Abstract
Inflammatory caspases, such as caspase-1 and -11, mediate innate immune detection of pathogens. Caspase-11 induces pyroptosis, a form of programmed cell death, and specifically defends against bacterial pathogens that invade the cytosol. During endotoxemia, however, excessive caspase-11 activation causes shock. We report that contamination of the cytoplasm by lipopolysaccharide (LPS) is the signal that triggers caspase-11 activation in mice. Specifically, caspase-11 responds to penta- and hexa-acylated lipid A, whereas tetra-acylated lipid A is not detected, providing a mechanism of evasion for cytosol-invasive Francisella. Priming the caspase-11 pathway in vivo resulted in extreme sensitivity to subsequent LPS challenge in both wild type and Tlr4-deficient mice, whereas caspase 11-deficient mice were relatively resistant. Together, our data reveal a new pathway for detecting cytoplasmic LPS.
Caspases are evolutionarily ancient proteases that are integral to basic cellular physiology. Although some caspases mediate apoptosis, the inflammatory caspases-1 and -11 trigger pyroptosis, a distinct form of lytic programmed cell death. In addition, caspase-1 processes IL-1β and IL-18 to their mature secreted forms. Caspase-1 is activated by the canonical inflammasomes, which signal via the adaptor ASC; NLRC4 and NLRP1a/1b can additionally activate caspase-1 directly (1, 2). In contrast to caspase-1, caspase-11 is activated independently of all known canonical inflammasome pathways; the hypothetical caspase-11 activating platform has been termed the non-canonical inflammasome (3). Casp1−/− mice generated from 129 background stem cells are also deficient in Casp11 due to a passenger mutation backcrossed from the 129 background into C57BL/6. Caspase-11 is responsible for certain phenotypes initially attributed to caspase-1, such as shock following endotoxin challenge (3). The physiologic function of caspase-11 is to discriminate cytosolic from vacuolar bacteria (4). In the absence of caspase-11, mice become acutely susceptible to infection by bacteria that escape the phagosome and replicate in the cytosol (4), such as Burkholderia pseudomallei and B. thailandensis. Caspase-11 also responds to vacuolar Gram-negative bacteria, albeit with delayed kinetics (3, 5–7), which may have relevance to its aberrant activation during sepsis. Although these studies demonstrated both detrimental and protective roles for caspase-11, the precise nature of the caspase-11 activating signal remained unknown.
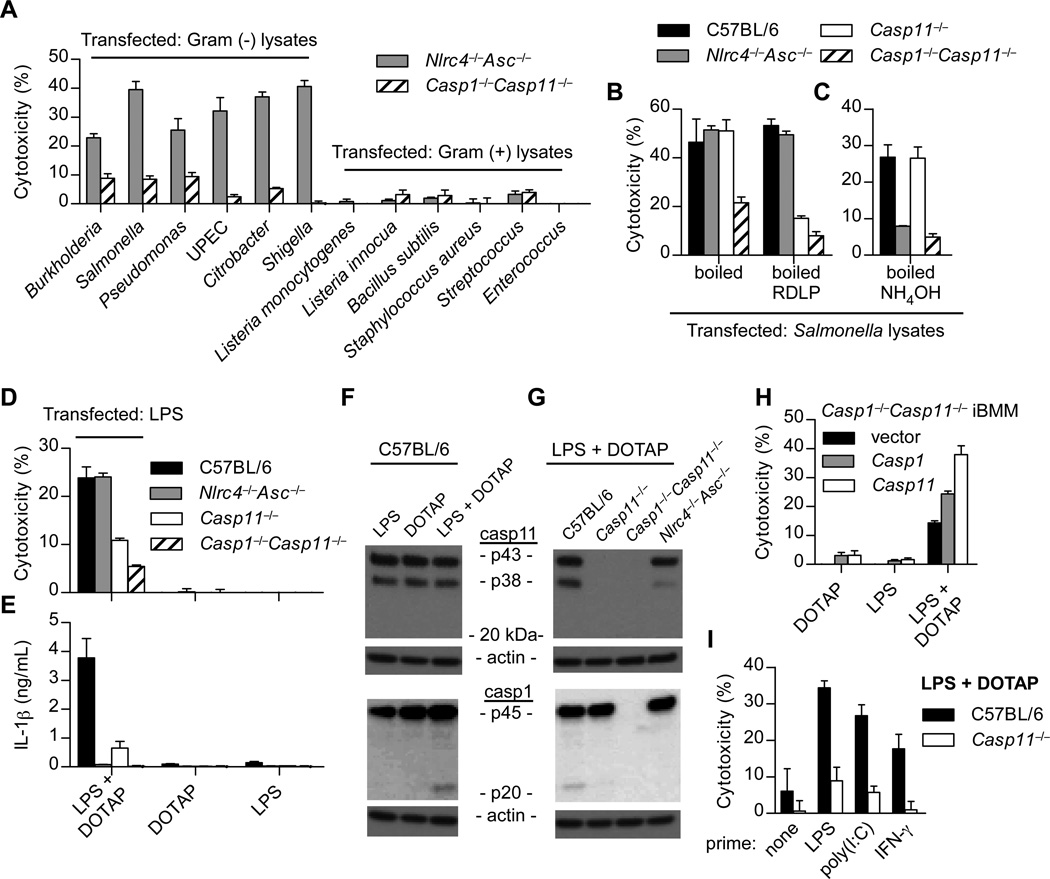
Because caspase-11 specifically responds to cytosolic bacteria, we hypothesized that detection of a conserved microbial ligand within the cytosol triggers caspase-11. To address this hypothesis, we generated lysates of Gram-negative and Gram-positive bacteria and transfected them into LPS primed Nlrc4−/−Asc−/−Casp11+/+ or Casp1−/−Casp11−/− bone marrow-derived macrophages (BMMs). By comparing these strains, we can examine caspase-11 activation in the absence of canonical inflammasome detection of flagellin and DNA (fig. S1). Although boiled Gram-negative bacterial lysates were detected through caspase-11 upon transfection into BMMs, Gram-positive lysates were not (Fig. 1A). RNase, DNase, lysozyme, and proteinase K digestion was sufficient to dispose of canonical inflammasome agonists, but failed to eliminate the caspase-11 activating factor(s) (Fig. 1B). We then treated boiled lysates with ammonium hydroxide, which is known to deacylate lipid species (8), and observed that the caspase-11 activating factor was degraded, whereas canonical inflammasome agonists persisted (Fig. 1C).
Fig. 1. Cytoplasmic LPS triggers caspase-11 activation.
(A–H) BMM were LPS primed overnight prior to transfection. (A–C) BMMs were transfected with the indicated bacterial lysates packaged in Lipofectamine 2000. Cytotoxicity was determined by lactate dehydrogenase release 4 hours later. Where indicated, lysates were treated with RNase, DNase, proteinase K, and lysozyme (RDLP) (B) or ammonium hydroxide (C). (D–E) BMMs were transfected with ultrapure LPS from S. minnesota RE595 packaged with DOTAP, a liposomal transfection reagent. Cytotoxicity (D) and IL-1β secretion by ELISA (E) were determined 4 h post transfection. (F–G) BMMs were stimulated as in (D) and caspase-1 and -11 processing by western blot were examined 2 h post transfection. (H) Immortalized Casp1−/−Casp11−/− BMMs (iBMMs) complemented by retroviral transduction of Casp1 or Casp11 were transfected with LPS from S. minnesota RE595. Cytotoxicity was determined after 4 h. (I) Macrophages were primed overnight with LPS (50ng/mL), poly(I:C) (1µg/mL), IFN-γ (8ng/mL), or left untreated. Cells were then transfected with LPS from S. minnesota RE595 and cytotoxicity was determined 2 h later. Data are representative of at least 3 experiments. Error bars indicate standard deviation of technical replicates.
These results suggested lipopolysaccharide (LPS) as the caspase-11 agonist. Consistent with this hypothesis, BMMs underwent caspase-11 dependent pyroptosis following transfection of ultra pure Salmonella minnesota RE595 LPS (Fig. 1D). Caspase-11 can promote IL-1β secretion by triggering the canonical NLRP3 pathway (3) (fig. S1). Consistently, IL-1β secretion and caspase-1 processing following transfection of LPS were also caspase-11 dependent (Fig. 1E to G). Moreover, caspase-11 alone promoted pyroptosis (Fig. 1H). In contrast to caspase-1, we were unable to convincingly visualize caspase-11 processing by western blot (Fig. 1F and G; fig. S2A), despite the vast majority of cells exhibiting pyroptotic morphology as seen by phase microscopy. Although these data do not exclude the possibility that processing of a small amount of caspase-11 is required for pyroptosis, they do indicate that processing is not a good proxy measure for rapid caspase-11 activation. This is consistent with direct caspase-1 activation by NLRC4, which is not accompanied by processing (9). These results suggest that the presence of LPS in the cytosol is sufficient to trigger caspase-11; however, we cannot rule out the formal possibility that this signaling arises from a membrane bound compartment such as the ER or golgi. Future identification of the non-canonical inflammasome will permit this determination.
The caspase-11 pathway is not responsive unless macrophages are previously stimulated (primed) with either LPS, poly(I:C), IFN-β, or IFN-γ, which likely induces multiple components of the non-canonical inflammasome pathway including caspase-11 (fig. S2B) (4–7, 10). LPS and poly(I:C) prime via TLR4 and TLR3, respectively, which both stimulate IFN-β production; IFN-β and IFN-γ signaling overlap in their activation of the STAT1 transcription factor, which is critical to caspase-11 activation (5, 7). In order to separate the priming and activation stimuli of caspase-11, we verified that poly(I:C) and IFN-γ could substitute for LPS as priming agents (Fig. 1I).
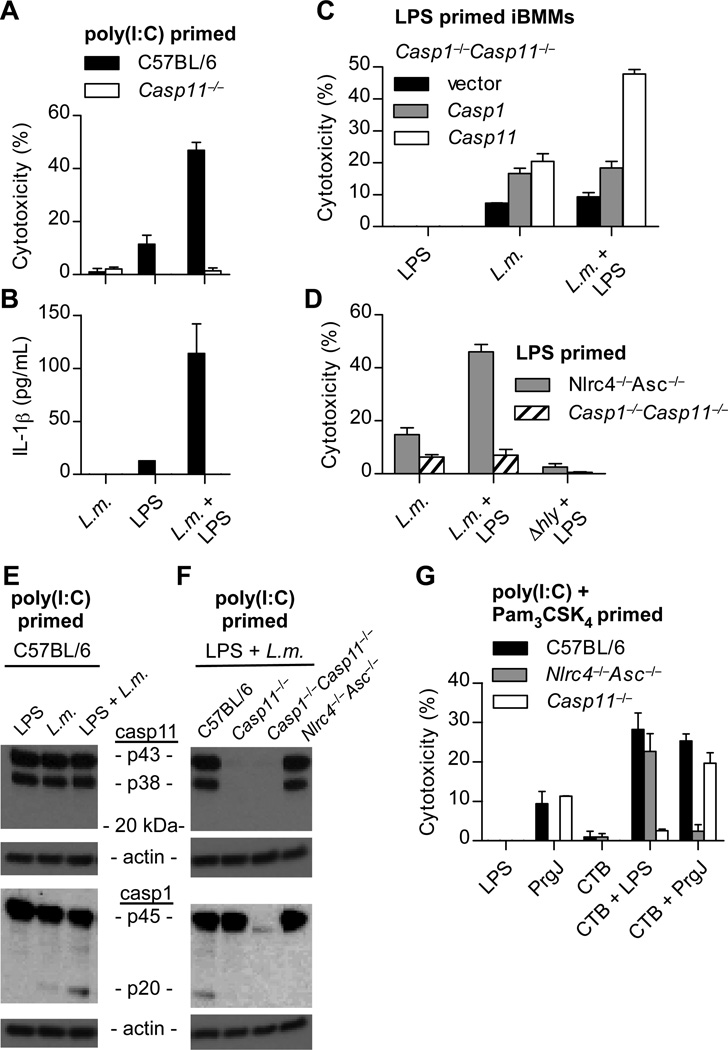
To corroborate our LPS transfection results, we sought another means to deliver LPS to the cytoplasm. Listeria monocytogenes lyses the phagosome via the pore forming toxin LLO, and as a Gram-positive bacterium does not contain LPS. L. monocytogenes infection did not activate caspase-11 in BMMs; however, co-phagocytosis of wild type, but not LLO mutant (∆hly), L. monocytogenes with exogenous LPS triggered pyroptosis, IL-1β secretion, and caspase-1 processing dependent upon caspase-11 (Fig. 2A–F). Despite this genetic evidence of caspase-11 activation, we again did not observe proteolytic processing of caspase-11 (Fig. 2E and F). In conjunction with our previous data indicating that caspase-11 discriminates cytosolic from vacuolar Gram-negative bacteria (4), these results indicate that detection of LPS in the cytoplasm triggers caspase-11 dependent pyroptosis.
Fig. 2. Listeria and CTB mediate caspase-11 activation by LPS.
(A–F) The indicated macrophages were primed with poly(I:C) or LPS and then infected by L. monocytogenes (MOI 5) in the presence or absence of LPS from S. minnesota RE595 (1µg/mL). Cytotoxicity (A, C, D), IL-1β secretion (B), or caspase-1 and caspase-11 processing (E–F) were examined 4 h post-infection. (G) Poly(I:C) and Pam3CSK4 primed macrophages were incubated with the indicated combinations of CTB (20µg/mL), LPS from E. coli O111:B4 (1µg/mL), and PrgJ (10µg/mL). Cytotoxicity was determined 16 h later. Data are representative of 3 (A, D, G) or 2 (B, C, E, F) experiments. Error bars indicate standard deviation of technical replicates.
Previous studies have shown that another agonist, cholera toxin B (CTB), activates caspase-11. However, LPS was present with CTB for the duration of these experiments (3), and caspase-11 failed to respond to CTB in the absence of LPS (Fig. 2G). The physiological function of CTB is to mediate the translocation of the enzymatically active cholera toxin A (CTA) into host cells. Therefore, we hypothesized that activation of caspase-11 by CTB results from delivery of co-phagocytosed LPS into the cytosol. Under this hypothesis, CTB should likewise be able to shuttle canonical inflammasome agonists, which are detected in the cytosol. Indeed, when LPS was replaced with PrgJ, an NLRC4 agonist (11), the pyroptotic response switched from caspase-11-dependence to NLRC4-dependence (Fig. 2G). Therefore, in these experiments CTB is not a caspase-11 agonist, but rather an LPS delivery agent. Whether CTB disrupts vacuoles during its use as an adjuvant, or whether complete cholera toxin (CTA/CTB) disrupts vacuoles during infection with Vibrio cholera remain to be examined.
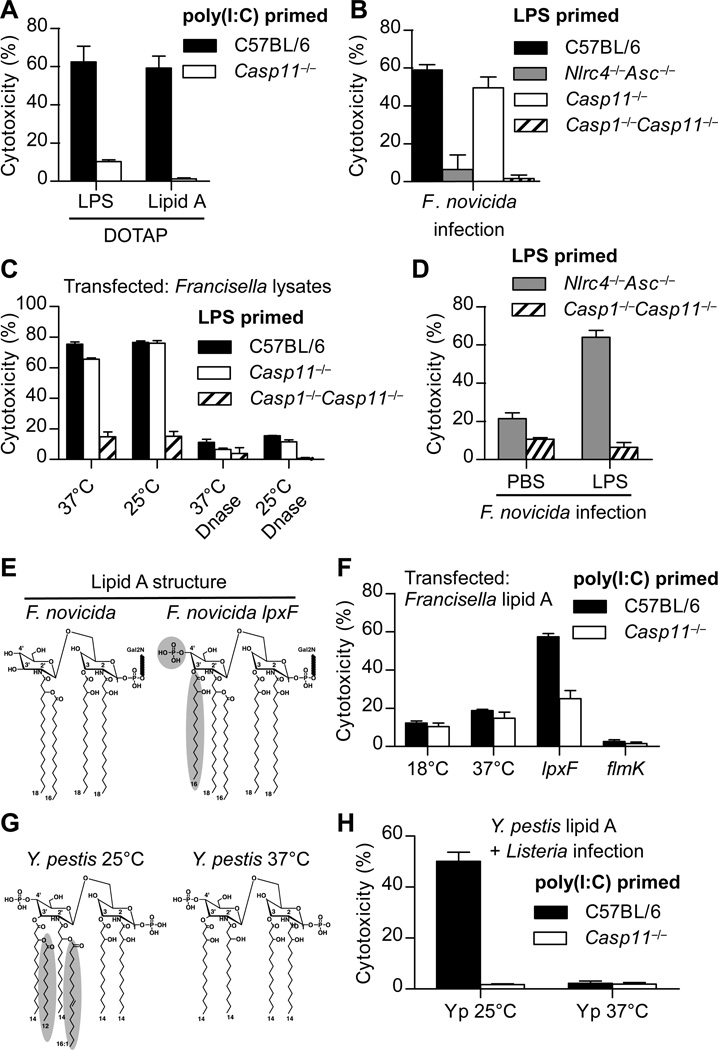
We next examined the LPS structural determinants required for detection through caspase-11, and found that the lipid A moiety alone was sufficient for activation (Fig. 3A). It is well established that lipid A modifications enable TLR4 evasion, and we therefore hypothesized that cytosolic pathogens could evade caspase-11 by a similar strategy. Indeed, Francisella novicidaa Gram-negative cytosolic bacteria, was not detected by caspase-11 (no signal in Nlrc4−/−Asc−/− BMMs; Fig. 3B). F. novicida lysates containing DNA activated caspase-1; however, after DNase digestion the remaining LPS failed to activate caspase-11, which was not restored by temperature-dependent alterations in acyl chain length (12) (Fig. 3C). As with L. monocytogenesco-phagocytosis of F. novicida with exogenous S. minnesota LPS resulted in caspase-11 activation (Fig. 3D). Together, these results suggest that Francisella species evade caspase-11 by modifying their lipid A. Francisella species have peculiar tetra-acylated lipid A unlike the hexa-acylated species of enteric bacteria (13). F. novicida initially synthesizes a penta-acylated lipid A structure with two phosphates and then removes the 4’ phosphate and 3’ acyl chain in reactions that do not occur in lpxF mutants (14, 15) (Fig. 3E). Conversion to the penta-acylated structure restored caspase-11 activation, whereas other modifications that maintained the tetra-acylated structures (flmK mutant or 18°C growth (12, 16)) did not (Fig. 3F). lpxF mutant lipid A is not detected by TLR4 (14), suggesting that the TLR4 and caspase-11 pathways have different structural requirements.
Fig. 3. Caspase-11 responds to distinct lipid A structures.
(A) Poly(I:C) primed BMMs were transfected with LPS from S. minnesota RE595 or S. typhimurium lipid A. Cytotoxicity was determined after 2 h. (B) Cytotoxicity in LPS primed BMMs was determined 4 hours after infection with F. novicida (MOI 200). (C) LPS primed BMMs were transfected with mock or DNase treated F. novicida lysates. Cytotoxicity was determined 4 hours later. (D) Macrophages were infected as in (B) in the presence or absence of LPS from S. minnesota RE595. (E) Structural comparison of lipid A from wild type F. novicida or the lpxF mutant. Structural changes are indicated. (F) Poly(I:C) primed macrophages were transfected with lipid A from F. novicida grown at 18°C or 37°C, or the indicated F. novicida mutants grown at 37°C. Cytotoxicity was determined after 2 h. (G) Structural comparison of lipid A from Y. pestis grown at 25°C or 37°C. (H) Poly(I:C) primed BMMs were infected with L. monocytogenes in the presence of lipid A from Y. pestis grown at 25°C or 37°C. Cytotoxicity was determined after 4 h. Data are representative of at least 3 (A, F, G) or 2 (B, C, D) experiments. Error bars indicate standard deviation of technical replicates.
Deacylation of lipid A is a common strategy employed by pathogenic bacteria. For example, Yersinia pestis removes two acyl chains from its lipid A upon transition from growth at 25°C to 37°C (17) (Fig. 3G). Consistent with our structural studies of F. novicida lipid A, caspase-11 detected hexa-acylated lipid A from Y. pestis grown at 25°C, but not tetra-acylated lipid A from bacteria grown at 37°C (Fig. 3H). Together, these data indicate that caspase-11 responds to distinct lipid A structures, and pathogens appear to exploit these structural requirements in order to evade caspase-11.
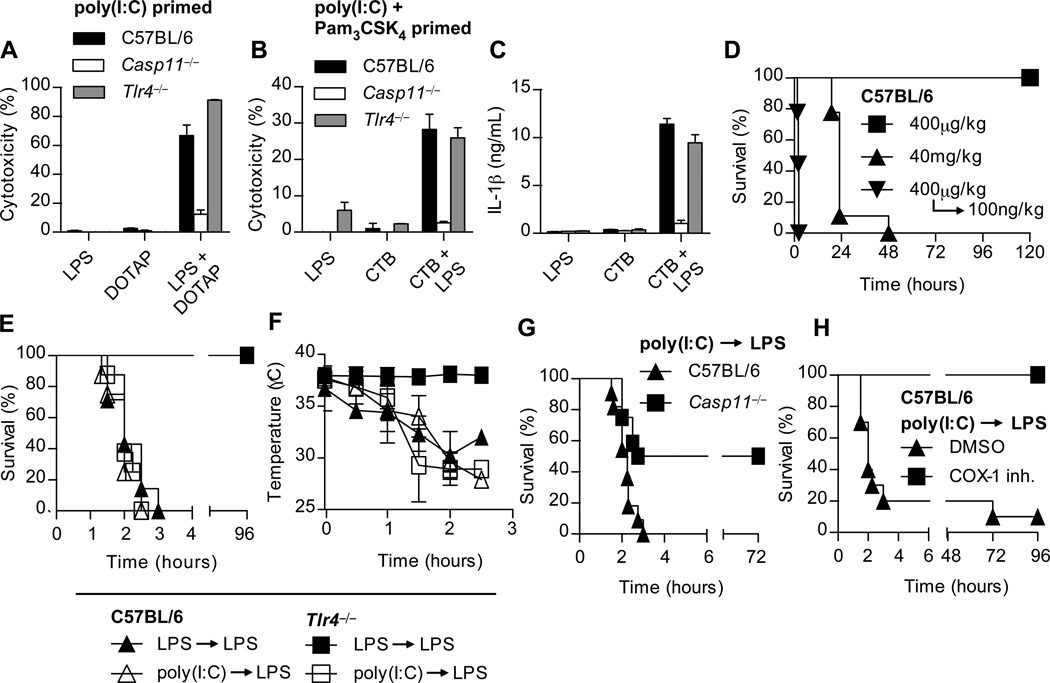
In addition to detection of extracellular/vacuolar LPS by TLR4, our data indicate that an additional sensor of cytoplasmic LPS activates caspase-11. These two pathways intersect, however, because TLR4 primes the caspase-11 pathway. However, Tlr4−/− BMMs responded to transfected or CTB-delivered LPS after poly(I:C) priming (Fig. 4A–C). Therefore, caspase-11 can respond to cytoplasmic LPS independently of TLR4.
Fig. 4. LPS detection and rapid induction of shock in primed mice occur independently of TLR4.
(A) BMMs were primed overnight with poly(I:C) and then transfected with S. minnesota RE595 LPS. Cytotoxicity was determined 2 hours later. (B–C) Poly(I:C) and Pam3CSK4 primed macrophages were incubated with the indicated combinations of CTB (20µg/mL) and LPS from E. coli O111:B4 (1µg/mL). Cytotoxicity (B) and IL-1β secretion (C) were determined 16 hours later. Data are representative of at least 3 experiments; error bars indicate standard deviation of technical replicates (A–C). (D) Survival of mice challenged with the indicated doses of Escherichia coli LPS, or primed with LPS and then re-challenged 7 hours later. Data are pooled from three experiments; n = 9 per condition. (E) Survival of mice primed with LPS (400μg/kg) or poly(I:C) (10µg/kg) and then challenged 7 hours later with LPS (100ng/kg). Data are pooled from three experiments; n = 7 per LPS prime group and n = 8 for poly(I:C) prime group. (F) Rectal temperatures of mice in panel (E) after LPS challenge. Data are representative of 3 experiments; n = 4 per condition. (G) Survival of poly(I:C) primed mice challenged 6 hours later with LPS (10ng/kg). Data are pooled from 3 experiments, n = 11 (C57BL/6) or 12 (Casp11−/−). (H) Mice were primed with poly(I:C) and then challenged 6 hours later with LPS (100ng/kg) and monitored for survival. 1 h before LPS challenge, mice were given 5mg/kg of COX-1 inhibitor or DMSO control. Data are pooled from 2 experiments; n = 11 per condition.
In established models of endotoxic shock, both Tlr4−/− and Casp11−/− mice are resistant to lethal challenge with 40–54 mg/kg LPS (3, 18, 19), whereas WT mice succumb in 18 to 48 hours (Fig. 4D). We hypothesized that TLR4 detects extracellular LPS and primes the caspase-11 pathway in vivo. Then, if high concentrations of LPS persist, aberrant localization of LPS within the cytoplasm could trigger caspase-11, resulting in the generation of shock mediators. We sought to separate these two events by priming and then challenging with otherwise sublethal doses of LPS. C57BL/6 mice primed with LPS rapidly succumbed to secondary LPS challenge in 2 hours (Fig. 4D). TLR4 was required for LPS priming, as LPS primed Tlr4−/− mice survived secondary LPS challenge (Fig. 4E). To determine whether alternate priming pathways could substitute for TLR4 in vivowe primed mice with poly(I:C), and observed that both C57BL/6 and Tlr4−/− mice succumbed to secondary LPS challenge (Fig. 4E). This was concomitant with hypothermia (Fig. 4F), seizures, peritoneal fluid accumulation, and occasionally intestinal hemorrhage. In contrast, poly(I:C) primed Casp11−/− mice were more resistant to secondary LPS challenge (Fig. 4G), demonstrating the consequences of aberrant caspase-11 activation. Collectively, our data indicate that activation of caspase-11 by LPS in vivo can result in rapid onset of endotoxic shock independent of TLR4.
Mice challenged with the canonical NLRC4 agonist flagellin coupled to the cytosolic translocation domain of anthrax lethal toxin also experience a rapid onset of shock (20). In this model, NLRC4-dependent caspase-1 activation triggers lethal eicosanoid production via COX-1 with similar kinetics to our prime-challenge model, suggesting convergent lethal pathways downstream of caspase-1 and caspase-11. Indeed, the COX-1 inhibitor SC-560 rescued poly(I:C) primed mice from LPS lethality (Fig. 4H).
Although physiological activation of caspase-11 is beneficial in defense against cytosolic bacterial pathogens (4), its aberrant hyperactivation becomes detrimental during endotoxic shock. Our data suggest that when LPS reaches critical concentrations during sepsis, aberrant LPS localization occurs, activating cytosolic surveillance pathways. Clinical sepsis is a more complex pathophysiologic state, where multiple cytokines, eicosanoids, and other inflammatory mediators are likely to be hyperactivated. Eicosanoid mediators and other consequences of pyroptotic cellular lysis (21) should be considered in future therapeutic options designed to treat Gram-negative septic shock. This underscores the concept that Gram-negative and Gram-positive sepsis may cause shock via divergent signaling pathways (22), and that treatment options should consider these as discreet clinical entities.
Supplementary Material
Acknowledgments
The authors thank V. Dixit for sharing key mouse strains (Casp11−/− and Nlrc4−/− Asc−/− mice were provided under an MTA agreement with Genentech). We also thank R. Flavell, M. Heise, and J. Brickey for sharing mice. We thank D. Mao, L. Zhou, and D. Trinh for managing mouse colonies. The data presented in this manuscript are tabulated in the main paper and in the supplementary materials. This work was supported by NIH grants AI007273 (JAH), AI097518 (EAM), AI057141 (EAM), and AI101685 (RKE).
References and Notes
- 1.Von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 2013;31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 2.Masters SL, et al. NLRP1 Inflammasome Activation Induces Pyroptosis of Hematopoietic Progenitor Cells. Immunity. 2012;37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 4.Aachoui Y, et al. Caspase-11 Protects Against Bacteria That Escape the Vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broz P, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurung P, et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. Journal of Biological Chemistry. 2012;287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathinam VAK, et al. TRIF Licenses Caspase-11-Dependent NLRP3 Inflammasome Activation by Gram-Negative Bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silipo A, Lanzetta R, Amoresano A, Parrilli M, Molinaro A. Ammonium hydroxide hydrolysis: a valuable support in the MALDI-TOF mass spectrometry analysis of Lipid A fatty acid distribution. J. Lipid Res. 2002;43:2188–2195. doi: 10.1194/jlr.d200021-jlr200. [DOI] [PubMed] [Google Scholar]
- 9.Broz P, Von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Case CL, et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proceedings of the National Academy of Sciences. 2013;110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao EA, et al. From the Cover: Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proceedings of the National Academy of Sciences. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, et al. LPS remodeling is an evolved survival strategy for bacteria. Proceedings of the National Academy of Sciences. 2012;109:8716–8721. doi: 10.1073/pnas.1202908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunn JS, Ernst RK. The structure and function of Francisella lipopolysaccharide. Annals of the New York Academy of Sciences. 2007;1105:202–218. doi: 10.1196/annals.1409.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Ribeiro AA, Guan Z, Abraham SN, Raetz CRH. Attenuated virulence of a Francisella mutant lacking the lipid A 4'-phosphatase. Proc Natl Acad Sci USA. 2007;104:4136–4141. doi: 10.1073/pnas.0611606104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanistanon D, et al. Role of Francisella lipid A phosphate modification in virulence and long-term protective immune responses. Infect Immun. 2012;80:943–951. doi: 10.1128/IAI.06109-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanistanon D, et al. A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLoS Pathog. 2008;4:e24. doi: 10.1371/journal.ppat.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebeil R, Ernst RK, Gowen BB, Miller SI, Hinnebusch BJ. Variation in lipid A structure in the pathogenic yersiniae. Mol Microbiol. 2004;52:1363–1373. doi: 10.1111/j.1365-2958.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi O, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 20.Von Moltke J, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamkanfi M, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. The Journal of Immunology. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao H, Evans TW, Finney SJ. Bench-to-bedside review: Sepsis, severe sepsis and septic shock – does the nature of the infecting organism matter? Crit Care. 2008;12:212. doi: 10.1186/cc6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shornick LP, et al. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J Exp Med. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda K, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 25.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 26.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nature Immunology. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 27.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 28.Warren SE, et al. Cutting Edge: Cytosolic Bacterial DNA Activates the Inflammasome via Aim2. The Journal of Immunology. 2010;185:818–821. doi: 10.4049/jimmunol.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura T, et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.