Abstract
Exposure to cold, dehydration, and aging are known to contribute to the development of decompression sickness (DCS) in divers. Hypertension and nicotine usage have also been suggested as risk factors. Vasoconstriction is an underlying mechanism associated with all of these risk factors. Vasoconstriction increases the degree of bubble formation which is believed to be the cause of DCS. Formed bubbles interfere with the production of nitric oxide which modulates vascular tone resulting in vasoconstriction. Divers commonly use sympathomimetic decongestants which induce vasoconstriction to prevent barotrauma of the ears and sinuses while diving and thus theoretically may contribute to the risk for developing DCS. The purpose of this case-control study was to explore the association between decongestant usage and development of DCS in 400 divers treated/evaluated at the University of Hawai‘i, John A. Burns School of Medicine between 1983 and 2010. Bivariate and logistic regression analyses were employed to evaluate differences between cases and controls. In addition to the variable of interest, other co-variables known to have significant influence in the development of DCS were appropriately controlled for during the analyses. In this study population, dehydration (OR = 2.7; 95% CI: 1.1, 7.4), repetitive diving (OR = 2.8; 95% CI: 1.8, 4.4), and violation of dive profiles (OR = 4.9; 95% CI: 3.1, 7.9) contributed independently and significantly to the development of DCS. The co-variables of cold, gender, obesity, and rapid ascents were not significant contributors to developing DCS in this study. There was a small but statistically insignificant risk associated with decongestant use (OR = 1.4; 95% CI: 0.8–2.6; P = .22). The inherent limitations associated with records-based studies may have underestimated this risk. It is important therefore that future research be undertaken to help clarify this concern.
Keywords: Decompression sickness, decongestants, epidemiology of diving accidents, diving health, safety
Introduction
Exposure to the underwater environment while diving with compressed gas incurs some risks. One of those risks is decompression sickness (DCS), which is believed to be caused by the formation of inert gas bubbles during decompression and is manifested by a broad array of symptoms which can result in severe morbidity, life-long disabilities, and even death.1, 2 Thus there are profound public health implications associated with these injuries. The precise incidence of decompression sickness is unknown. Researchers at the Diver's Alert Network (DAN) at Duke University have demonstrated that the incidence rates for DCS have ranged from 1/10,000 to 1/20,000 dives with over 1,000 cases of DCS reported annually in the United States alone.3 In Hawai‘i, the average yearly number of cases treated for decompression sickness is about 50 cases.4 Approximately 25% of these are classified as severe injuries resulting in significant residual morbidity.5 Epidemiological researchers have shown that cold exposure, as well as dehydration and aging, increase the risk for developing DCS.6–10 Other epidemiological studies have also implicated hypertension and nicotine use from smoking as risk factors for developing DCS.11–13 Koteng, et al, demonstrated that increased peripheral resistance resulting from vasoconstriction caused a greater degree of bubble formation during decompression.14 Each of these risk factors are associated with vasoconstriction which can alter the inert gas kinetics potentially leading to slower rates of off-gassing, increased bubble formation, and the development of DCS. Bubbles formed during decompression damage the endothelial lining of blood vessels and lower the production of nitric oxide which modulates vascular tone; in turn, the absence of nitric oxide results in vasoconstriction.15–18 The integrity and optimal functioning of the vasculature is a key factor in both on-gassing and off-gassing while diving.19 Several additional risk factors have been associated with the development of DCS including adiposity, gender, repetitive diving, violation of recommended depth and time limits (dive profile), and rapid ascent rates.1, 2, 8, 9
A second and more common affliction in divers is that of barotrauma to the ears and sinuses.20–22 This results from poor equalization of the external water or ambient pressure with the internal pressures of the middle ear and sinus cavities. This occurs because of blockage of the ostia of the sinuses and/or the eustachian tubes of the ears, which normally provide ventilation to those air spaces.23 Up to 65% of divers report this type of injury.24 In order to prevent barotrauma, many divers use a sympathomimetic decongestant drug such as pseudoephedrine prior to diving. Researchers have found that 6% to 25% of divers routinely use these drugs while diving and another 30% occasionally use them.25–27 These drugs mimic the sympathetic nervous system by stimulating increased production of norepinephrine from nerve endings or by direct stimulation of vascular smooth muscle to induce vasoconstriction. Specifically, these decongestants stimulate alpha-adrenergic receptors embedded in the vascular smooth muscle and are referred to as alpha agonists.28 However, their effects are not confined solely to those tissues for which they are targeted, but also produce systemic effects as well.29 Westerveld, et al, demonstrated in an in vitro study of rat lung macrophages, that the nasal decongestants oxymetazoline and xylometazoline inhibited nitric oxide synthetase production, thereby reducing nitric oxide production.30 As was previously mentioned, the absence or low levels of nitric oxide result in vasoconstriction.16
Lambertsen suggested that vasoactive medications might adversely impact the incidence of DCS.31 Thus, a hypothetical question ensues. Since vasoconstriction appears to be an underlying mechanism associated with other established risk factors for DCS, could the use of sympathomimetic drugs while diving enhance the risk for developing DCS? The conventional thinking among diving medical experts is that it is probably safe to employ these drugs while diving.32 However, there is no scientific evidence to support this position despite these theoretical concerns. While these drugs have been studied to assess their effects on cognitive function and mental alertness while diving, no studies have been done to investigate their potential contribution to developing DCS. 33, 34 This study was undertaken to explore the possibility that use of sympathomimetic drugs during diving might increase the risk for DCS. Given the potential for long-term and sometimes catastrophic sequelae which may ensue from a case of DCS, there is a need to determine whether the use of these decongestants while diving poses additional risk for development of this malady. Data on this relationship will allow appropriate safety policies to follow regarding their usage while diving in an effort to lessen risk potential.
Methods
A records-based, case-control study of 400 scuba divers was undertaken to compare sympathomimetic decongestant usage in divers treated for DCS (cases) with those who did not exhibit signs or symptoms of DCS (controls) after diving. Cases and controls were drawn from a cohort population of over 1600 divers' records resulting from evaluation at the Hyperbaric Treatment Center (HTC) at the University of Hawai‘i, John A. Burns School of Medicine between the years 1983 through 2010. The study population consisted of recreational divers. The same data had been collected from each patient relative to their immediate diving history irrespective of whether they were diagnosed with DCS or something else. This study was approved by the university institutional review board and human use committee.
The independent variable was decongestant use during an incident dive. Decongestants were defined as those medications which contained any of the following compounds: pseudoephedrine, oxymetazoline, phenylephrine, or xylometazoline.29 Because most of these drugs are short acting, usually less than 12 hours, drug use during the incident dive was defined as usage within 12 hours prior to the documented incident dive. For this particular exploratory study, no distinction was made between topical usage versus oral administration of the sympathomimetic drug, and equivalency of dose and route of administration was assumed. The dependent variable was DCS.
The estimated sample size for this study was determined using Cohen's d. The effect size of .20 was selected based upon published studies of decongestant usage by divers which demonstrated that anywhere from 6% to 40% of divers may use these drugs.25–27 This effect size was a conservative estimate and led to a larger sample size requirement. By convention, an alpha level of .05 and power of .80 were utilized.
Cases were defined as those who were diagnosed and treated for DCS with records coded as 993.3 (ICD-9). Cases were restricted to those who were 18 years and older, and had completed at least one dive immediately prior to presenting for evaluation. Sampled cases came from randomly selected calendar years until 200 cases were identified. The years selected were 1988, 1993, 1994, 1995, 1997, 2000, 2001, and 2007.
Controls were those divers, 18 years and older, who had completed at least one dive immediately prior to presenting for evalaution, and were diagnosed with a diving related problem other than DCS. Controls came from the same randomly sampled years as the cases. They were randomly selected and matched with replacement on a 1:1 basis to cases based on age +/− 5 years following a cumulative incidence sampling model.35 This procedure effectively limited the risk for selection bias while ensuring representativeness of both cases and controls.
Specific information collected from each subject record was the ICD-9 code of the final diagnosis, age, gender, dive profile (depth and time length of dive[s]), history of rapid ascent, whether the diver made repetitive dives, temperature at admission or whether the diver complained of being cold during the incident dive, height and weight, whether the diver dehydrated as measured by a urine specific gravity of 1.025 or greater and/or receiving intravenous fluids, and whether the diver used decongestants during the incident dive(s).
Dive profiles were evaluated using the US Navy Diving Manual to determine whether the diver may have violated that profile36. This approach was taken to standardize the assessment of the dive profile. Height and weight were used to determine BMI and if that value was greater than 30, the subject was classified as being obese. All collected data, aside from age and gender, were converted to either “yes” or “no” dichotomous nominal values for data entry into EpiInfo version 3.5.3 (Centers for Disease Control and Prevention Atlanta, Georgia).
The ultimate aim of this study was to compare the proportion of decongestant users in cases, to that in controls, to ascertain whether the odds ratio suggested a potential association between sympathomimetic drug usage and the occurrence of DCS. Since other confounding variables such as obesity, being cold during the dive, rapid ascents, repetitive diving, and violation of dive profiles also influence whether a diver develops DCS, these were analyzed as well. Initial analysis assessed the frequencies with which these variables were found in both the cases and the controls. Each was subjected to bivariate analysis to assess their independent impact within this study population. Odds ratios were determined. As this study analyzed proportions as well as nominal data, the level of significance was determined using chi-square or Fisher's Exact Test as required. Because the other co-variables may have played a role in combination with each other in the development of DCS, further analysis used logistic regression to determine the relative contribution of each co-variable in addition to the independent variable of this study.
Results
For the entire study population, the ages ranged from 18 to 66 years with a mean age of 35.9 years and a mode of 34 years. There were 285 males (71.2%) and 115 females (28.8%). Sixty-nine (17.3%) had used decongestants. Violation of dive profiles occurred with 153 (38.3%) divers. Rapid ascents were identified in 111 (27.8%) divers. Two hundred sixty-three (65.8%) made repetitive dives. Only six (1.5%) were categorized as being cold; 74 (18.5%) were found to be obese, and 25 (6.3%) were classified as being dehydrated (Figure 1).
Figure 1.
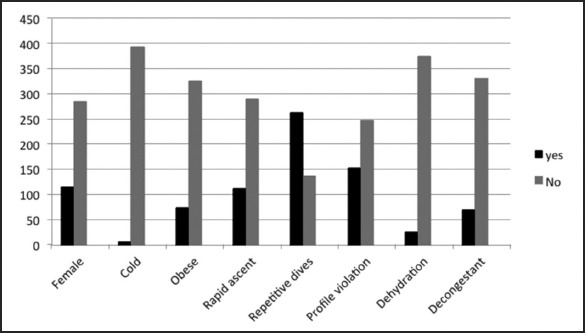
Frequencies of All Variables for Entire Study Population
Bivariate Analysis
The average age for cases was 36.1 years with a mode of 34 years while the average age for controls was 35.7 years with a mode of 34 years. Contingency tables were employed to analyze bivariate relationships between the independent and confounding variables and DCS and the consolidated results are depicted in Table 1. Significant findings are shown in bold.
Table 1.
Results Bivariate Analyses Variables vs DCS
| Variable | Odds Ratio | 95% CI | .2 | P-value |
| Gender | 0. | 0.6, 1.4 | 0.304 | .581 |
| Cold | 5.1 | 0.6, 116.5 | ** | .212 |
| Obese | 0.7 | 0.4, 1.2 | 1.658 | .198 |
| Rapid Ascent | 1.2 | 0.8, 1.9 | 0.609 | .434 |
| Repetitive Dives | 2.8 | 1.8, 4.4 | 22.424 | .000 |
| Profile Violation | 4.9 | 3.1, 7.9 | 53.223 | .000 |
| Dehydration | 2.7 | 1.1, 7.4 | 5.149 | .023 |
| Decongestant Use | 1.5 | 0.9, 2.6 | 2.113 | .145 |
Fisher's Exact Test
The bivariate analyses indicate that the co-variables of dehydration, repetitive diving, and profile violation resulted in odds ratios which were statistically significant (OR = 2.7 95% CI: 1.1, 7.4; P = .023; OR = 2.8 95% CI: 1.8, 4.4; P = .000; OR = 4.9 95% CI: 3.1, 7.9; P = .000 respectively). The resulting odds ratios for the independent variable of decongestant use as well as the co-variables of cold, rapid ascent, and gender did not achieve statistical significance. To further assess these variables to investigate any interactions which may have influenced these findings, logistic regression was performed using all the variables at first and reducing their number until the appropriate final model was ascertained.
Logistic Regression
Logistic regression was carried out to assess the inter-relationships of the relative contribution and probabilities of each of the co-variables and the independent variable to the development of DCS in this study population. For this analysis, backward logistic regression was undertaken by initially entering all the co-variables and running repeated calculations after elimination of non-significant variables until the final model was arrived at which contained only variables with significance. The final model is shown in Table 2 which also includes the independent (study) variable of decongestant use for comparison.
Table 2.
Final Logistic Regression Model including Independent Variable (Decongestant Use)
| Co-variable | OR | 95% CI | Coefficient | SE | Z-statistic | P-value |
| Decongest Use | 1.4 | 0.8, 2.6 | 0.36 | 0.30 | 1.21 | .225 |
| Dehydration | 3.2 | 1.2, 8.5 | 1.17 | 0.50 | 2.35 | .018 |
| Repetitive Dives | 2.4 | 1.5, 3.7 | 0.85 | 0.24 | 3.56 | .000 |
| Profile violation | 4.3 | 2.7, 6.7 | 1.45 | 0.23 | 6.22 | .000 |
The results of the logistic regression analysis indicate that dehydration, repetitive diving, and violation of dive profiles all contributed significantly to the development of DCS in this study population whereas the use of decongestants did not.
Discussion
Historically, the demographics and types, nature, and distribution of diving injuries seen at the HTC have mirrored those reported throughout the country.4 The total study population of 400 was comprised of 28.8% females and 71.2% males. This distribution of study subjects is consistent with the percentages of certified scuba divers based upon gender within the general diving population.37 No statistical differences were identified during the analysis of this data for any of the variables based upon gender. The average age for subjects who developed DCS was nearly identical to that of the non-DCS controls because of age matching, thus there is no concern that age differences may have influenced the outcomes.
The findings for this case-control study indicate that only dehydration, repetitive diving, and violation of dive profiles were statistically significant contributors for the development of DCS. This was established in both the bivariate and multivariate analyses of the variables. These findings do not imply that those variables other than the independent variable of decongestant use, which have been previously established as risk factors, are not of import to developing DCS, but rather indicate that in this particular study population, they did not play a significant role. With respect to the variable of interest, decongestant usage, it was shown that 17.3% of the studied divers used these medications. This was slightly less than the expected 20% usage used to calculate the sample size for this study. It is unlikely that increasing the sample size for this study would have appreciably affected the overall percentage of divers who used decongestants. There were significant limitations in this study which may have influenced the outcome. Records-based studies are dependent upon the quality and thoroughness of the records.38 This cohort was amassed over a time period of 27 years during which time there were multiple evaluators who documented their findings in the records and the quality of the efforts to solicit all the patient information may not have been uniformly comprehensive. A lack of notation in the records of the use of decongestants was interpreted in this study as non-use so as to avoid overestimation. It is possible however that some of those undocumented subjects had actually used these drugs which would lead to underestimation of the actual use and risk and in turn would favor the null hypothesis of this study and represents possible information bias.39
Berkson's bias, or hospital admission bias, must also be considered.39 This situation extends from the fact that clinic records were used for this study for both cases and controls. It invites the question of representativeness of the sampling of the study population. With respect to cases, the vast majority of divers with DCS in Hawai‘i who develop DCS are treated at the HTC which is the only facility other than the US Navy which treats these injuries. So for cases of DCS, the data is assumed to be representative of the diving population; this is further suggested by the comparability of HTC's DCS data to national trends for such injuries.4 For controls however, Berkson's bias may be in play. Certainly it could be argued that those who served as controls were different than those in the general diving population in Hawai‘i. Because this was a retrospective study, it would have been impossible to obtain the specific, reliable data needed to conduct this study dating back to 1983 without very significant recall bias. To mitigate Berkson's bias, controls were randomly chosen from the same randomly chosen years from which the cases were drawn. This also aided in ensuring that trends in sympathomimetic decongestant usage over the time span of this study would be similar in both cases and controls.
This study also made no attempt to distinguish between topical agents or those taken orally. Additionally, no effort was made to assess the specific dosages of the medications which were used or the specific timing of the administration of the drug within a 12 hour window prior to diving. Conceivably, any or all of these considerations might have a bearing on the outcome. However, this approach allowed for the opportunity to take an initial broad look utilizing the greatest number of exposures to determine whether the hypothesis had merit. Had this exploratory study demonstrated that use of sympathomimetic decongestants while diving did increase the risk for developing DCS, a more focused analysis looking at those additional parameters would be warranted.
Conclusions
Given the sample size and power of this study, it is reasonable to conclude that the use of sympathomimetic decongestants did not increase the risk for developing DCS whereas dehydration, repetitive dives, and violation of dive profiles were shown to significantly contribute to the development of DCS. The inherent limitations associated with records-based studies may have biased the outcome and underestimated the risk associated with decongestant usage while diving in this study. It is important, therefore, that future research be undertaken to further clarify this concern.
Conflict of Interest
The author identifies no conflict of interest.
References
- 1.Francis T, Mitchell S. Manifestations of decompression disorders. In: Neuman T, Brubakk A, editors. Physiology and Medicine of Diving. 5 ed. New York, NY: W. B. Saunders Company; 2003. pp. 578–599. [Google Scholar]
- 2.Levett D, Millar I. Bubble trouble: a review of diving physiology and disease. Postgrad Med J. 2008;84:571–578. doi: 10.1136/pgmj.2008.068320. [DOI] [PubMed] [Google Scholar]
- 3.Vann R, Uguccioni D. Divers Alert Network. Durham, NC: 2000. Report on diving accidents and fatalities, 2000. [Google Scholar]
- 4.Nakayama H, Smerz R. Descriptive epidemiological analysis of diving accidents in Hawaii, 1983–2001. Hawaii Med J. 2003;62(8):165–170. [PubMed] [Google Scholar]
- 5.Smerz R, Overlock R, Nakayama H. Hawaiian deep tables: efficacy and outcomes, 1983–2003. Undersea Hyperc Med. 2005;32(5):363–373. [PubMed] [Google Scholar]
- 6.Bove A, Hardenbergh E, Miles J. Effect of heat and cold stress on inert gas (133 xenon) exchange in the rabbit. Undersea Biomed Res. 1978;5:149–158. [PubMed] [Google Scholar]
- 7.Broome J. Climatic and environmental factors in the aetiology of decompression sickness in divers. J R Nav Med Serv. 1993;79:68–74. [PubMed] [Google Scholar]
- 8.Dunford R, Hayward J. The effects of cold stress on venous gas bubble production in man following a no-decompression dive. Undersea Biomed Res. 1981;8:41–49. [PubMed] [Google Scholar]
- 9.Gempp E, Blatteau J, Pontier J, Balestra C, Louge P. Preventive effect of pre-dive hydration on bubble formation in divers. Br J Sports Med. 2009;43:224–228. doi: 10.1136/bjsm.2007.043240. [DOI] [PubMed] [Google Scholar]
- 10.Malette W, Fitzgerald J, Cockett A. Dysbarism: A review of thirty-five cases with suggestion for therapy. Aerosp Med. 1962;33:1132. [PubMed] [Google Scholar]
- 11.Dutka A, Pearson R. Spontaneously hypertensive rats are more likely to develop decompression sickness than Sprague-Dawley rats. Undersea Hyperb Med. 1992;19:20. [Google Scholar]
- 12.Yoshida T, Sakane N, Umekawa T, Kondo M. Effect of nicotine on sympathetic nervous system activity of mice subjected to immobilization stress. Physiology and Behavior. 1994;55(1):53–57. doi: 10.1016/0031-9384(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 13.Buch D, Habib E, Dovenbarger J, Uguccioni D, Moon R. Cigarette smoking and decompression illness severity: A retrospective study in recreational divers. Aviation, Space, and Environmental Medicine. 2003;74(12):1271–1274. [PubMed] [Google Scholar]
- 14.Koteng S, Reinertsen R, Holman J, Ustad A, Flook V, Brubakk A. The effect of reduced peripheral circulation on formation of venous gas bubbles and decompression. Undersea Hyperb Med. 1996;23(suppl):38. [Google Scholar]
- 15.Brubakk A, Duplancic D, Valic Z, Palada I, Obad A, Bakovic D, et al. A single air dive reduces arterial endothelial function in man. J Physiol. 2005;566(Pt 3):901–906. doi: 10.1113/jphysiol.2005.089862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galley H, Webster N. Physiology of the endothelium. Br J Anaesth. 2004;93:105–113. doi: 10.1093/bja/aeh163. [DOI] [PubMed] [Google Scholar]
- 17.Nossum V, Hjelde A, Brubakk A. Small aounts of venous gas embolism cause delayed impairment of endothelial function and increase polymorphonuclear neutrophil infiltration. Eur J Appl Physiol. 2002;86:209–214. doi: 10.1007/s00421-001-0531-y. [DOI] [PubMed] [Google Scholar]
- 18.Nossum V, Koteng S, Brubakk A. Endothelial damage by bubbles in the pulmonary artery of the pig. Undersea Hyperb Med. 1999;26:1–8. [PubMed] [Google Scholar]
- 19.Bondi M, Cavaggioni A, Michieli P, Schiavon M, Travain G. Delayed effect of nitric oxide synthetase inhibition on the survival of rats after acute decompression. Undersea Hyperb Med. 2005;32(2):121–128. [PubMed] [Google Scholar]
- 20.Klingmann C, Praetorius M, Baumann I, Plinkert P. Otorhinolaryngologic disorders and diving accidents: an analysis of 306 divers. Eur Arch Otorhinolaryngol. 2007;264:1243–1251. doi: 10.1007/s00405-007-0353-6. [DOI] [PubMed] [Google Scholar]
- 21.Taylor D, O'Toole K, Ryan C. Experienced scuba divers in Australia and the United States suffer considerable injury and morbidity. Wilderness Environ Med. 2003;14:83–88. doi: 10.1580/1080-6032(2003)014[0083:esdiaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Fagan P, McKenzie B, Edmonds C. Sinus barotrauma in divers. Ann Otol Rhinol Laryngol. 1976;85:61–64. doi: 10.1177/000348947608500110. [DOI] [PubMed] [Google Scholar]
- 23.Magnuson B, Falk B. Diagnosis and management of eustachian tube malfunction. Otolaryngol Clin North Am. 1984;17:659–671. [PubMed] [Google Scholar]
- 24.Mawle S, Jackson C. An investigation of ear trauma in divers including ear barotrauma and ear infection. Eur J Underwater Hyperb Med. 2002;3:47–50. [Google Scholar]
- 25.Dowse M, Cridge C, Smerdon G. The use of drugs by UK recreational divers: prescribed and over-the-counter medications. Diving Hyperb Med. 2011;41:16–21. [PubMed] [Google Scholar]
- 26.Smerz R. Drugs downed divers did. Undersea Hyperb Med. 2007;34:117. [Google Scholar]
- 27.Taylor S, Taylor D, O'Toole K, Ryan C. Medications taken daily and prior to diving by experienced scuba divers. SPUMS Journal. 2002;33:129–135. [Google Scholar]
- 28.Kanfer I, Dowse R, Vuma V. Pharmacokinetics of oral decongestants. Pharmacotherapy. 1993;13:116S–128S. [PubMed] [Google Scholar]
- 29.Hoffman B. Adrenoceptor-activating & other sympathomimetic drugs. In: Katzung B, editor. Basic and Clinical Pharmacology. 8 ed. Norwalk, CT: Appleton and Lange; 2000. pp. 120–137. [Google Scholar]
- 30.Westerveld G, Voss R, van der Hee G, de Hann-Koelewjin G, den Hartog R, Schneeren A. Inhibition of nitric oxide synthetase by nasal decongestants. Eur Resp J. 2000;16:437–444. doi: 10.1034/j.1399-3003.2000.016003437.x. [DOI] [PubMed] [Google Scholar]
- 31.Lambertsen C. Basic requirements for improving diving depth and decompression tolerance. In: Lambertsen C, editor. Proceedings of the Third Symposium on Underwater Physiology. Baltimore, MD: Williams & Wilkins; 1967. pp. 223–240. [Google Scholar]
- 32.Thalmann E. Diving Medicine Articles: Pseudoephedrine & Enriched-Air Diving? Divers Alert Network. (n.d.) http://www.diversalertnetwork.org.
- 33.McGeoch G, Davis F, Fletcher L. The effects on performance of cyclizine and pseudoephedrine during dry chamber dives breathing air to 30 metres depth. SPUMS Journal. 2005;35(4):178–182. [Google Scholar]
- 34.Taylor D, O'Toole K, Auble T, Ryan C, Sherman D. The psychometric and cardiac effects of pseudoephedrine and antihistamines in the hyperbaric environment. SPUMS Journal. 2001;31(1):50–57. [Google Scholar]
- 35.Mietinnen O. The “case-control” study: valid selection of subjects. J Chronic Dis. 1985;7:543–548. doi: 10.1016/0021-9681(85)90039-6. [DOI] [PubMed] [Google Scholar]
- 36.US Navy Dive Manual. 6th Edition. Washington, DC: US Navy; 2008. Naval Sea Systems Command. [Google Scholar]
- 37.Professional Association of Diving Instructors, author. PADI Statistics. 2009. PADI: http://www.padi.com/scuba/about-padi/PADI-statistics.
- 38.Checkoway H, Pearce N, Kriebel D. Overview of study designs. In: Checkoway H, Pearce N, Kriebel D, editors. Research Methods in Occupational Epidemiology. 2nd ed. New York: Oxford University Press; 2004. pp. 59–81. [Google Scholar]
- 39.Gerstman B. Types of epidemiologic study. In: Gerstman B, editor. Epidemiology Kept Simple. 2nd ed. San Jose, CA: Wiley-Liss; 2003. pp. 173–230. [Google Scholar]


