Abstract
Enhancing β-cell proliferation is a major goal for type 1 and type 2 diabetes research. Unraveling the network of β-cell intracellular signaling pathways that promote β-cell replication can provide the tools to address this important task. In a previous Perspectives in Diabetes article, we discussed what was known regarding several important intracellular signaling pathways in rodent β-cells, including the insulin receptor substrate/phosphatidylinositol-3 kinase/Akt (IRS-PI3K-Akt) pathways, glycogen synthase kinase-3 (GSK3) and mammalian target of rapamycin (mTOR) S6 kinase pathways, protein kinase Cζ (PKCζ) pathways, and their downstream cell-cycle molecular targets, and contrasted that ample knowledge to the small amount of complementary data on human β-cell intracellular signaling pathways. In this Perspectives, we summarize additional important information on signaling pathways activated by nutrients, such as glucose; growth factors, such as epidermal growth factor, platelet-derived growth factor, and Wnt; and hormones, such as leptin, estrogen, and progesterone, that are linked to rodent and human β-cell proliferation. With these two Perspectives, we attempt to construct a brief summary of knowledge for β-cell researchers on mitogenic signaling pathways and to emphasize how little is known regarding intracellular events linked to human β-cell replication. This is a critical aspect in the long-term goal of expanding human β-cells for the prevention and/or cure of type 1 and type 2 diabetes.
Introduction
Induction of proliferation in human β-cells is a major goal of current research in both types 1 and 2 diabetes. Over the last 20 years, dramatic progress has occurred in understanding transcriptional control of key genes required for mouse and human β-cell specification. More recently, advances have been made in coaxing human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells to differentiate to endocrine lineage. Concurrently, major advances have been made in understanding control of cell-cycle progression in mouse and human β-cells. In contrast, one large area that remains poorly studied, particularly in human β-cells, is the network of intracellular signaling pathways that link extracellular nutrient and growth factor actions at the β-cell surface to cell-cycle machinery.
In a recent Perspectives in Diabetes article, we discussed what is known regarding several important intracellular signaling pathways in rodent β-cells, and contrasted that ample body of data to the relative paucity of complimentary data on human β-cell intracellular signaling pathways (1). That Perspectives focused on the insulin receptor substrate/phosphatidylinositol-3 kinase/Akt (IRS-PI3K-Akt) pathway, glycogen synthase kinase-3 (GSK3) and mammalian target of rapamycin (mTOR)-S6 kinase pathways, protein kinase Cζ (PKCζ) pathways, and their downstream cell-cycle molecular targets. In this article, we now turn attention to additional important signaling pathways linked to β-cell proliferation. Our goals are twofold. First, we provide a “primer” or resource for β-cell researchers on intracellular signaling pathways linked to proliferation in β-cells. Second, we emphasize how little is known regarding intracellular events in human β-cells and how important it is to understand this understudied area if we are ever going to be able to expand human β-cells ex vivo or in vitro for therapeutic exploitation.
Glucose and Metabolic Mitogenic Signaling
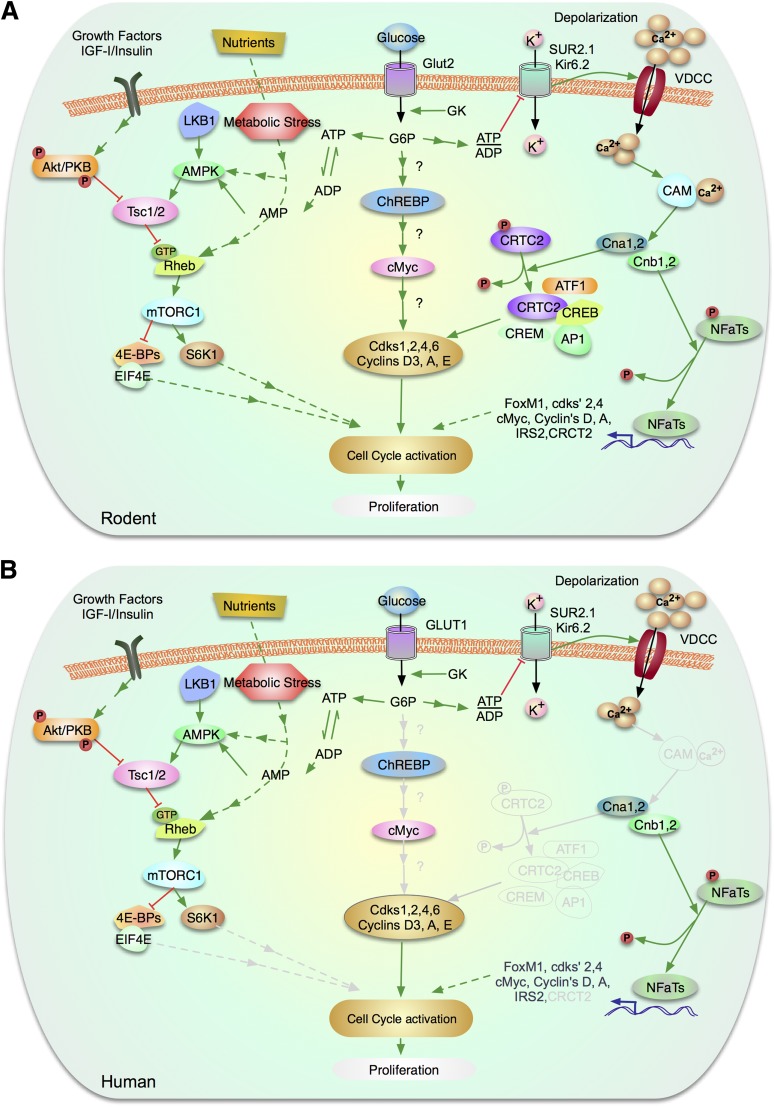
Glucose, under some physiological circumstances, is clearly a mitogenic nutrient in rodent β-cells, as glucose infusion and in vitro glucose exposure have been demonstrated repeatedly to drive replication in mouse and rat β-cells. In this model, glucose enters the β-cell via GLUT2 in rodents (or GLUT1 in humans) and is phosphorylated by glucokinase (GK). GK acts as the β-cell’s glucose sensor as a result of a Km that lies in the center of the physiological range of blood glucose. Glucose-6-phosphate (G-6-P) generated by GK enters the glycolytic pathway to generate ATP and other metabolic signals such as pyruvate, consuming AMP, and ADP. These metabolic signals activate the three parallel downstream mitogenic pathways depicted in Fig. 1. That these downstream mitogenic effects are mediated by glucose and GK is clear, as β-cell proliferation fails to occur in hyperglycemic GK−/− mice (2). Conversely, even hypoglycemic mice treated with pharmacologic GK activators display increases in β-cell proliferation (3).
Figure 1.
Glucose signaling pathways to β-cell proliferation via mTOR, via ChREBP/cMyc, and via NFATs. A: Signaling mechanisms in rodent β-cells. B: Signaling molecules confirmed in human β-cells. Molecules and arrows in gray denote pathways that are known to exist in rodents, but are unstudied in human β-cells. Briefly, glucose enters the β-cell via GLUT2 (in rodents) or GLUT1 (in humans) whose kinetics ensure that phosphorylation and subsequent catabolism is proportional to blood glucose levels. Glucose is phosphorylated by GK to G-6-P and enters glycolysis. In the right side of the figure, this generates ATP, depleting ADP and AMP, which permits suppression of AMP-kinase, with resultant activation of mTOR signaling to proliferation. In parallel, in the middle of the figure, metabolism of glucose activates ChREBP, which leads to activation of cMyc and then cyclins with β-cell proliferation. In the right side of the figure, in parallel with the other pathways, glucose metabolism to generate ATP blocks potassium entry via the potassium inward rectifier/sulfonyurea receptor complex, which leads to depolarization of voltage-dependent calcium channels, and resultant calcium entry. This leads to activation of calmodulin, and thus the phosphatase, calcineurin, with resultant dephosphorylation of proliferative molecules such as the NFAT and CRTC2 families. Upon growth factor and insulin stimulation, Akt and ERK phosphorylates and inactivates TSC2, releasing the inhibition of Rheb and activation of mTOR complex 1 (mTORC1). In contrast, phosphorylation and activation of TSC2 by AMPK and GSK3β inhibits mTOR signaling. mTORC1 controls growth (cell size) and proliferation (cell number) by modulating mRNA translation through phosphorylation of 4E-BP 1, 2, and 3 and the ribosomal protein S6 kinases (S6K1 and 2). Phosphorylation of the 4E-BPs triggers their release from eIF4E and initiates cap-dependent translation. See the text for more detail. (A high-quality color representation of this figure is available in the online issue.)
The majority of information presented above is derived from rodent models (Fig. 1A), but it is important to emphasize two points relating to the human β-cell (Fig. 1B). First, in rodents, GLUT2 is the principal β-cell glucose transporter. In contrast, in humans, GLUT1 serves as the major glucose transporter (4,5). Second, additional support for the importance of GK in human β-cell proliferation comes from human neonates with activating GK mutations who demonstrate increases in β-cell proliferation and mass, with resultant hypoglycemia (6).
Carbohydrate Response Element–Binding Protein and Proliferation
Carbohydrate response element–binding protein (ChREBP) is expressed in many tissues involved in energy balance and lipid metabolism pathways, including β-cells, fat, skeletal muscle, intestine, and brain (7). The catabolism of glucose in β-cells promotes nuclear translocation and activation of ChREBP where it binds to DNA with its partner, Mlx, at carbohydrate response elements and stimulates glucose-responsive target genes (8–10) (Fig. 1).
ChREBP exists in two isoforms, a full-length α isoform induced by glucose metabolism and a shortened β isoform, which is constitutively active (11). In adipose tissue, expression of the β isoform of ChREBP correlates with increased GLUT4 expression, de novo lipogenesis, and insulin sensitivity; whereas in the liver, increased ChREBP β correlates with insulin resistance and steatosis (11–13). Much work needs to be done to determine the role of the β isoform in rodent and human β-cells. ChREBP appears to be required for glucose-stimulated β-cell proliferation: depletion of ChREBP blocks glucose-stimulated β-cell proliferation in mouse and rat β-cells; conversely, overexpression of the full-length form of ChREBP results in an amplification of glucose-mediated proliferation (14).
In human β-cells, ChREBP is present and required for glucose-stimulated β-cell proliferation, and is found in the nuclei of cadaveric β-cells derived from type 2 diabetic patients, suggesting a role in glucotoxicity (14,15).
Liver Kinase B1, AMP-Activated Protein Kinase, mTOR, and Proliferation
Glucose (and also fatty acids and amino acids) can act on multiple nutrient sensing pathways and affect β-cell proliferation. The mTOR pathway has been considered in our previous review (1). Here, we focus on two other glucose-responsive nutrient-sensing mitogenic pathways, the liver kinase B1 (LKB1) and AMP-activated protein kinase (AMPK) pathways (Fig. 1). These pathways integrate signals from nutrients and growth factors such as insulin and are also responsible for conserving cellular energy during times of nutrient restriction.
LKB1
The serine/threonine kinase gene LKB1 (also known as STK11) is a tumor suppressor gene that acts in a complex with a pseudokinase (STRAD) and the scaffold protein (MO25). This kinase is mutated in patients with familial polyposis or Peutz-Jeghers syndrome. LKB1 phosphorylates several substrates, including phosphatase and tensin homolog (PTEN), lysosomal acid lipase 1, AMPK, and several other kinases, with a T-loop activation domain similar to AMPK. Phosphorylation of these kinases controls cellular and organismal metabolism, cell polarity, proliferation, senescence, apoptosis, DNA damage response, and differentiation (16). The effects of LKB1 on cell growth and proliferation are mediated, in part, by AMPK modulation of mTOR signaling and by PTEN regulation of PI3K/Akt (17–19). In addition, it is possible that the interaction of LKB1 with GSK3 and PKCζ (1) may also play a role, although it remains unclear if this occurs in β-cells.
Insights into the functions of this kinase in β-cells emerge from conditional as well as constitutive inactivation of LKB1. In both models, deletion of LKB1 in adult or developing β-cells resulted in enhanced insulin secretion and β-cell mass (20–22). The changes in β-cell mass resulted from both increased proliferation and cell size and were associated with both reduced AMPK activity as well as enhanced mTOR C1 signaling. These studies make it clear that LKB1 acts on AMPK/mTOR C1 signaling to modulate β-cell proliferation, size, and mass.
In human β-cells, LKB1 expression has been demonstrated in islets at the mRNA level, but little is known about the role of this kinase in regulation of human β-cell proliferation. Mutations of LKB1 in patients with Peutz-Jeghers syndrome are associated with an increased susceptibility to endocrine tumors, but insulinoma has not been described in these patients.
AMPK
AMPK is an energy-sensing heterotrimeric serine/threonine kinase that mediates the adaptation to decreased nutrients (low ATP/ADP + AMP ratio) by promoting energy production and limiting energy utilization. AMPK contains a catalytic α-subunit (AMPKα-1 or -2), a scaffold β-subunit (β-1 or -2) and a regulatory γ-subunit (γ-1, -2, or -3). Activation of AMPK inhibits mTOR C1 activity by acting on its upstream negative regulators, tuberous sclerosis complex proteins 1 and 2 (TSC1/2), and thereby inhibits β-cell proliferation and hypertrophy.
The importance of AMPK in regulation of mature β-cell function has recently been demonstrated in conditional mice in which AMPKα-1 and -2 were disrupted in β-cells (23). These mice displayed impaired glucose tolerance, decreased insulin secretion, and enhanced insulin sensitivity. These mice also showed normal β-cell mass, decreased β-cell size, and increased β-cell proliferation. The mechanisms responsible for this surprising proliferative response were not explored. Experiments in transgenic mice overexpressing either constitutively active or dominant negative AMPKα in β-cells failed to show major defects in mass but underscore the role of AMPK on insulin secretion (22). The phenotypes of models with loss of AMPK function are in marked contrast with the observations in LKB1-deficient mice described above. Thus, it is possible that AMPK-independent effects downstream of LKB1 regulate β-cell proliferation, but further studies using inducible mice and β-cell–specific Cre lines could determine the role of AMPK on β-cell proliferation.
In human β-cells, AMPKα-1 and -2 are present and AMPK activity is negatively regulated by glucose treatment (24). While activation of AMPK inhibits insulin secretion, its role in β-cell proliferation is unclear and warrants research given the extensive therapeutic use of metformin.
Calcium, Nuclear Factor of Activated T Cells, Calcineurin, and Proliferation
In addition to the LKB1 and ChREBP glucose-driven mitogenic pathways, a third calcium-mediated signaling pathway related to β-cell proliferation exists (Fig. 1). In this pathway, the same canonical signals that link glucose entry into cells, activating calcium-mediated insulin secretion, also can activate β-cell proliferation. In the β-cell mitogenic pathway, this increment in intracellular calcium binds to and activates calmodulin, which then phosphorylates and activates the catalytic (CnA) and regulatory (CnB) subunits of the phosphatase, calcineurin (25–31). Calcineurin has a number of substrates. One of the substrates is CREB-regulated transcription coactivator-2 (CRTC2) (also called Transducer of Regulated CREB activity-2 [TORC2]) (32), which is bound to and retained in the cytoplasm by the scaffolding protein 14-3-3. Dephosphorylation of CRTC2 by calcineurin releases TORC2 from 14-3-3 and permits nuclear translocation and association of CRTC2 with other transcription factors and coactivators such as CREB, cAMP-response element modulator (CREM), and ATF1 on the promoters of cell-cycle–activating genes, such as the cyclin A promoter (32,33).
A similar scenario applies to a second calcineurin substrate, the nuclear factor of activated T cells (NFAT) family of transcription factors (25,26,28–30,34). NFATs are phosphorylated and thereby constrained to the cytoplasm in quiescent cells. With a rise in intracellular calcium, calmodulin phosphorylates and activates calcineurin, which dephosphorylates NFATs, allowing them to translocate to the nucleus. There, they bind to the promoter of a number of cell-cycle activators (e.g., D and A cyclins, cyclin-dependent kinase 2 and 4, cMyc, and FoxM1 among others) (26,30), thereby activating proliferation.
This calcium-mitogenic paradigm may employ multiple additional variations. For example, NFAT action is often enhanced by association with transcription factors such as CREM, CREB, and AP1, Jun, or Fos (34). NFATs may also transcriptionally repress the promoters of certain cell-cycle inhibitors, with p21 and p27 being examples. Also, they may bind to the promoter of IRS-2, which can then recruit PI3K, Akt/ PKB, and Ras/Raf/MAP kinase pathways to activate proliferation (25). In another variation, ChREBP is retained in the cytoplasm in an inactive state, bound to the binding protein, sorcin, under conditions of low glucose and low calcium, thereby preventing its nuclear localization. Intracellular calcium increments release ChREBP from sorcin, and allow it to enter the nucleus and drive proliferation. In a third variant of calcium-mediated β-cell mitogenesis, activation of GABA receptors, which activate extracellular calcium entry, can also serve to activate these pathways (35).
While the majority of information described above is derived from rodent β-cells or cell lines, it is likely that many of the phenomena also apply to human β-cells (Fig. 1B). For example, it is known from human GK mutations and experiments using calcium-channel activators, calcium-channel inhibitors, and calcineurin inhibitors that glucose activates intracellular calcium in human β-cells, that human β-cells contain calcineurins and NFATs, and that calcium entry is associated with, and to some extent required for, human β-cell proliferation (6,31,34). Indeed, calcineurin inhibitors such as cyclosporin and tacrolimus, used as immunosuppressive agents in human organ transplantation, lead to diabetes, in part as a result of loss of proliferation, failure of insulin secretion, and de-differentiation in rodent as well as human β-cells (34,36,37).
Epidermal Growth Factor and Platelet-Derived Growth Factor and Proliferation
The epidermal growth factor (EGF) family of proteins has attracted much attention in the β-cell replication field since 1993, when betacellulin (BTC) was identified in mouse insulinoma cell lines (38), suggesting that BTC could be a contributor to the development of the insulinoma phenotype. Indeed, BTC is a potent mitogen in vitro for INS-1 insulinoma cells (39) and rodent pancreatic β-cells in vivo (40,41). BTC-mediated mitogenic effects in β-cells require activation of both EGF/ErbB1 and ErbB2 receptors and also upregulation of IRS2 (42) (Fig. 2A).
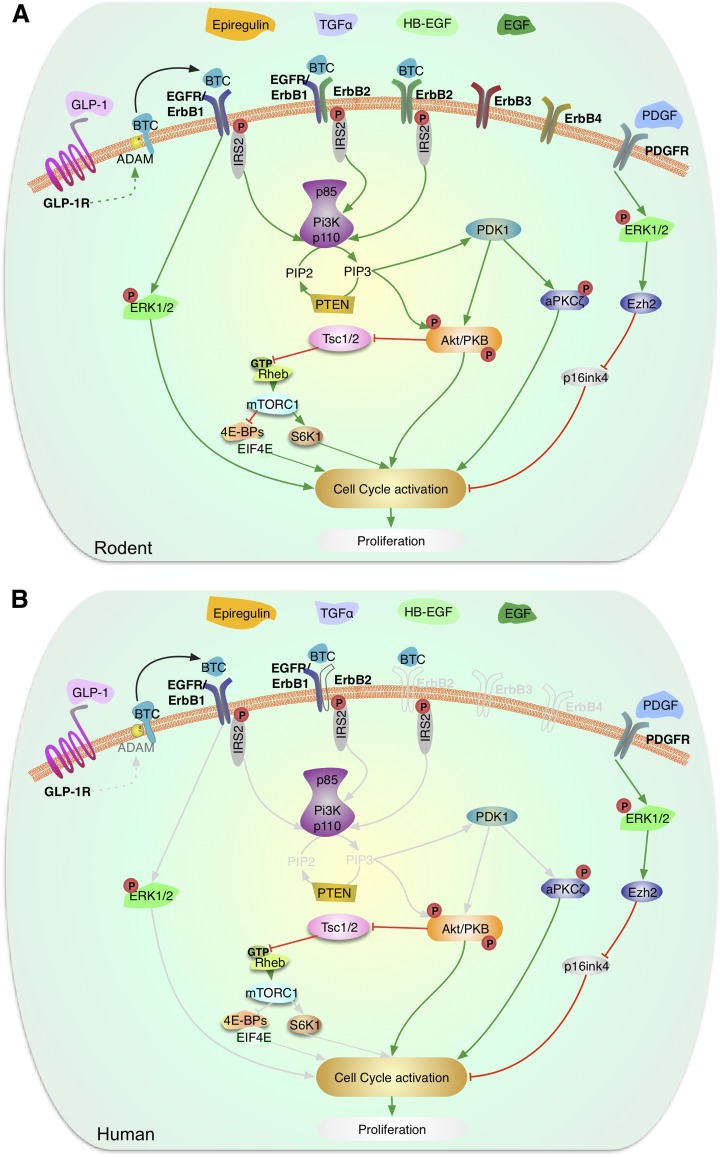
Figure 2.
Signaling by EGF and PDGF in the regulation of β-cell proliferation. Schematic representation of the signaling pathways activated by EGF family of proteins and PDGF in rodent (A) and human (B) β-cells. Multiple members of the EGF family of proteins, including BTC, EGF, HB-EGF, TGF-α, and epiregulin, have been shown to act in β-cells. ErbB family of receptors is expressed in β-cells and BTC binding to ErbB1, also called EGFR, and ErbB2 has been reported to activate the IRS2/PI3K pathway, which in turn signals via PDK-1 to modulate Akt and PKCζ. EGF binding to ErbB receptors in β-cells leads to activation of Akt and ERK signaling pathways. GLP-1 through activation of ADAM proteins can lead to the secretion of BTC from β-cells that act upon ErbB receptors. Activation of these pathways leads to enhanced rodent β-cell proliferation, an aspect unknown in human β-cells. PDGF receptors are expressed in β-cells but their expression is attenuated during aging. PDGF binding to PDGFR leads to activation of ERK1/2, increased expression of the histone methyltransferase, Ezh2, repression of the cell-cycle inhibitor p16INK4, and increased β-cell proliferation. This pathway is preserved in juvenile human β-cells and leads to enhanced replication. Gray lines are molecules and pathways that are known to exist in rodents but are unknown in human β-cells. (A high-quality color representation of this figure is available in the online issue.)
EGF receptor (EGFR) deficiency in mouse models causes markedly reduced β-cell proliferation and diabetes, associated with reductions in extracellular signal–related kinase (ERK) and Akt activity (43–45). Further, EGFR-deficient mice fail to expand β-cell mass following high-fat feeding or during pregnancy, in which β-cell replication is the primary mechanism for compensatory β-cell growth (43). In addition, the mitogenic effects of glucagon-like peptide 1 (GLP-1) in INS-1 cells require EGFR activation (46). Therefore, an intact EGFR pathway is required for β-cell proliferation postnatally, when induced by GLP-1 and in insulin-resistant states. However, and in contrast to BTC, two EGFR ligands, EGF or transforming growth factor (TGF)-α only modestly increase rodent β-cell proliferation (39,46). In addition, transgenic overexpression of heparin-binding (HB)-EGF did not lead to increased β-cell proliferation (47). Taken together, these results suggest that although EGFR activation is required for normal β-cell proliferation and expansion, EGF, HB-EGF, or TGFα may not be the natural ligands that activate this receptor. Recently, another EGFR ligand, epiregulin, has been shown to increase β-cell proliferation in vitro (48). These studies highlight the complicated nature of β-cell mitogenic effects involving this large family of ligands and receptors and point out the need to analyze the role of ErbB receptors and ligands in β-cell proliferation and expansion.
In vitro experiments in the 1990s demonstrated that cotransfection of platelet-derived growth factor (PDGF) with PDGF receptor (PDGFR) increases islet cell proliferation (49,50). More recently, an elegant study by Chen et al. (51) has shown that PDGFR expression decreases in an age-dependent manner, reducing the proliferative potential of this growth factor in adult β-cells. Exogenous addition of PDGF-AA to juvenile mouse islets leads to increased expression of the histone methyltransferase, Ezh2 (a repressor of p16INK4), and increased β-cell proliferation (51). However, these effects were not observed in adult mouse islets. Conditional inactivation of PDGFR-α in β-cells of young mice leads to a remarkable decrease in β-cell proliferation and mass and the development of hyperglycemia and glucose intolerance (51). Importantly, PDGFR inactivation impairs β-cell regeneration and restoration of β-cell mass after streptozotocin treatment in adult mice. On the other hand, overexpression of an active form of the PDGFR-α in the β-cell of transgenic mice leads to remarkable β-cell proliferation in old mice. Collectively, these studies demonstrate that PDGFR signaling is required for physiologic β-cell proliferation and expansion, and its activation is sufficient to sustain adult β-cell expansion in vivo in mice. Activation of Erk1/2, but not PI3K or phospholipase C-γ, is responsible for PDGFR signaling–mediated β-cell proliferation (Fig. 2A).
For human β-cells (Fig. 2B), information on EGF/EGFR family members relating to proliferation is almost nonexistent. It is known that BTC and EGFR are expressed specifically in human α-cells, β-cells, and ductal cells (52). In addition, it has been shown that human insulinomas also express BTC, suggesting that BTC might be important for human islet growth (52). Interestingly, the PDGFR-α is present in juvenile human islets, but not in adult human islets, suggesting that PDGF signaling attenuation is a preserved feature of aging human β-cells (51). PDGF induces a remarkable increase in the proliferation of juvenile human β-cells, an effect potentially mediated by Erk1/2 activation, but was ineffective in adult human β-cells (51). Unfortunately, it is unknown whether the transfer of PDGFR to adult human islets could lead to enhanced β-cell proliferation in the presence of PDGF.
Wnt, β-Catenin, and Proliferation
Genome-wide association studies have implicated a role for the Wnt signaling pathway in the pathogenesis of type 2 diabetes and variants of the transcription factor 7-like 2 (TCF7L2) convey the strongest genetic risk factor for type 2 diabetes. The canonical Wnt pathway is activated by binding of Wnt ligands to the Frizzled receptor, inducing a cascade of events that result in nuclear localization of β-catenin and transcriptional activation by interacting with T-cell–specific factor/lymphoid enhancer–binding factor (TCF/LEF) transcription factors (Fig. 3) (53,54). Absence of Wnt signaling permits β-catenin proteosomal degradation by activation of a complex of proteins that include axins, GSK3β, and APC (55). In addition to responses to Wnt ligands, this pathway is induced by activation of the GLP-1 receptor in β-cells (56). This signaling pathway plays a major role in pancreas development and β-cell proliferation (57–62). Wnt3a induces expression of cyclin D2, D1, and cdk4 and leads to increased β-cell proliferation in vitro (63). Increased proliferation by Wnt appeared to be mediated by Pitx2-induced cyclin D2 transcription. In vivo studies demonstrated that overexpression of an active mutant of β-catenin increases β-cell proliferation (63). In contrast, inhibition of Wnt signaling by overexpression of axin prevented β-cell expansion and deletion of β-catenin in β-cells using RIP-Cre or Pdx1-Cre mice also had a detrimental impact on islet mass and proliferation (57,63,64). Interestingly, conditional deletion of the Wnt coreceptor TCF7L2 in the pancreas using Pdx1-Cre or RIP2-CreERT2 failed to show a clear defect in β-cell mass in normal conditions (65,66). However, it is possible that TCF7 could play a role in β-cell proliferation and regeneration during diabetogenic conditions but the mechanisms and cell-cycle components are largely unexplored (65,67,68).
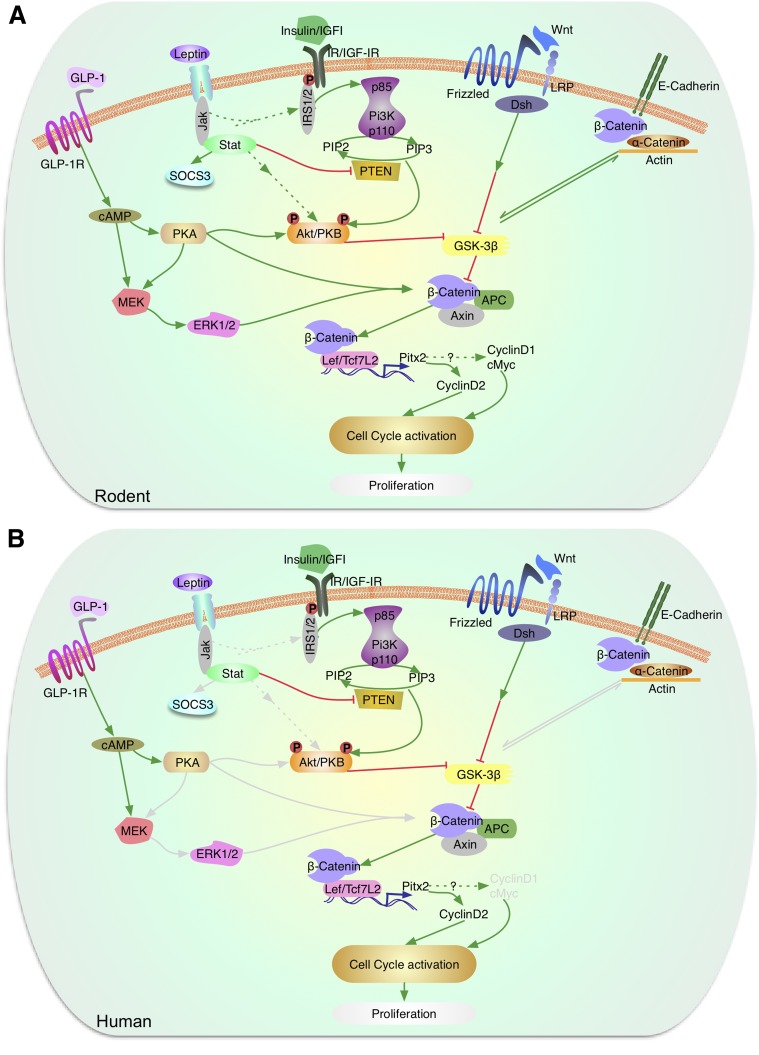
Figure 3.
Signaling by leptin, Wnt, and β-catenin in the regulation of β-cell proliferation. A: In murine models, leptin acts via the JAK-STAT pathway to inhibit PTEN and also modulate Akt/PKB and p70S6k. Akt/PKB, which is also activated by growth factor (insulin/IGF-1) signaling, modulates GSK3β. The Wnt/frizzled pathway also regulates GSK3β, which blocks phosphorylation of β-catenin to control the expression of Lef/Tcf7L2 and cyclin D2 and potentially cyclin D1 and cMyc to activate the cell cycle and regulate proliferation. GLP-1 receptor signaling activated by GLP-1 or exendin-4 leads to elevation of cAMP and activation of protein kinase A, which can directly or indirectly via the MEK/ERK1/2 pathway phosphorylate β-catenin. Activation of the insulin or IGF-1 receptors leads to phosphorylation of serine/threonine residues in IRS2, activation of PI3K and Akt/PKB, which can, in turn, phosphorylate and inactivate GSK3β. B: In human β-cells, leptin receptors, GLP-1 receptors, and elements of the Wnt signaling pathway have been reported. However, the downstream proteins (marked in gray) that link to the proliferation response are not fully understood. (A high-quality color representation of this figure is available in the online issue.)
In humans, most of the studies implicating a role for Wnt signaling in β-cells have focused on TCF7L2 (Fig. 3B). Depletion of TCF7L2 in human islets was associated with reduced β-cell proliferation under basal conditions (69). In contrast, TCF7L2 overexpression reversed the decrease in β-cell proliferation induced by chronic high glucose (69). Exposure of human islets to Wnt3a stimulated expression of Pitx2 and cyclin D2, although β-cell expansion has not been demonstrated (63).
Leptin and Proliferation
Leptin receptors have been reported to be expressed in primary murine β-cells and in insulinoma cell lines (70–72). The major isoform, ObRb, is considered to mediate the actions of leptin in β-cells. Leptin has been shown to inhibit insulin gene transcription by directly or indirectly (via Janus kinase/signal transducers and activators of transcription [JAK-STAT]) activating SOCS3, which, in turn, inhibits insulin promoter activity. The effects of leptin on β-cell proliferation occur by inhibition of PTEN that activates PI3K/Akt. Alternatively, leptin activation of JAK-STAT has also been reported to directly modulate β-cell proliferation (Fig. 3A).
In vivo models also provide evidence for a direct effect of leptin on islet biology without impacting hypothalamic function. Mice with islet-specific deletion of the leptin receptor (73,74) do not show changes in body weight or food intake, but exhibit improved glucose tolerance indicating the removal of a tonic inhibitory effect of leptin on insulin release. These studies indicate that absence of leptin action promotes β-cell growth by affecting phosphorylation of p70S6k and Akt. These data are consistent with an increase in β-cell mass in Zucker fatty rats, which also lack leptin action (75).
Human β-cells are also known to express ObRb receptors and exogenous leptin is known to suppress insulin secretion in vitro in human islets (71,76). However, leptin effects on β-cell mass remain unexplored (Fig. 3B). The effects of leptin in vivo in humans with type 2 diabetes and obesity is not explicit given the effects of hyperinsulinemia that can in turn lead to leptin release from adipocytes and promote leptin resistance (77).
Estrogen, Progesterone, and Proliferation
Estrogen
In rodent models, the main female estrogen, 17β-estradiol (E2), protects functional β-cell mass against injury associated with both type 1 and type 2 diabetes, including oxidative stress, amyloid polypeptide toxicity, glucolipotoxicity, and apoptosis (78). The role of E2 in β-cell proliferation is less clear, although E2 may also promote β-cell proliferation under specific physiological and experimental conditions (Fig. 4). Historically, an effect of estrogen on islet regeneration was suggested 60 years ago by Houssay et al. (79), who observed that subtotal pancreatectomy followed by implantation of an estrogen pellet in the remaining pancreas induced regeneration of surrounding islets. Further, a stimulatory effect of estrogen on islet and β-cell regeneration was also observed in the alloxan and streptozotocin diabetic rat models (80). E2 also increases cultured rat islet cell proliferation (81). In these studies, however, estrogen was used at pharmacological concentrations, so it is unclear if these observations are relevant to physiology. Nevertheless, in one study, physiological doses of estrogen have been reported to increase β-cell proliferation and restore the decrease in β-cell mass observed in ovariectomized rodents with subtotal pancreatectomy (82). This effect was associated with an increase in the expression of IRS-2 and Pdx1 proteins via the activation of CREB (Fig. 4A) (82). Thus, in classical models of β-cell regeneration, or at high doses, E2 can induce β-cell proliferation. In contrast, in most studies, E2 used at doses leading to physiological serum concentrations in either male or female rodents, β-cell proliferation was not significantly induced (83–85).
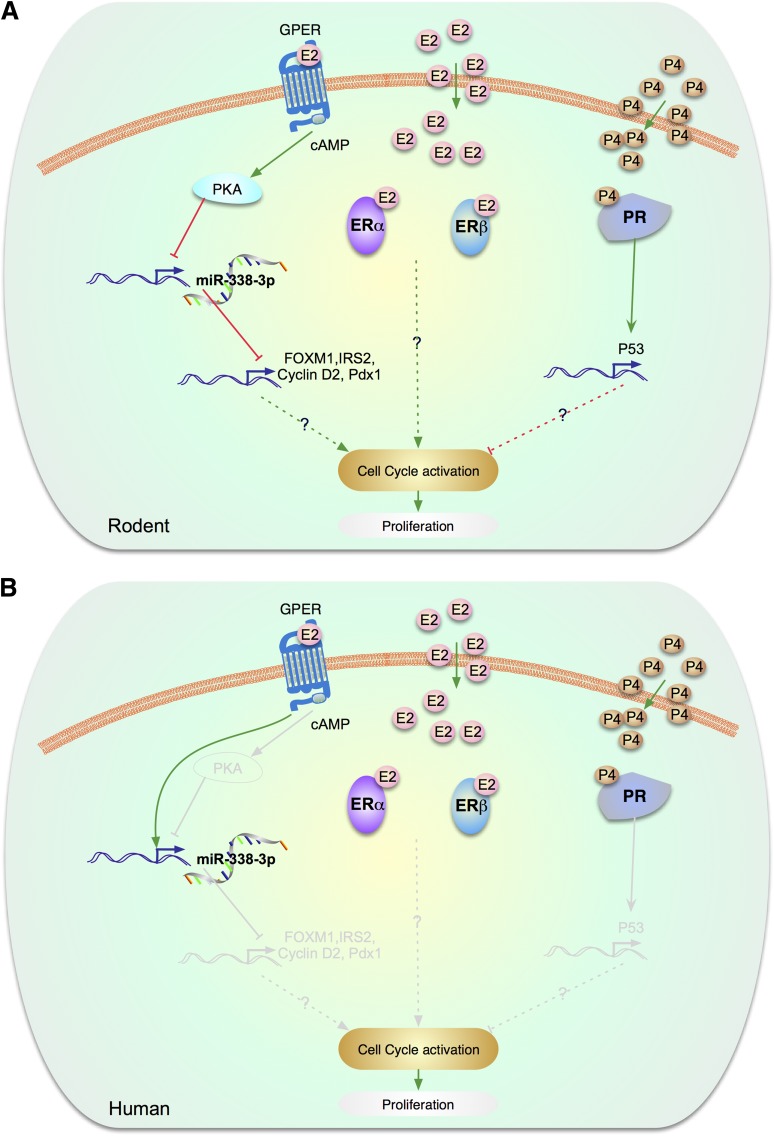
Figure 4.
Estrogen/progesterone signaling pathways involved in proliferation. A: In rodent β-cells, GPER has been implicated in β-cell proliferation during pregnancy. In rodents, GPER expression is upregulated during pregnancy, which leads to a decrease in the expression of the islet microRNA, miR-338–3p, leading to increased mRNA expression of IRS-2, Pdx1, FOXM1, and cyclin D2 and stimulation of β-cell proliferation. These effects of E2/GPER are cAMP- and PKA-dependent. E2 has also been reported to increase β-cell proliferation in ovariectomized rodents with subtotal pancreatectomy. This effect was associated with an increase in the expression of IRS-2 and PDX1 proteins. B: Human β-cells. The gray lines are molecules and pathways that are known to exist in rodents but are unknown in human β-cells. The human β-cell signaling road map is underdeveloped. Although FOXM1, IRS-2, cyclin D2, PDX-1 are known to be present in human β-cell, their involvement in estrogen signaling has not been studied. Exposure to E2 reduces the level of miR-338–3p in human islet cells. However, neither E2 nor silencing of miR-338–3p elicited replication of cultured human β-cells. In addition, progesterone signaling has not been studied in human β-cells.
Three estrogen receptors (ERs), ERα, ERβ, and the G-protein–coupled ER (GPER) (also called GPR30), have been identified in rodent β-cells. Unlike the classical nuclear ERs that function as ligand-activated transcription factors in the nucleus, β-cell ERs reside mainly in extranuclear locations. They exert their effect via cytosolic interactions with kinases such as Src, ERK, and AMPK or with transcription factors such as STAT3 (78,86,87). Interestingly, GPER has recently been implicated in β-cell proliferation (88). Pregnancy is associated with an expansion of the functional β-cell mass to adapt to the increased metabolic demand. In rodents, GPER expression is strongly upregulated during pregnancy. In addition, β-cell mass expansion during pregnancy is associated with a decrease in the expression of the islet microRNA, miR-338–3p. In isolated rat islets, exposure to E2 or the GPER agonist, G1, decreased miR-338–3p to levels observed in gestation, which was associated with increased β-cell proliferation. These effects of E2 are cAMP-dependent and are blocked by cAMP-dependent protein kinase (PKA) inhibitors.
In human β-cells, the three ERs identified in rodents are also present (78,84,85,89,90) (Fig. 4B). Beneficial effects of E2 and ER ligands on β-cell survival, function, and nutrient homeostasis are all observed in cultured human islets (84,85,89,90). Further, the antiapoptotic action of physiological doses of E2 and ER agonists is maintained in human islets transplanted into mice in an in vivo hyperglycemic environment (84). However, in these conditions, there is no proliferation of human β-cells (84). Exposure to E2 reduces the level of miR-338–3p in human islet cells (88). However, neither E2 nor silencing of miR-338–3p elicited replication of human β-cells in culture. Thus, as in the case of other molecules described in this review, the impressive effect of E2, GPER, and miR-338–3p observed in rodent β-cell proliferation is not observed in human β-cells. Finally, E2 has been reported to promote the proliferation and inhibit the differentiation of adult human islet-derived precursor cells via ERα (91).
Progesterone
Progesterone treatment in vivo stimulates α- and β-cell proliferation in male and female mice. This effect is not observed in gonadectomized mice, suggesting that progesterone-induced islet cell proliferation requires intact gonadal function (92). Indeed, this effect is not observed in cultured rat islet cells (81). In contrast, Picard et al. (93) have shown that female progesterone receptor–deficient mice have lower fasting blood glucose and higher fasting insulin associated with greater glucose clearance. The enhanced pancreatic function in these mice is attributed to higher islet mass with enhanced β-cell proliferation. This is not associated with differences in the islet expression level of the cell-cycle regulators, p21, p27, cyclin D1, cyclin B1, and cyclin E (Fig. 4A). In contrast, the protein levels of the tumor-suppressor p53 were markedly decreased in progesterone receptor–deficient islets. Progesterone did not affect miR-338–3p levels in INS-1 cells (88).
Although the presence of progesterone receptors in the endocrine pancreas in humans suggests a direct role of progesterone on pancreatic islet function (94), no effect of progesterone or its receptor in human β-cell proliferation has been described (Fig. 4B).
Conclusions
As is clear from the preceding sections, intracellular signaling pathways connecting cell surface receptors and channels to proliferative machinery in the β-cell are complex. Interestingly, as emphasized previously (1), signaling pathways can access cell-cycle machinery in multiple, as well as distinct ways, with some pathways activating cdks, others activating early or late cyclins, and others principally repressing cell-cycle inhibitors, and still others acting on several of these targets. An aspect of signaling that has not been emphasized in these two Perspectives, but is very important, is the complex cross talk between and among signaling pathways. For example, GSK3β is a constitutively active kinase that is involved in both PI3K/Akt signaling as well as Wnt-β-catenin signaling. And IRS2 may activate downstream signals in both the PI3K and RAS-MAPK pathways. Prolactin receptor–signaling may activate not only its canonical downstream JAK2-STAT5 pathway, but also PI3K and MAPK signaling. Moreover, these pathways, which we have depicted as being linear, top-down pathways, in fact are replete with autoinhibitory as well as amplifying limbs. Thus, insulin and IGF2 signaling via IRS2 activates PI3K/mTOR pathways that feed back to inhibit IRS2, to attenuate IRS2 signaling. This complexity is challenging, but also provides multiple and rich targets for small molecules that can activate β-cell proliferation.
It is also important to emphasize that additional important intracellular signaling pathways have not been covered in these two Perspectives because of space limitations. Examples include GH, Epo, ICA-512, JAK2, STAT5, SOCS-CISH signaling; stem cell factor, c-kit signaling; GPCR, cAMP, PKA, CREB, CREM signaling; TGF-β, BMP, activin, inhibin, myostatin, SMAD signaling; details of RAS-MAPK signaling; muscarinic, adrenergic, and cannabinoid signaling; cadeherins, integrins, and focal adhesion kinases; and signaling via purinergic receptor and adenosine kinase pathways that have recently become relevant as a result of high-throughput small molecule β-cell screens. These and others merit attention in future reviews.
Finally, it is important to underscore how little research has been done on these pathways in the adult human β-cell. While broadly similar in juvenile rodent and adult human β-cells, these pathways differ in important details. Elucidating these differences may provide clues explaining why adult human β-cells are recalcitrant to induction of proliferation, as well as therapeutic opportunities for inducing human β-cell proliferation. With the advent of techniques to purify human β-cells, there is great current opportunity to explore and define the unique detailed “anatomy” of human β-cell proliferation. Thus, in human β-cells, we are still driving in the dark without headlights. It is time to turn on the floodlights.
Article Information
Acknowledgments. The authors apologize to the many authors whose important publications were not cited because of lack of space. The authors would like to thank Corentin Cras-Meneur for assistance in preparing the figures.
Funding. The authors wish to thank the funding agencies for their essential contribution to this work, which was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants DK084236 and DK073716 and the Juvenile Diabetes Research Foundation (JDRF) International grant 46-2010-758 to E.B.-.M.; NIDDK grants DK067536 and DK055523, the Harvard Stem Cell Institute, the JDRF/Sanofi Strategic Alliance, and AstraZeneca to R.N.K.; NIDDK grant DK065149, American Diabetes Association grant 7-11-BS-128, and JDRF grant 17-2011-598 to D.K.S.; NIDDK grants DK069362, DK074970, and HD044405, JDRF grant 1-2006-837, and the March of Dimes grant 6-FY07-678 to F.M.-.J.; the NIDDK Beta Cell Biology Consortium grants U01 DK089538 and DK55023, JDRF grants 1-2008-39, 17-2011-598, and 34-2008-630 to A.F.S.; and NIDDK grants DK77096 and DK67351 and JDRF grant 47-2012-750 to A.G.-O.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.B.-M., R.N.K., D.K.S., F.M.-J., A.F.S., and A.G.-O. wrote the manuscript, reviewed and edited the manuscript, and contributed to the discussion. E.B.-M., R.N.K., D.K.S., F. M.-J., A.F.S., and A.G.-O. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Kulkarni RN, Mizrachi E-B, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 2012;61:2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PubMed] [Google Scholar]
- 3.Nakamura A, Terauchi Y, Ohyama S, et al. Impact of small-molecule glucokinase activator on glucose metabolism and beta-cell mass. Endocrinology 2009;150:1147–1154 [DOI] [PubMed] [Google Scholar]
- 4.Guillam MT, Hümmler E, Schaerer E, et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2 [published correction appears in Nat Genet 1997;17:503]. Nat Genet 1997;17:327–330 [DOI] [PubMed] [Google Scholar]
- 5.De Vos A, Heimberg H, Quartier E, et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 1995;96:2489–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassem S, Bhandari S, Rodríguez-Bada P, et al. Large islets, beta-cell proliferation, and a glucokinase mutation. N Engl J Med 2010;362:1348–1350 [DOI] [PubMed] [Google Scholar]
- 7.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA 2004;101:7281–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies MN, O’Callaghan BL, Towle HC. Glucose activates ChREBP by increasing its rate of nuclear entry and relieving repression of its transcriptional activity. J Biol Chem 2008;283:24029–24038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci USA 2003;100:5107–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem 2006;281:28721–28730 [DOI] [PubMed] [Google Scholar]
- 11.Herman MA, Peroni OD, Villoria J, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012;484:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eissing L, Scherer T, Tödter K, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat Commun 2013;4:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kursawe R, Caprio S, Giannini C, et al. Decreased transcription of ChREBP-α/β isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: associations with insulin resistance and hyperglycemia. Diabetes 2013;62:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metukuri MR, Zhang P, Basantani MK, et al. ChREBP mediates glucose-stimulated pancreatic β-cell proliferation. Diabetes 2012;61:2004–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poungvarin N, Lee JK, Yechoor VK, et al. Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in beta cell glucotoxicity. Diabetologia 2012;55:1783–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaahtomeri K, Mäkelä TP. Molecular mechanisms of tumor suppression by LKB1. FEBS Lett 2011;585:944–951 [DOI] [PubMed] [Google Scholar]
- 17.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev 2004;18:1533–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005;310:1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ylipaasto P, Kutlu B, Rasilainen S, et al. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 2005;48:1510–1522 [DOI] [PubMed] [Google Scholar]
- 20.Fu A, Ng AC-H, Depatie C, et al. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab 2009;10:285–295 [DOI] [PubMed] [Google Scholar]
- 21.Granot Z, Swisa A, Magenheim J, et al. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab 2009;10:296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun G, Tarasov AI, McGinty JA, et al. LKB1 deletion with the RIP2.Cre transgene modifies pancreatic beta-cell morphology and enhances insulin secretion in vivo. Am J Physiol Endocrinol Metab 2010;298:E1261–E1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun G, Tarasov AI, McGinty J, et al. Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia 2010;53:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leclerc I, Woltersdorf WW, da Silva Xavier G, et al. Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 2004;286:E1023–E1031 [DOI] [PubMed] [Google Scholar]
- 25.Demozay D, Tsunekawa S, Briaud I, Shah R, Rhodes CJ. Specific glucose-induced control of insulin receptor substrate-2 expression is mediated via Ca2+-dependent calcineurin/NFAT signaling in primary pancreatic islet β-cells. Diabetes 2011;60:2892–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodyer WR, Gu X, Liu Y, Bottino R, Crabtree GR, Kim SK. Neonatal β cell development in mice and humans is regulated by calcineurin/NFAT. Dev Cell 2012;23:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardie DG. Signal transduction: How cells sense energy. Nature 2011;472:176–177 [DOI] [PubMed] [Google Scholar]
- 28.Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature 2006;443:345–349 [DOI] [PubMed] [Google Scholar]
- 29.Kahl CR, Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev 2003;24:719–736 [DOI] [PubMed] [Google Scholar]
- 30.Nguidjoe E, Sokolow S, Bigabwa S, et al. Heterozygous inactivation of the Na/Ca exchanger increases glucose-induced insulin release, β-cell proliferation, and mass. Diabetes 2011;60:2076–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salpeter SJ, Klochendler A, Weinberg-Corem N, et al. Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic β-cells through glycolysis and calcium channels. Endocrinology 2011;152:2589–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansson D, Ng AC, Fu A, Depatie C, Al Azzabi M, Screaton RA. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc Natl Acad Sci USA 2008;105:10161–10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song WJ, Schreiber WE, Zhong E, et al. Exendin-4 stimulation of cyclin A2 in beta-cell proliferation. Diabetes 2008;57:2371–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence MC, Naziruddin B, Levy MF, Jackson A, McGlynn K. Calcineurin/nuclear factor of activated T cells and MAPK signaling induce TNF-alpha gene expression in pancreatic islet endocrine cells. J Biol Chem 2011;286:1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soltani N, Qiu H, Aleksic M, et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci USA 2011;108:11692–11697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Talavera JC, Garcia-Ocaña A, Sipula I, Takane KK, Cozar-Castellano I, Stewart AF. Hepatocyte growth factor gene therapy for pancreatic islets in diabetes: reducing the minimal islet transplant mass required in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology 2004;145:467–474 [DOI] [PubMed] [Google Scholar]
- 37.Weir MR, Fink JC. Risk for posttransplant diabetes mellitus with current immunosuppressive medications. Am J Kidney Dis 1999;34:1–13 [DOI] [PubMed]
- 38.Shing Y, Christofori G, Hanahan D, et al. Betacellulin: a mitogen from pancreatic beta cell tumors. Science 1993;259:1604–1607 [DOI] [PubMed] [Google Scholar]
- 39.Huotari MA, Palgi J, Otonkoski T. Growth factor-mediated proliferation and differentiation of insulin-producing INS-1 and RINm5F cells: identification of betacellulin as a novel beta-cell mitogen. Endocrinology 1998;139:1494–1499 [DOI] [PubMed] [Google Scholar]
- 40.Li L, Seno M, Yamada H, Kojima I. Promotion of beta-cell regeneration by betacellulin in ninety percent-pancreatectomized rats. Endocrinology 2001;142:5379–5385 [DOI] [PubMed] [Google Scholar]
- 41.Tokui Y, Kozawa J, Yamagata K, et al. Neogenesis and proliferation of beta-cells induced by human betacellulin gene transduction via retrograde pancreatic duct injection of an adenovirus vector. Biochem Biophys Res Commun 2006;350:987–993 [DOI] [PubMed] [Google Scholar]
- 42.Oh YS, Shin S, Lee YJ, Kim EH, Jun HS. Betacellulin-induced beta cell proliferation and regeneration is mediated by activation of ErbB-1 and ErbB-2 receptors. PLoS One 2011;6:e23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hakonen E, Ustinov J, Mathijs I, et al. Epidermal growth factor (EGF)-receptor signalling is needed for murine beta cell mass expansion in response to high-fat diet and pregnancy but not after pancreatic duct ligation. Diabetologia 2011;54:1735–1743 [DOI] [PubMed] [Google Scholar]
- 44.Miettinen PJ, Huotari M, Koivisto T, et al. Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development 2000;127:2617–2627 [DOI] [PubMed] [Google Scholar]
- 45.Miettinen PJ, Ustinov J, Ormio P, et al. Downregulation of EGF receptor signaling in pancreatic islets causes diabetes due to impaired postnatal beta-cell growth. Diabetes 2006;55:3299–3308 [DOI] [PubMed] [Google Scholar]
- 46.Krakowski ML, Kritzik MR, Jones EM, et al. Transgenic expression of epidermal growth factor and keratinocyte growth factor in beta-cells results in substantial morphological changes. J Endocrinol 1999;162:167–175 [DOI] [PubMed] [Google Scholar]
- 47.Means AL, Ray KC, Singh AB, et al. Overexpression of heparin-binding EGF-like growth factor in mouse pancreas results in fibrosis and epithelial metaplasia. Gastroenterology 2003;124:1020–1036 [DOI] [PubMed] [Google Scholar]
- 48.Kuntz E, Broca C, Komurasaki T, et al. Effect of epiregulin on pancreatic beta cell growth and insulin secretion. Growth Factors 2005;23:285–293 [DOI] [PubMed] [Google Scholar]
- 49.Mares J, Claesson-Welsh L, Welsh M. A chimera between platelet-derived growth factor beta-receptor and fibroblast growth factor receptor-1 stimulates pancreatic beta-cell DNA synthesis in the presence of PDGF-BB. Growth Factors 1992;6:93–101 [DOI] [PubMed] [Google Scholar]
- 50.Welsh M, Claesson-Welsh L, Hallberg A, et al. Coexpression of the platelet-derived growth factor (PDGF) B chain and the PDGF beta receptor in isolated pancreatic islet cells stimulates DNA synthesis. Proc Natl Acad Sci USA 1990;87:5807–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Gu X, Liu Y, et al. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 2011;478:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyagawa J, Hanafusa O, Sasada R, et al. Immunohistochemical localization of betacellulin, a new member of the EGF family, in normal human pancreas and islet tumor cells. Endocr J 1999;46:755–764 [DOI] [PubMed] [Google Scholar]
- 53.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 2004;303:1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nusse R. Wnt signaling in disease and in development. Cell Res 2005;15:28–32 [DOI] [PubMed] [Google Scholar]
- 55.Welters HJ, Kulkarni RN. Wnt signaling: relevance to beta-cell biology and diabetes. Trends Endocrinol Metab 2008;19:349–355 [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. J Biol Chem 2008;283:8723–8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dabernat S, Secrest P, Peuchant E, Moreau-Gaudry F, Dubus P, Sarvetnick N. Lack of beta-catenin in early life induces abnormal glucose homeostasis in mice. Diabetologia 2009;52:1608–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murtaugh LC, Law AC, Dor Y, Melton DA. Beta-catenin is essential for pancreatic acinar but not islet development. Development 2005;132:4663–4674 [DOI] [PubMed] [Google Scholar]
- 59.Papadopoulou S, Edlund H. Attenuated Wnt signaling perturbs pancreatic growth but not pancreatic function. Diabetes 2005;54:2844–2851 [DOI] [PubMed] [Google Scholar]
- 60.Heiser PW, Lau J, Taketo MM, Herrera PL, Hebrok M. Stabilization of beta-catenin impacts pancreas growth. Development 2006;133:2023–2032 [DOI] [PubMed] [Google Scholar]
- 61.Wells JM, Esni F, Boivin GP, et al. Wnt/beta-catenin signaling is required for development of the exocrine pancreas. BMC Dev Biol 2007;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dessimoz J, Bonnard C, Huelsken J, Grapin-Botton A. Pancreas-specific deletion of beta-catenin reveals Wnt-dependent and Wnt-independent functions during development. Curr Biol 2005;15:1677–1683 [DOI] [PubMed] [Google Scholar]
- 63.Rulifson IC, Karnik SK, Heiser PW, et al. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci USA 2007;104:6247–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elghazi L, Gould AP, Weiss AJ, et al. Importance of β-catenin in glucose and energy homeostasis. Sci Rep 2012;2:693 [DOI] [PMC free article] [PubMed]
- 65.da Silva Xavier G, Mondragon A, Sun G, et al. Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice. Diabetologia 2012;55:2667–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boj SF, van Es JH, Huch M, et al. Diabetes risk gene and Wnt effector Tcf7l2/TCF4 controls hepatic response to perinatal and adult metabolic demand. Cell 2012;151:1595–1607 [DOI] [PubMed] [Google Scholar]
- 67.Figeac F, Uzan B, Faro M, Chelali N, Portha B, Movassat J. Neonatal growth and regeneration of beta-cells are regulated by the Wnt/beta-catenin signaling in normal and diabetic rats. Am J Physiol Endocrinol Metab 2010;298:E245–E256 [DOI] [PubMed] [Google Scholar]
- 68.Shu L, Zien K, Gutjahr G, et al. TCF7L2 promotes beta cell regeneration in human and mouse pancreas. Diabetologia 2012;55:3296–3307 [DOI] [PubMed] [Google Scholar]
- 69.Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes 2008;57:645–653 [DOI] [PubMed] [Google Scholar]
- 70.Kieffer TJ, Heller RS, Habener JF. Leptin receptors expressed on pancreatic beta-cells. Biochem Biophys Res Commun 1996;224:522–527 [DOI] [PubMed] [Google Scholar]
- 71.Kulkarni RN, Wang ZL, Wang RM, et al. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 1997;100:2729–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes 1997;46:313–316 [DOI] [PubMed] [Google Scholar]
- 73.Morioka T, Asilmaz E, Hu J, et al. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest 2007;117:2860–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Covey SD, Wideman RD, McDonald C, et al. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 2006;4:291–302 [DOI] [PubMed] [Google Scholar]
- 75.Jetton TL, Lausier J, LaRock K, et al. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes 2005;54:2294–2304 [DOI] [PubMed] [Google Scholar]
- 76.Seufert J, Kieffer TJ, Leech CA, et al. Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab 1999;84:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee YH, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism 2011;60:1664–1672 [DOI] [PubMed] [Google Scholar]
- 78.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol 2012;8:342–351 [DOI] [PubMed] [Google Scholar]
- 79.Houssay BA, Foglia VG, Rodriguez RR. Production or prevention of some types of experimental diabetes by oestrogens or corticosteroids. Acta Endocrinol (Copenh) 1954;17:146–164 [PubMed] [Google Scholar]
- 80.Goodman MN, Hazelwood RL. Short-term effects of oestradiol benzoate in normal, hypophysectomized and alloxan-diabetic male rats. J Endocrinol 1974;62:439–449 [DOI] [PubMed] [Google Scholar]
- 81.Sorenson RL, Brelje TC, Roth C. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology 1993;133:2227–2234 [DOI] [PubMed] [Google Scholar]
- 82.Choi SB, Jang JS, Park S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology 2005;146:4786–4794 [DOI] [PubMed] [Google Scholar]
- 83.Le May C, Chu K, Hu M, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 2006;103:9232–9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu S, Kilic G, Meyers MS, et al. Oestrogens improve human pancreatic islet transplantation in a mouse model of insulin deficient diabetes. Diabetologia 2013;56:370–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tiano JP, Delghingaro-Augusto V, Le May C, et al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J Clin Invest 2011;121:3331–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong WP, Tiano JP, Liu S, et al. Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci USA 2010;107:13057–13062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tiano JP, Mauvais-Jarvis F. Molecular mechanisms of estrogen receptors’ suppression of lipogenesis in pancreatic β-cells. Endocrinology 2012;153:2997–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jacovetti C, Abderrahmani A, Parnaud G, et al. MicroRNAs contribute to compensatory β cell expansion during pregnancy and obesity. J Clin Invest 2012;122:3541–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar R, Balhuizen A, Amisten S, Lundquist I, Salehi A. Insulinotropic and antidiabetic effects of 17β-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology 2011;152:2568–2579 [DOI] [PubMed] [Google Scholar]
- 90.Liu S, Le May C, Wong WP, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 2009;58:2292–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren Z, Zou C, Ji H, Zhang YA. Oestrogen regulates proliferation and differentiation of human islet-derived precursor cells through oestrogen receptor alpha. Cell Biol Int 2010;34:523–530 [DOI] [PubMed] [Google Scholar]
- 92.Nieuwenhuizen AG, Schuiling GA, Liem SM, Moes H, Koiter TR, Uilenbroek JT. Progesterone stimulates pancreatic cell proliferation in vivo. Eur J Endocrinol 1999;140:256–263 [DOI] [PubMed] [Google Scholar]
- 93.Picard F, Wanatabe M, Schoonjans K, Lydon J, O’Malley BW, Auwerx J. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to beta-cell proliferation. Proc Natl Acad Sci USA 2002;99:15644–15648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doglioni C, Gambacorta M, Zamboni G, Coggi G, Viale G. Immunocytochemical localization of progesterone receptors in endocrine cells of the human pancreas. Am J Pathol 1990;137:999–1005 [PMC free article] [PubMed] [Google Scholar]