate change and to consider the contributions of biological, behavioral, affective, and other variables that provide the context in which these mechanisms operate.
This study has several strengths, including a relatively large community sample, use of validated measures, 12-month longitudinal data with modest attrition, and replication of earlier findings with a different patient sample. Several limitations should be kept in mind. First, although validated measures were used to assess behavioral self-management, the study could have been strengthened by the use of a more comprehensive battery of behavioral measures. Second, because level of HbA1c was not an eligibility criterion, the mean baseline level was relatively low (7.4%), thus limiting potential group change over time. Third, the study sample was relatively well educated and had computer access. Both the study eligibility criteria and the larger study context, an RCT aimed at reducing distress, may limit the generalizability of the findings.
With the use of both cross-sectional and longitudinal analyses, this study provides additional evidence for a relationship among RD, behavioral self-management, and glycemic control. Multiple explanatory pathways are likely among these variables, including biological, behavioral, and affective mechanisms. The findings suggest that improvements in management and HbA1c co-occur with improvements with RD. The findings underscore the importance of addressing RD in clinical care as a routine part of diabetes management.
Article Information
Acknowledgments. The authors thank the following medical groups and diabetes education centers for their collaboration: Alta Bates Diabetes Education Center, Brown and Toland Medical Group, California Pacific Medical Center Diabetes Education Center, Hill Physicians Medical Group, and University of California, San Francisco, Lakeshore Medical Group.
Funding. This research was funded by National Institute of Diabetes and Digestive and Kidney Diseases grant DK-061937.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.H. and L.F. reviewed the research data and wrote the manuscript. R.E.G., P.A.A., and U.M. reviewed and edited the manuscript. L.A.S. and L.M.D. conducted the data analyses, wrote the Data Analysis section, and reviewed and edited the manuscript. D.H. is the guarantor of this work, and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A slide set summarizing this article is available online.
Clinical trial reg. no. NCT00714441, clinicaltrials.gov.
The opinions expressed are those of the authors and do not necessarily represent those of the National Institutes of Health, National Cancer Institute, or the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Gonzalez JS, Fisher L, Polonsky WH. Depression in diabetes: have we been missing something important? Diabetes Care 2011;34:236–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsujii S, Hayashino Y, Ishii H, Diabetes Distress and Care Registry at Tenri Study Group Diabetes distress, but not depressive symptoms, is associated with glycaemic control among Japanese patients with type 2 diabetes: Diabetes Distress and Care Registry at Tenri (DDCRT 1). Diabet Med 2012;29:1451–1455 [DOI] [PubMed] [Google Scholar]
- 5.Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care 2007;30:542–548 [DOI] [PubMed] [Google Scholar]
- 6.Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care 2012;35:2472–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–1799 [DOI] [PubMed] [Google Scholar]
- 8.Rhee MK, Slocum W, Ziemer DC, et al. Patient adherence improves glycemic control. Diabetes Educ 2005;31:240–250 [DOI] [PubMed] [Google Scholar]
- 9.Peyrot MF. Theory in behavioral diabetes research. Diabetes Care 2001;24:1703–1705 [DOI] [PubMed] [Google Scholar]
- 10.Peyrot M, McMurry JF, Jr, Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav 1999;40:141–158 [PubMed] [Google Scholar]
- 11.Hanson CL, Henggeler SW, Burghen G. Model of associations between psychosocial variables and health-outcome measures of adolescents with IDDM. Diabetes Care 1987;10:752–758 [DOI] [PubMed]
- 12.Lloyd C, Smith J, Weinger K. Stress and diabetes: a review of the links. Diabetes Spectrum 2005;18:121–127 [Google Scholar]
- 13.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008;299:2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008;31:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med 2008;6:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 17.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher L, Hessler DM, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care 2013;36:2551–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwarenstein M, Treweek S, Gagnier JJ, et al. CONSORT group. Pragmatic Trials in Healthcare (Practihc) group Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasgow RE, Kurz D, King DK, et al. Outcomes of minimal and moderate support versions of an internet-based diabetes self-management support program. J Gen Intern Med 2010;25:1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasgow RE, Christiansen SM, Kurz D, et al. Engagement in a diabetes self-management website: usage patterns and generalizability of program use. J Med Internet Res 2011;13:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arean P, Hegel M, Vannoy S, Fan MY, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. Gerontologist 2008;48:311–323 [DOI] [PubMed] [Google Scholar]
- 24.Nezu AM. Problem solving and behavior therapy revisited. Behav Ther 2004;35:1–33 [Google Scholar]
- 25.Masharani U. Diabetes De-mystified: A Self-teaching Guide. New York, McGraw-Hill Books, 2007 [Google Scholar]
- 26.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001;33:1126–1141 [DOI] [PubMed] [Google Scholar]
- 27.Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc 2001;33:962–970 [DOI] [PubMed] [Google Scholar]
- 28.Thompson FE, Kipnis V, Subar AF, et al. Performance of a short instrument to estimate usual dietary intake of percent calories from fat. Eur J Clin Nutr 1998;52:S63 [Google Scholar]
- 29.Krousel-Wood M, Muntner P, Jannu A, Desalvo K, Re RN. Reliability of a medication adherence measure in an outpatient setting. Am J Med Sci 2005;330:128–133 [DOI] [PubMed] [Google Scholar]
- 30.NORM: Multiple Imputation of Incomplete Multivariate Data Under a Normal Model (Version 2) [Software]. University Park, PA, The Methodology Center, Penn: State, 1999 [Google Scholar]
- 31.Neuhaus JM, Kalbfleisch JD. Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics 1998;54:638–645 [PubMed] [Google Scholar]
- 32.Hedeker D, Gibbons R. Mixed-effects regression models for continuous outcomes In Longitudinal Data Analyses Hedeker D, Gibbons R, Eds. Hoboken, NJ, Wiley & Sons, 2006, p. 69–76 [Google Scholar]
- 33.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 2007;21:901–912 [DOI] [PubMed]
- 34.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 2007;133:25–45 [DOI] [PubMed] [Google Scholar]
- 35.Skaff MM, Mullan JT, Almeida DM, et al. Daily negative mood affects fasting glucose in type 2 diabetes. Health Psychol 2009;28:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bocchieri LE, Meana M, Fisher BL. A review of psychosocial outcomes of surgery for morbid obesity. J Psychosom Res 2002;52:155–165 [DOI] [PubMed] [Google Scholar]
- 37.Goodwin RD, Friedman HS. Health status and the five-factor personality traits in a nationally representative sample. J Health Psychol 2006;11:643–654 [DOI] [PubMed] [Google Scholar]
- 38.Chapman BP, Duberstein PR, Sörensen S, Lyness JM. Personality and perceived health in older adults: the five factor model in primary care. J Gerontol B Psychol Sci Soc Sci 2006;61:362–365 [DOI] [PubMed] [Google Scholar]


