A growing body of evidence has demonstrated significant and sustained improvement in glycemic control in type 2 diabetes after bariatric surgery. However, there are limited data on the impact of bariatric surgery in type 1 diabetes (T1D). Only fewer than 10 cases of bariatric surgery in patients with T1D have been reported in the literature, which show a significant weight reduction and improvement in glycemic control (1–3). A recent experimental study in a rat model of spontaneous development of T1D has also shown that a particular type of gastrointestinal bypass (duodenal-jejunal bypass surgery) lowers blood glucose concentration within 2 days after surgery (4). The aim of this study was to assess the metabolic outcomes, including the glycemic status, of patients with T1D after bariatric surgery.
Clinical outcomes and metabolic parameters of 10 morbidly obese patients with poorly controlled T1D who underwent laparoscopic bariatric surgery between 5 January 2005 and 12 December 2012 were retrieved from a database approved by an institutional review board. The diagnosis of T1D was verified for all patients by the presence of pancreatic autoantibodies (islet cell and GAD), absence of C-peptide, and/or documented history of diabetic ketoacidosis. Baseline characteristics, intraoperative data, and postoperative outcomes were assessed, including changes in weight, A1C, daily insulin requirements, lipid panel, and blood pressure. A paired t test was used to analyze changes at the last follow-up point from baseline.
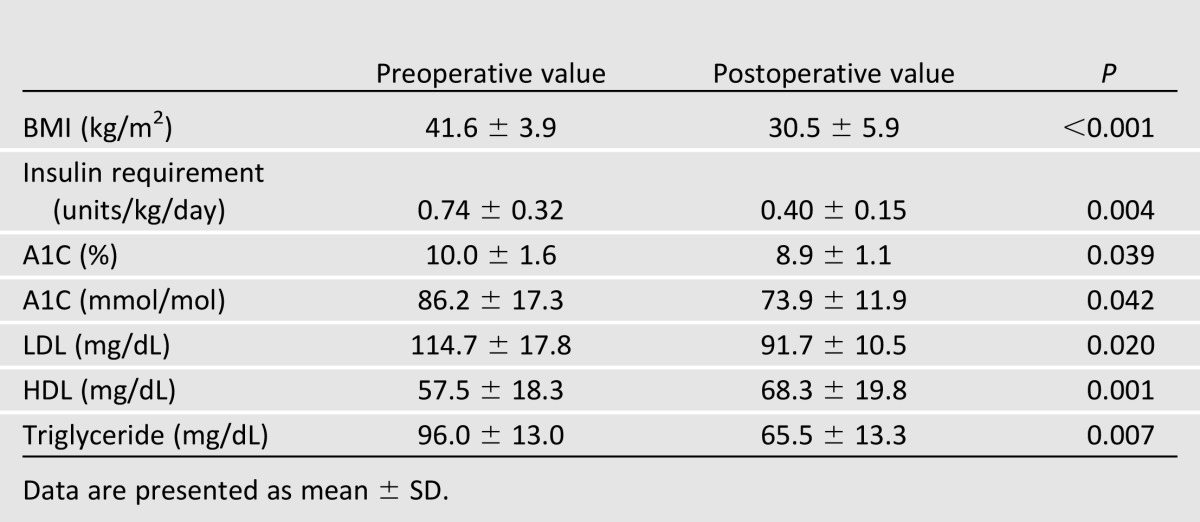
Patients had a male-to-female ratio of 1:9, a mean age of 45.6 ± 10.9 years, a mean baseline BMI of 41.6 ± 3.8 kg/m2, a median duration of T1D of 22 years (range 2–43), and a median of 10 (range 5–13) obesity- or T1D-related comorbidities. One patient had history of coronary bypass and one had history of failed kidney-pancreas transplant. Bariatric procedures included laparoscopic Roux-en-Y gastric bypass (n = 7), adjustable gastric banding (n = 2), and sleeve gastrectomy (n = 1). There were no intraoperative complications and no need for conversion to laparotomy. In total, five postoperative complications occurred, including diabetic ketoacidosis on postoperative day 10, deep vein thrombosis, ulcer at gastrojejunal anastomosis, esophageal dysmotility, and persistent nausea. At a mean follow-up of 36.8 ± 32.3 months, excess weight loss >60% was achieved in all patients except one case of adjustable gastric banding. The mean reduction in BMI of 27.0 ± 9.6% was associated with a significant mean reduction in A1C (10.0 ± 1.6 vs. 8.9 ± 1.1%, P = 0.039) and daily insulin requirement (0.74 ± 0.32 vs. 0.40 ± 0.15 units/kg/day, P = 0.004). There were also favorable changes in LDL (−23.0 ± 19.3 mg/dL, P = 0.02), HDL (10.8 ± 3.4 mg/dL, P = 0.001), and triglyceride (−30.5 ± 17.1 mg/dL, P = 0.007) following surgery (Table 1). Hypertension resolved or improved in 5 of 7 (71%) hypertensive patients. Albuminuria resolved in one of two patients with preoperative microalbuminuria.
Table 1.
Metabolic profile of patients with T1D after bariatric surgery (n = 10)
The findings of this study, which is the largest case series to date, indicate that bariatric surgery leads to a remarkable and sustained weight loss in severely obese patients with T1D and results in significant improvement in their glycemic status and comorbid conditions, despite having prolonged diabetes and undetectable C-peptide. The favorable metabolic effects of bariatric surgery may facilitate medical management of T1D in the setting of morbid obesity. The true role of bariatric surgery in patients with T1D awaits longer follow-up studies in a larger cohort.
Article Information
Funding. This research was supported in part by National Institutes of Health grant RO1-DK-089547 to P.R.S., J.P.K., and S.R.K.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Prior Presentation. These data were presented at Obesity Week, a conference of The Obesity Society and the American Society for Metabolic and Bariatric Surgery, Atlanta, GA, 11–16 November 2013.
Author Contributions. S.A.B. and A.A. were responsible for concept development and study design. S.A.B., R.J.R., S.R.K., and P.R.S. were involved in the management of patients. A.A. performed data collection and analysis and drafted the manuscript. S.A.B., A.A., R.J.R., J.P.K., S.R.K., and P.R.S. reviewed the data. S.A.B., R.J.R., J.P.K., S.R.K., and P.R.S. revised and edited the final manuscript. S.A.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Czupryniak L, Wiszniewski M, Szymański D, Pawłowski M, Loba J, Strzelczyk J. Long-term results of gastric bypass surgery in morbidly obese type 1 diabetes patients. Obes Surg 2010;20:506–508 [DOI] [PubMed] [Google Scholar]
- 2.Mendez CE, Tanenberg RJ, Pories W. Outcomes of Roux-en-Y gastric bypass surgery for severely obese patients with type 1 diabetes: a case series report. Diabetes Metab Syndr Obes 2010;3:281–283 [PMC free article] [PubMed] [Google Scholar]
- 3.Reyes Garcia R, Romero Muñoz M, Galbis Verdú H. Bariatric surgery in type 1 diabetes. Endocrinol Nutr 2013;60:46–47 [DOI] [PubMed] [Google Scholar]
- 4.Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung GW, Lam TK. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med 2012;18:950–955 [DOI] [PubMed] [Google Scholar]


