Abstract
Aims: The immune system is critical for protection against infections and cancer, but requires scrupulous regulation to limit self-reactivity and autoimmunity. Our group has utilized a manganese porphyrin catalytic antioxidant (MnTE-2-PyP5+, MnP) as a potential immunoregulatory therapy for type 1 diabetes. MnP has previously been shown to modulate diabetogenic immune responses through decreases in proinflammatory cytokine production from antigen-presenting cells and T cells and to reduce diabetes onset in nonobese diabetic mice. However, it is unclear whether or not MnP treatment can act beyond the reported inflammatory mediators. Therefore, the hypothesis that MnP may be affecting the redox-dependent bioenergetics of diabetogenic splenocytes was investigated. Results: MnP treatment enhanced glucose oxidation, reduced fatty acid oxidation, but only slightly decreased overall oxidative phosphorylation. These alterations occurred because of increased tricarboxylic acid cycle aconitase enzyme efficiency and were not due to changes in mitochondrial abundance. MnP treatment also displayed decreased aerobic glycolysis, which promotes activated immune cell proliferation, as demonstrated by reduced lactate production and glucose transporter 1 (Glut1) levels and inactivation of key signaling molecules, such as mammalian target of rapamycin, c-myc, and glucose-6-phosphate dehydrogenase. Innovation: This work highlights the importance of redox signaling by demonstrating that modulation of reactive oxygen species can supplant complex downstream regulation, thus affecting metabolic programming toward aerobic glycolysis. Conclusion: MnP treatment promotes metabolic quiescence, impeding diabetogenic autoimmune responses by restricting the metabolic pathways for energy production and affecting anabolic processes necessary for cell proliferation. Antioxid. Redox Signal. 19, 1902–1915.
Introduction
The control of self-reactivity is critical for prevention and potential reversal of autoimmunity. In particular, regulating immune cells in type 1 diabetes can lead to reduced pancreatic beta cell damage. Beta cells are highly susceptible to reactive oxygen species (ROS) and damage, as they contain very low antioxidant levels (46). In the face of autoimmunity, vulnerable beta cells and redox-activated immune cells create the perfect storm for mediating destruction. As a strategy to control immune and autoreactive responses, pentacationic, Mn(42) N-ethyllpyridylporphyrin, MnTE-2-PyP5+ (MnP), a manganese porphyrin with catalytic antioxidant and ROS-scavenging (73, 74) capabilities, has been extensively explored (2, 3, 23, 24a). Many major metabolic pathways that are involved in immune responses are redox-based such as hypoxia-inducible factor (HIF)-1α, nuclear factor kappa B (NF-κB), AP-1, and SP-1. MnP reportedly inactivates all of these transcription factors (54, 73, 80) and other signaling proteins, likely via oxidation and glutathionylation of exposed redox-active cysteines (41). In the context of immunity, MnP-mediated inhibition of NF-κB in innate immune cells decreases proinflammatory cytokine production (73). Moreover, MnP treatment also controls diabetogenic TH1 cell activation through inhibition of metalloprotease-mediated lymphocyte activation gene 3 (LAG-3) cleavage, a negative regulator of T cell function (23, 24), reduces CD8 T cell effector function (72), and enhances long-term allograft survival through cytoprotection of transplanted islets (71). All of these effects contribute to protection against type 1 diabetes development, which has been demonstrated upon MnP treatment of an adoptive transfer model of diabetes and in nonobese diabetic (NOD) mice, which spontaneously develop disease (24, 62).
Innovation.
The metalloporphyrin antioxidant used in this study is catalytic with high bioavailability (3) and displays oxidoreductase properties, oxidizing and inhibiting NF-κB binding in the nucleus (73) yet reducing thiols in the cytoplasm (24). This is the first study characterizing the effects of MnP treatment on immune cell metabolism. Further, these results are novel for diabetogenic splenocytes and help deduce the reduced effector function and diabetogenic potential seen previously (24, 62). Overall, redox modulation provides immunoregulation of bioenergetics in the absence of cytotoxicity (7, 8, 71, 72) and has implications in other pathologies, including cancer, as MnP treatment also displays anti-Warburg effect characteristics.
Metalloporphyrins were originally produced to be very electron-deficient to possess nearly identical potency as the superoxide dismutase (SOD) enzyme. MnP has been optimized with respect to its thermodynamics and kinetics, so that it is involved in cellular redox-based pathways and readily interacts with reactive species and/or cellular reductants. The metal-centered reduction potential of MnP for the MnIII/Mn2+ redox couple is +228 mV versus NHE (3). In different oxidation states of Mn+3 and +4, MnTE-2-PyP5+ reacts with cellular reductants and couples this reactivity to the scavenging of superoxide (O2−) and peroxynitrite (ONOO−) (1, 20, 73). Alternatively, if the cell is under excessive oxidative stress, such as a cancer cell, and levels of hydrogen peroxide are already high or are further increased due to radiation or chemotherapy, MnP would act as glutathione peroxidase or thiol oxidase, glutathionylating protein thiols (40). The prevailing action of MnP would critically depend upon the cellular redox status, that is, levels of reactive species/reductants and the co-localization with reactive species/proteins. At the present state of knowledge and due to extreme complexity of the cell and MnP reactivity, it is close to impossible to single out the predominant reaction in vivo. It is very likely that multiple reactions, beyond those discussed above, are involved in the redox-based activity of MnP.
Therefore, the possibility of MnP also affecting the metabolic response of diabetogenic immune cells was tested. Although frequently associated with inhibiting cancer progression (60, 65), metabolic regulation is relatively unexploited but promising in the context of autoreactivity. Both innate and adaptive immune cells depend on specific metabolic pathways for survival. Aerobic glycolysis is the primary metabolic pathway utilized during both antigen-presenting cell (APC) and T cell activation (44, 59). On the other hand, survival of naive and central memory T cells is highly dependent on mitochondrial respiration via oxidative phosphorylation (29, 66). Resting T cells undergo oxidative phosphorylation as they traverse the body, building up reserves of adenosine triphosphate (ATP) in preparation for an upcoming response (29). However, instead of relying solely on the slow process of oxidative phosphorylation, activated immune cells predominantly use the much faster (100×) glycolytic pathway to meet their energy and macromolecule synthesis demands (17, 59). Despite lower ATP generation from glycolysis than mitochondrial respiration (2 vs. 38 ATP molecules, respectively), immune cells upregulate the glucose transporter Glut1 to compensate and maximize glucose uptake, offsetting the difference in energy currency (29). In NOD mice, which spontaneously develop type 1 diabetes, self-reactive T cells are not efficiently deleted in the thymus, allowing for the escape of autoreactive cells into the periphery (27). These T cells then require aerobic glycolysis, which allows rapid macromolecule synthesis, cell growth, and TH1 effector function (58), to effectively target and destroy the pancreatic beta cells.
The alteration of undesired immune responses can be beneficial in the context of a plethora of diseases, including autoimmunity. Unlike cancer and infections, controlling self-reactivity requires a reduction in immune cell activities. A number of endogenous pathways exist to directly or indirectly adjust immune responses, such as cytokine skewing, regulatory T (Treg) cells, and indoleamine 2,3-dioxygenase induction (4, 48, 57). However, when these mechanisms fail, treatment options are warranted. Anti-glycolytics and agents to inhibit oxidative phosphorylation have been utilized in cancer, transplantation, and neurodegenerative diseases (14, 26, 31). Metabolic control can theoretically affect both innate and adaptive immune cells, making it a viable option for autoimmune therapy. In autoimmune models, HIF-1 blockade leads to decreased glycolysis, resulting in diminished TH17-mediated murine experimental autoimmune encephalomyelitis (EAE) (19). Moreover, blocking glycolysis via rapamycin treatment can augment Treg cell development in type 1 diabetes patients and in humanized mouse allograft recipients (4). The bioenergetic demands of diabetogenic immune cells during survival and activation highlight the potential for immunometabolic control.
Other groups have demonstrated the ability of various antioxidants to suppress TH17-mediated arthritis (78), enhance Treg cells for the resolution of EAE (77), and reduce cancer cell glycolysis and growth (69). However, it is unknown whether MnP can act as an immunometabolic treatment for the bioenergetic regulation of autoreactive immune cell responses. In this study, oxidative phosphorylation and aerobic glycolysis are measured upon redox-active MnP treatment of diabetogenic splenocytes.
Results
Redox modulation of immune cells is reversible
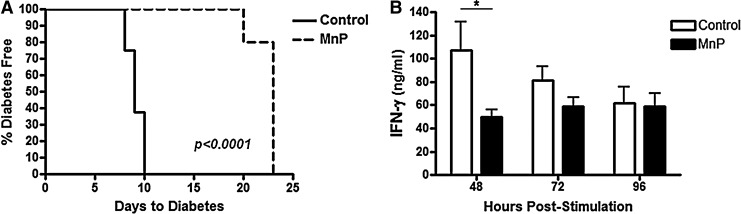
Our lab previously demonstrated the ability of chronic MnP administration to prevent type 1 diabetes onset in NOD mice; however, removing systemic MnP treatment allowed for spontaneous diabetes to progress (24). Based on these studies, the reversibility of MnP treatment in a rapid adoptive transfer model of type 1 diabetes was tested. BDC-2.5.TCR.Tg mice do not get spontaneous diabetes, like the NOD mice, but instead, splenocytes from these animals are highly diabetogenic when adoptively transferred into NOD.scid recipients (24, 25). MnP or Hank's balanced salt solution (HBSS) (control) was administered to BDC-2.5.TCR.Tg mice for 7 days and on day 8, splenocytes were transferred into NOD.scid recipients. Control recipients succumbed to diabetes by day 10, consistent with historical data (24, 62). Compared to control, recipients of splenocytes from MnP-treated donors exhibited significantly delayed diabetogenic potential (p<0.0005) (Fig. 1A). However, treatment of the donor BDC-2.5.TCR.Tg mouse did not afford durable protection against disease onset past 20 days post-transfer. This is in stark contrast to previously reported results in which chronic treatment of the NOD.scid recipient allowed for stable prevention of diabetes (24). The current data suggest a clearance of MnP following the final donor injection and a return of diabetogenic potential, indicating that the treatment is effective, while not permanent once therapeutic levels of MnP trough. Additionally, in vitro stimulation of BDC-2.5.TCR.Tg splenocytes after 7 days of in vivo MnP administration also depicted restoration of the TH1 effector cytokine, interferon (IFN)-γ (Fig. 1B). Stopping MnP treatment thus retains cell viability and permits reversibility of immunomodulatory effects.
FIG. 1.
Stopping redox modulation leads to finite autoreactive immune cell control. BDC-2.5.TCR.Tg mice were treated for 7 days i.p. with MnP or HBSS at 10 mg/kg. (A) On day 8, splenocytes were harvested and adoptively transferred intravenously into NOD.scid recipients. Recipients were monitored by glucosuria and blood glucose and considered diabetic after two consecutive blood glucose readings of >300 mg/dL. n=3 transfers/group, p<0.0001. (B) On day 8, splenocytes were harvested for in vitro stimulation with 2.5 mimotope±MnP. At 48–96 h, supernatants were collected and used in an IFN-γ ELISA. Data show the average of independent experiments performed in triplicate from n=5 mice/group, *p<0.05. BDC, Barbara Davis Center; ELISA, enzyme-linked immunosorbent assay; HBSS, Hank's balanced salt solution; i.p., intraperitoneal; NOD, nonobese diabetic; Tg, transgenic; IFN, interferon.
Redox modulation minimally decreases overall oxygen consumption of diabetogenic splenocytes
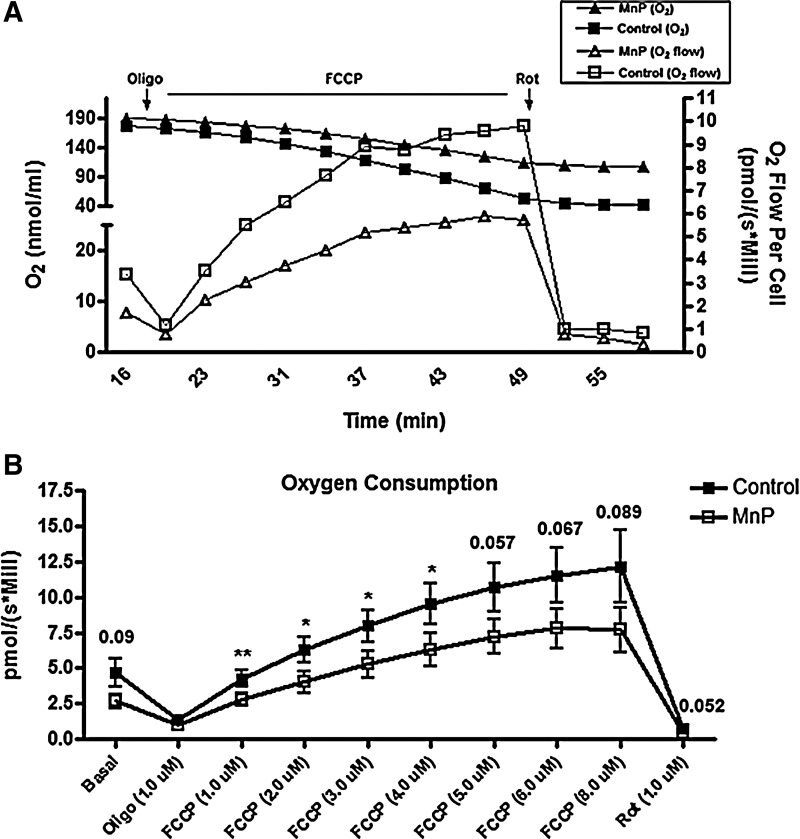
Redox modulation can inhibit TH1 effector function and block immune cell NF-κB activation (24, 73, 74). Once MnP treatment ceases, there is a cumulative regeneration of diabetogenic potential (Fig. 1), which raised the question of how redox modulation may be affecting the progression to effector function in immune cells. Because MnP can scavenge ROS, which are a constant byproduct of cellular respiration, metabolic regulation may be contributing to the delayed adoptive transfer of diabetes (Fig. 1A) and the previously reported reductions in diabetogenic immune cell effector function (24, 74). Accordingly, the bioenergetics of diabetogenic splenocytes as a source of immune cells were interrogated. The majority of spleen cells are lymphocytes, with monocytes making up less than 7% of the total cells; therefore, splenocytes in the BDC-2.5.TCR.Tg mouse reflect an abundant population of self-reactive CD4+ T cells. First, oxidative phosphorylation, important for producing abundant ATP reserves (29), was measured. To compare respiratory capacity, overall oxygen concentration and the rate of its consumption in control versus MnP-treated BDC-2.5.TCR.Tg splenocytes using an Oroboros Respirometer were detected, as represented in Figure 2A. Oligomycin, an ATPase inhibitor, causes a drop in oxygen consumption that reflects the respiration needed to sustain ATP production in the splenocytes. Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), on the other hand, uncouples ATP synthesis from oxygen consumption, allowing for maximal respiration to occur. To stop all mitochondrial respiration at the end of the experiment, rotenone, an inhibitor of complex I, was added. Upon first glance, MnP treatment seemed to affect oxygen consumption, as shown in the representative Oroboros respirometry graph (Fig. 2A); however, the average of multiple experiments (Fig. 2B) refuted this interpretation. Although uncoupling respiration from ATP synthesis with additive FCCP, which was utilized to reach a maximum respiratory response without exhibiting inhibitory action, elicited significant decreases in MnP-treated splenocytes at specific doses, redox modulation did not significantly reduce respiration at baseline (Basal) or upon maximal uncoupling (FCCP 8.0 μM), the major indicators of experimental oxygen consumption rates. Therefore, overall oxidative phosphorylation was only minimally affected by MnP treatment.
FIG. 2.
Respiration is not significantly reduced following in vivo redox modulation. BDC-2.5.TCR.Tg mice were treated for 7 days i.p. with MnP or HBSS at 10 mg/kg. On day 8, splenocytes were harvested and analyzed in an Oroboros Respirometer. Dulbecco's minimal essential medium was used as an incubation media with 20 mM glucose as a substrate for oxidation. Oxygen consumption was measured as pmol/(s*Mill). (A) Respiration (O2 flow per cell; open squares=control, open triangles=MnP) was determined at basal conditions and after the addition of mitochondrial inhibitors, oligomycin (Oligo) and rotenone (Rot), and additive amounts of the mitochondrial uncoupler FCCP. Total oxygen concentration also displayed (nmol/ml O2; closed squares=control, closed triangles=MnP). Graph representative of typical Oroboros respirometry measurements. (B) Data show the average of independent experiments performed in triplicate from n=5 mice/group, *p<0.05, **p<0.005. FCCP, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone; pmol/(s*Mill), pmol/second/million cells.
MnP treatment induces more efficient glucose oxidation while reducing fatty acid oxidation
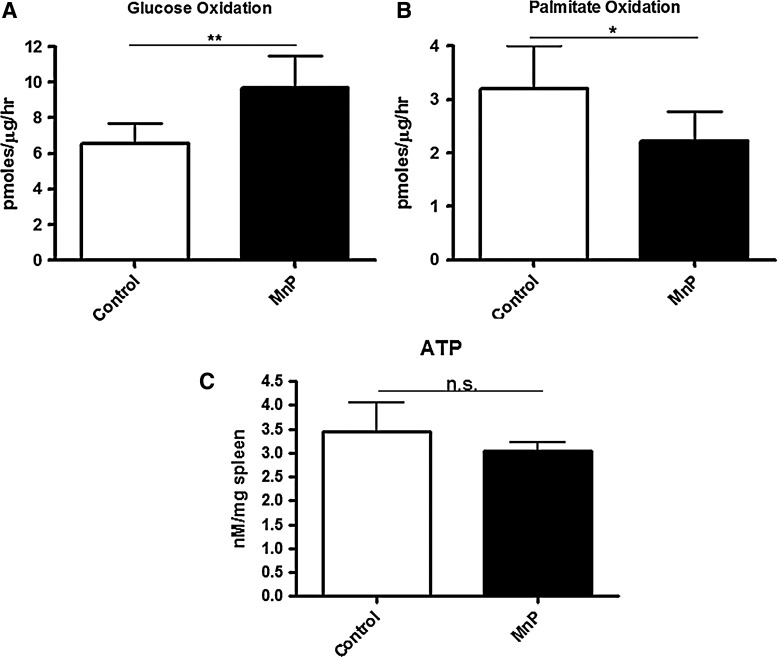
Glucose and fatty acids can be used by immune cells in the tricarboxylic acid (TCA) cycle for oxidative phosphorylation and ATP production. To parse out differences in substrate oxidation, in contrast to overall oxygen consumption (Fig. 2), glucose and the fatty acid palmitate were used in splenocyte uptake assays. In MnP-treated splenocytes, glucose oxidation, as measured by radiolabeled CO2 production, was significantly enhanced (p<0.005) in comparison to control cells (Fig. 3A). Conversely, fatty acid oxidation, which would increase in the absence of sufficient glucose oxidation (9), was decreased following redox modulation (Fig. 3B). However, spleen ATP levels remain unchanged between the groups (Fig. 3C), suggesting an augmentation of glucose-driven TCA cycle efficiency after MnP administration.
FIG. 3.
Glucose oxidation efficiency is enhanced after redox modulation. BDC-2.5.TCR.Tg mice were treated for 7 days i.p. with MnP or HBSS at 10 mg/kg. On day 8, spleens were harvested. (A) Isolated splenocytes were given D-[6-14C] glucose and 2.5 μM cold glucose, incubated for 1 h, and 14CO2 was measured. (B) Isolated splenocytes were given [1-14C] palmitic acid, incubated for 1 h, and 14CO2 was measured. (C) Spleens were weighed, homogenized, boiled, and used in an ATP determination assay. ATP (nM) was quantified per mg of spleen. Data show the average of independent experiments performed in triplicate from n=3 mice/group, *p<0.05, **p<0.005. ATP, adenosine triphosphate.
MnP treatment does not significantly alter mitochondrial complex or biogenesis molecules
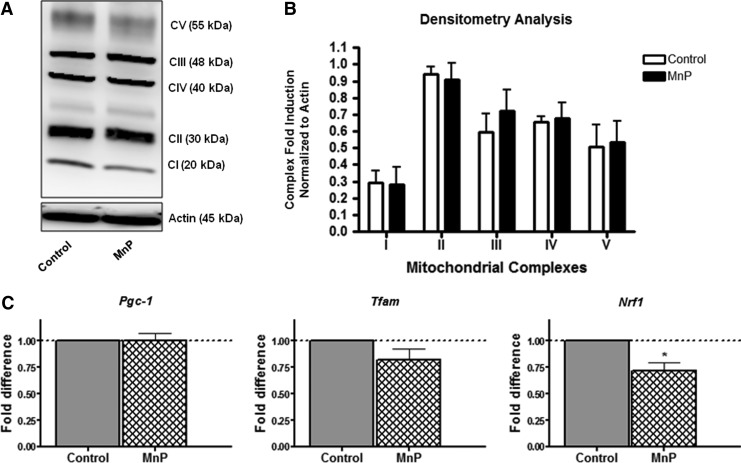
Based on the increase in glucose oxidation, differences in mitochondrial abundance, which may allot for this enhancement, were detected following MnP treatment. To first assess this possibility, an antibody cocktail was used to measure mitochondrial complex proteins (Complexes I–V) from whole cell splenocyte lysates after 7 days of in vivo treatment. The cocktail contains antibodies to complex subunits that are labile if not assembled properly; therefore, an accurate depiction of the mitochondrial complexes should be feasible with this probe. In general, mitochondrial complex proteins were not significantly decreased after MnP treatment (Fig. 4A, B). Further, mitochondrial biogenesis transcription factors, which regulate the production of the mitochondrial complexes, were also measured by quantitative real-time–polymerase chain reaction (qRT-PCR). Pgc-1 and Tfam were not statistically different, whereas nuclear respiratory factor 1 (Nrf1) was significantly reduced following MnP treatment (Fig. 4C). Nrf1, however, not only contributes to mitochondrial biogenesis, but also helps to control phase II antioxidant enzyme production (5) and cellular growth (11). Since the expression of other biogenesis molecules is unchanged, reduced Nrf1 after redox modulation may therefore correlate with a decreased need for antioxidant transcription, as MnP acts as an effective scavenger of ROS. However, splenocyte transcript levels of thioredoxin 1 and 2, glutathione synthase, and glutathione peroxidase 1 did not change following MnP treatment (data not shown), suggesting that Nrf1 regulation of the cell cycle is more likely targeted in correlation to the effects on aerobic glycolysis (discussed below). Overall, these results indicate that differences in glucose oxidation are not due to variations in mitochondrial abundance between control and MnP-treated splenocytes.
FIG. 4.
MnP treatment does not significantly alter the amount of mitochondria. BDC-2.5.TCR.Tg mice were treated for 7 days i.p. with MnP or HBSS at 10 mg/kg. On day 8, splenocytes were harvested for protein lysates. (A) Whole cell lysates were probed for MitoOXPHOS antibody cocktail by western blot. Actin was probed as a loading control. Each complex is indicated based on its molecular weight. Data are representative of 3 independent experiments. (B) Densitometry was quantified for each complex by normalizing control and MnP-treated cells to actin. Expression of complex proteins quantified from 3 independent experiments. (C) Mitochondrial biogenesis mRNA levels were measured by qRT-PCR. The fold change of control samples were set arbitrarily to 1 and compared to MnP treatment. All samples were normalized to the endogenous GAPDH control. Data show the average of independent experiments performed in triplicate from n=4–5 mice/group, *p<0.05. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
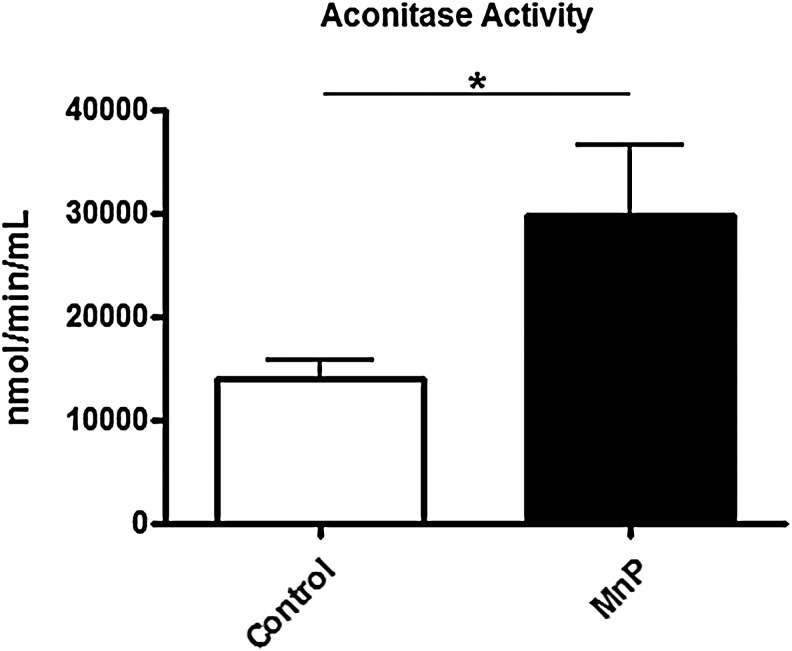
MnP treatment stabilizes aconitase activity in diabetogenic splenocytes
To determine TCA cycle efficiency, aconitase activity was measured in splenocytes from MnP-treated and control BDC-2.5.TCR.Tg mice. Over time, superoxide produced from the mitochondrial electron transport chain is able to damage TCA enzymes, such as aconitase (79). Aconitase catalyzes the conversion of citrate to isocitrate in the TCA cycle, making it necessary for normal metabolic function. However, the iron-sulfur cluster within its active site renders aconitase highly susceptible to superoxide-mediated damage (32), causing a subsequent decrease in enzymatic activity and less efficient glucose oxidation. An accumulation of aconitase inactivation has been correlated with oxidative stress present in diabetes (64) and aging (79). Upon MnP treatment, diabetogenic splenocytes have increased aconitase activity compared with controls (Fig. 5). In correlation with the enhanced glucose oxidation, these results indicate more efficient TCA cycle function and possibly better coupling in the electron transport chain. As a scavenger of superoxide, MnP treatment likely reduces the level of free radicals leaked from the respiratory chain, leading to better glucose utilization despite similar ATP production (Fig. 3C).
FIG. 5.
Redox modulation sustains aconitase activity. BDC-2.5.TCR.Tg mice were treated for 7 days i.p. with MnP or HBSS at 10 mg/kg. On day 8, spleens were harvested. Spleens were homogenized and diluted to a concentration of 1000 μg/ml based on BCA assay. Aconitase activity was measured kinetically over 15 min. Median activity of independent experiments performed in triplicate from n=3 mice/group,*p<0.05.
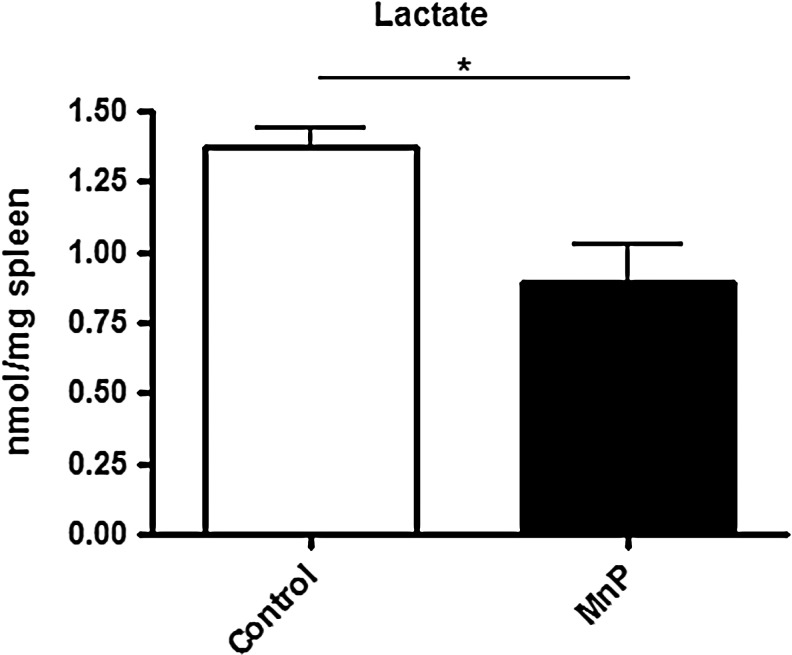
Redox modulation decreases aerobic glycolysis of diabetogenic splenocytes
Activated immune cells undergo aerobic glycolysis as their main form of metabolism (6, 59). Based on the results indicating a significant increase in glucose oxidation and aconitase activity (Figs. 3 and 5), the level of aerobic glycolysis following MnP treatment was next ascertained. To determine glycolytic activity, lactate production was measured in mouse spleens after 7 days of in vivo treatment. Lactate production is indicative of immune cell activation (17). Specifically, when a proliferating cell's glycolytic rate exceeds its energy needs, pyruvate and nicotinamide adenine dinucleotide (NADH) convert into lactate, regenerating the NAD+ pool (30). Lactate levels were significantly decreased in spleens from MnP-treated mice (p<0.05) (Fig. 6), suggesting that reduced aerobic glycolysis may contribute to immune cell quiescence and stunted diabetogenic potential.
FIG. 6.
MnP treatment decreases lactate production. BDC-2.5.TCR.Tg mice were treated for 7 days i.p. with MnP or HBSS at 10 mg/kg. On day 8, spleens were harvested. Spleens were weighed, homogenized, and used in a lactate assay. Lactate (nmol) was quantified per mg of spleen. Data show the average of independent experiments performed in triplicate from n=3 mice/group,*p<0.05.
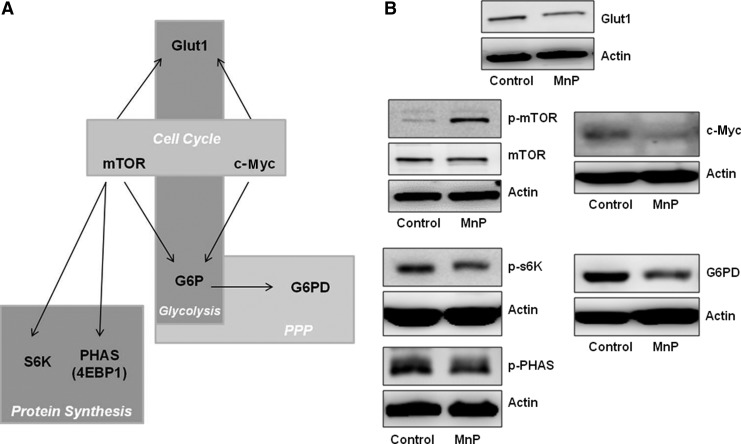
Redox modulation decreases signaling important for aerobic glycolysis and the pentose phosphate pathway
In accordance with the decrease in lactate production, MnP-mediated signaling effects were determined. Although redox reactions are known to regulate kinases and phosphatases that are crucial for immune signaling (15), those mediators that are important for driving aerobic glycolysis and the pentose phosphate pathway (PPP) were specifically measured. To initiate growth and proliferation, immune cells must switch their metabolic programming to the glycolytic pathway and PPP (45). In response to a mitogen, for example, aerobic glycolysis allows for rapid generation of ATP, facilitating immediate cell activation and immune responses (58, 66, 75). Moreover, aerobic glycolysis provides the necessary cues for protein synthesis, and PPP is responsible for producing macromolecule building blocks, which are also necessary for rapid cell division (21, 45). Certain signaling mediators are critical for both of these programs, as displayed in Figure 7A. Therefore, the levels of each of these signaling molecules were measured. Mammalian target of rapamycin (mTOR) and c-Myc activation can initiate cell cycle. Decreased activation of mTOR blocks expansion of cells, especially in times of starvation or when glycolysis is low (38). c-Myc regulates cyclin-dependent kinases, distinguishing it as an inducer of cell cycle and an amplifier of oncogenic gene expression in cancer biology (39, 47). After MnP treatment, mTOR phosphorylation at Ser2448 is enhanced (Fig. 7B), which is considered an indicator of mTOR repression (67). Additionally, c-Myc expression is decreased, suggesting a potential reduction in glycolysis and a blockade in splenocyte expansion. Both c-Myc and mTOR regulate Glut1 expression, a glucose transporter protein, for glucose uptake to further aerobic glycolysis during times of cellular proliferation (37, 56). Glut1 expression is reduced following MnP administration, suggesting diminished glycolysis in response to lowered c-Myc and mTOR activation.
FIG. 7.
Redox modulation leads to decreased aerobic glycolysis and PPP signaling. (A) Signaling important for the collaboration of glycolysis, cell cycle, protein synthesis, and PPP. (B) BDC-2.5.TCR.Tg mice were treated for 7 days i.p. with MnP or HBSS at 10 mg/kg. On day 8, splenocytes were harvested for protein lysates. Whole cell lysates were probed for Glut-1; c-Myc; phospho-mTOR and mTOR; phospho-p70s6K and phospho-PHAS; and G6PD by western blot. Actin was probed as a loading control for all panels. Data show representative blots of independent experiments from n=3–4 mice/group. G6PD, glucose-6-phosphate dehydrogenase; mTOR, mammalian target of rapamycin; PPP, pentose phosphate pathway; PHAS-1 (4E-BP1), eukaryotic initiation factor 4E-binding protein.
To further investigate mTOR activity, downstream signaling targets p70S6K (S6 kinase) and eukaryotic initiation factor 4E-binding protein (PHAS-I) (also known as 4E-BP1) were measured, which are important for triggering protein synthesis (36, 67). Upon MnP treatment, phosphorylation of both S6 kinase and PHAS is decreased, suggesting reduced mTOR activity, diminished cell cycle, and confirming previously reported data, where CD4+ T cell proliferation was also blunted by redox modulation (24). Lastly, expression of glucose-6-phosphate dehydrogenase (G6PD), the rate limiting step in the PPP and a regulator of ribose 5-phosphate and nicotinamide adenine dinucleotide phosphate (NADPH) generation for nucleotide biosynthesis (45) was assessed. Redox modulation also reduced G6PD levels, correlating with the overall decrease in signaling necessary for driving immune cell activation. These data in conjunction with reduced lactate production (Fig. 6) together highlight the potential of MnP treatment to lower immune cell bioenergetics, which most likely contributes to decreased activation and delayed diabetes onset upon transfer.
Discussion
Treatment with MnP is known to reduce BDC-2.5.TCR.Tg diabetogenicity and TH1 IFN-γ responses (24, 72, 74). As shown previously (24), MnP treatment causes a significant delay in diabetes onset compared to control upon adoptive transfer of BDC-2.5.TCR.Tg splenocytes into NOD.scid mice. In the present study, however, recipients of MnP-treated cell transfers ultimately succumbed to disease at day 23, whereas previous data demonstrated long-lasting tolerance against diabetes only when the recipients were treated (24). The eventual disease manifestation after MnP-treated cell transfer prompted investigation of what was changing immunologically after treatment ceases, which would enable better determination of necessary adjunct therapies to compliment MnP treatment in the future. In vitro stimulated IFN-γ production from splenocytes treated for 7 days in vivo demonstrated a quick restoration of TH1 cytokines to control levels, characteristic of unrestricted diabetogenic responses. These data highlighted not only sustained cell viability but also rapid changes in diabetogenic potential when treatment is stopped, providing rationale for elucidating whether redox modulation regulates the bioenergetics of diabetogenic immune cells.
Adequate energy is crucial for directing activation and expansion of immune cells. Resting T cells rely primarily on oxidative phosphorylation, whereas activated T cells depend mainly on glycolysis. Innate cells, on the other hand, predominately utilize glycolysis during all activation states (6). Although some increases in mitochondrial respiration are detected upon activation (52), oxidative phosphorylation is chiefly utilized for ATP storage in immune cells, preparing them for efficient function upon activation (29). Conversely, the macromolecule synthesis necessary for immune cell expansion is a result of the quicker metabolic route of glycolysis, in conjunction with the PPP (59). Redox modulation demonstrated only a minimal reduction in overall oxygen consumption of diabetogenic splenocytes, yet, upon parsing out different oxidation substrate pathways (glucose and palmitate), glucose oxidation was significantly enhanced while fatty acid oxidation was decreased compared with controls. While glucose-driven oxidative phosphorylation is necessary for preparing the cell for activation (29), fatty acid oxidation is critical for driving chronic inflammation, as in the case of EAE (70). Moreover, when glucose oxidation is utilized, fatty acid oxidation is historically downregulated, as the two pathways are reciprocally controlled (9). Regardless of these differences in oxidation, overall ATP production remains unchanged between spleens from control and MnP-treated mice. These results do not necessarily mean that more glucose is oxidized than in control cells, but may instead indicate more mitochondria and/or better TCA cycle function and coupling in the electron transport chain.
Upon further investigation, no differences were detected in mitochondrial complex proteins of the electron transport chain or biogenesis transcripts, indicating equal amounts of mitochondria in each sample. However, Nrf1, a transcription factor important for biogenesis and for activating the antioxidant response element, was significantly decreased after MnP treatment. Because of the ROS scavenging properties of MnP, the need for increased endogenous antioxidants is likely reduced. Moreover, alleviating ROS burden leads to reduced oxidative stress, which is known to upregulate Nrf1 gene transcription (35), and the significant reduction in Nrf1 may be related to the stress level of the cell, not differences in mitochondrial biogenesis. Further, Nrf1 plays a role in regulating cell cycle (15), and the aerobic glycolysis signaling results suggest that cell proliferation is reduced. Lowered Nrf1 upon MnP treatment may thus contribute to decreased cell growth.
TCA cycle function was next evaluated, and aconitase activity was enhanced in MnP-treated diabetogenic splenocytes. ROS produced during the electron transport chain are able to damage iron-sulfur-containing enzymes, such as aconitase (79). As aconitase activity is lost, less efficient glucose oxidation results (32). Further, aconitase dysfunction has been linked to diabetes (64) and aging (79), in connection with the high oxidative stress and accumulating ROS found in both conditions. In type 1 diabetes, a combination of hyperglycemia and high ROS causes beta cell and kidney mitochondrial oxidative damage, which reduces aconitase activity (64) and ultimately disturbs bioenergetics and triggers long-term complications. The effects of aconitase dysfunction in immune cells are less understood. In an SOD2−/− mouse, T cells have elevated superoxide production, impaired aconitase activity, and do not efficiently protect against influenza virus (12). Our results in autoimmune cells are counterintuitive to studies in infection. Protection of aconitase activity via redox modulation in a diabetogenic model may indeed maintain immune cell viability, as it does in pancreatic beta cells (63); however, in conjunction with scavenging of ROS and previously reported reductions in cytokine production (i.e., interleukin-1β) (8, 73), MnP treatment is not promoting increased responsiveness and effector function through aconitase augmentation, but instead it is keeping the cells alive yet quiescent. Enhanced efficiency of glucose oxidation via protected aconitase activity is likely mediated by MnP administration.
Contrary to mitochondrial respiration, aerobic glycolysis in combination with the PPP is necessary for providing metabolic intermediates to propel immune cell activation and proliferation. Although mitochondrial respiration provides greater amounts of ATP, the quicker production of energy and biosynthetic precursors skews immune cells toward glycolysis for reaching their energy and macromolecule demands, especially during infection (51). The same holds true for diabetogenic T cells, which rapidly destroy pancreatic beta cells upon adoptive transfer (24, 62). In aerobic glycolysis, the metabolic intermediate pyruvate is fermented to lactate, subsequently replenishing NAD+ for macromolecule synthesis and allowing the continuation of glycolysis (30). Lactate levels increase during T cell activation (17, 30), illustrating how immune cells heavily rely on aerobic glycolysis for transition from a resting state. Moreover, diabetic immune cells are known to produce greater amounts of lactate than their non-autoimmune counterparts (28). Upon MnP treatment, lactate levels in the spleen were significantly lower than control mice, suggesting that aerobic glycolysis is decreased upon redox modulation of diabetogenic splenocytes, potentially resetting their metabolic program to that of non-autoimmune cells. Additionally, lactate is known to induce ROS-responsive genes, such as Nrf1 (35), providing another potential reason for its downregulation upon MnP treatment.
In addition to lactate levels, glycolytic and PPP signaling molecules were also measured. mTOR and c-Myc were first investigated, as they are both important for cell cycle and glycolysis (36, 39). mTOR is activated downstream of the phosphatidylinositide 3-kinase (PI3K)-Akt pathway, which is stimulated during T, B, and APC activation, glycolysis, and oxidative stress (30). During times of starvation or low glycolysis, activated mTOR is decreased, repressing its own catalytic activity, augmenting cyclin-dependent kinase inhibitors and promoting hyporesponsive T cells (67, 81). MnP treatment boosted the amount of phosphorylated mTOR compared to control splenocytes. The specific serine probed, Ser2448, may contribute to a mTOR repressor domain (67); however, controversial literature exists characterizing Ser2448 as an activator of mTOR (55). Based on this discrepancy, downstream targets were investigated to better understand the activation status of mTOR after MnP treatment. For efficient cell cycle progression and proliferation, activated mTOR regulates p70S6K and PHAS-I (36), which both require phosphorylation to control ribosome biogenesis and translation initiation, respectively. S6 kinase is especially important for entry of T cells into S-phase, and intriguingly, blockade of S6 kinase increases lifespan, highlighting its role in aerobic glycolysis, ROS production, and aging (68). PHAS-I, on the other hand, is a repressor of translation and upon phosphorylation, releases the translational initiator eukaryotic initiation factor-4E (eIF-4E) (49). MnP treatment reduced both S6 kinase and PHAS-I phosphorylation, indicating that the phosphorylation of Ser2448 inhibits mTOR activation in our experimental model of diabetogenic splenocytes and results in reduced aerobic glycolysis and immune cell expansion. This result also positively correlates with previous studies displaying decreased CD4+ T cell proliferation and TH1 effector function following redox modulation (24), as mTOR activation is crucial for both (22). Further studies are currently being done to characterize several phosphorylation sites within mTOR.
c-Myc is a direct regulator of cell cycle machinery, making it a critical contributor to cellular growth. In T cells, c-Myc expression is greatly increased upon activation to induce genes involved in aerobic glycolysis, such as lactate dehydrogenase (18). Similar to the effects seen with the antioxidant α-tocopherol (69), MnP treatment of mice reduced c-Myc expression in splenocytes, again correlating with reduced aerobic glycolysis and autoreactive immune cell proliferation. Further, c-Myc is downregulated by transcription factors for programming quiescence in lymphocytes (10), suggesting that redox modulation may similarly be triggering this phenomenon.
Although c-Myc can provide the necessary cues for effective T cell activation (18), it is widely described as an oncogene (76). Similar to immune cell proliferation, cancer cells rely heavily on aerobic glycolysis, a phenomenon called the Warburg effect. c-Myc, mTOR, Glut1, and G6PD are all involved in signaling pathways that contribute to the Warburg effect (18, 36, 76). Both c-Myc and mTOR regulate glucose uptake through Glut1 expression (37, 56). Glut1, the only glucose transporter expressed on immune cells (30), was lower on MnP-treated splenocytes than control cells. Glucose uptake is essential for initiating T cell activation and proliferation, and Glut1 expression is highly increased upon T cell stimulation (52). Although counterintuitive, when glucose is in excess, glycolysis potentiates ATP production in greater quantities and at a faster rate compared with oxidative phosphorylation (33). However, lower Glut1 levels upon redox modulation does not cause cell death (72), suggesting that the nutrient supply and more efficient glucose oxidation allows for MnP-treated diabetogenic splenocytes to remain viable yet quiescent. Regulation of Glut1 expression is indeed essential for controlling hyperresponsive lymphocytes so as not to cause pathology, and in our model, may contribute to reduced diabetogenic potential and decreased IFN-γ secretion (current study) (13, 24). Lastly, MnP treatment also reduced G6PD expression, the rate-limiting enzyme in the PPP and, like c-Myc and mTOR, is important for cell growth (45). Although PPP and G6PD expression have been linked to oxidative stress control, as they provide NADPH reducing equivalents (53), their overexpression has been targeted for cancer therapies (34), suggesting a necessary balance to avoid pathologies.
Overall, MnP treatment can control autoimmunity through both immunological (24, 72–74) and metabolic mechanisms. Redox modulation maintained efficient glucose oxidative phosphorylation, yet reduced aerobic glycolysis, leading to decreased autoreactive immune cell activation and diabetogenic potential. ROS have been well established as critical components for controlling cell cycle in health and disease (47, 64). Controlling aerobic glycolysis via redox modulation may be a viable option for regulating immune responses, and MnP treatment, which is non-toxic (3, 7, 72), islet cytoprotective (7, 8), and reversible, displays anti-Warburg effect characteristics, largely promoting immune cell quiescence. Bioenergetic regulation is thus a possible therapeutic option for controlling self-reactivity and may hold promise for prevention or reversal of T cell-mediated autoimmune diseases, such as type 1 diabetes.
Materials and Methods
Materials
NOD.BDC-2.5.TCR.Tg and NOD.scid mice were bred and housed under specific pathogen-free conditions in the Animal Facility of Rangos Research Center at Children's Hospital of Pittsburgh of UPMC (Pittsburgh, PA). Standard chow-fed female mice were used at 4–10 weeks of age in all experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Pittsburgh and were in compliance with the laws of the United States of America. All animals were sacrificed by CO2 asphyxiation per AALC requirements. MnP was a generous gift from James Crapo, MD at National Jewish Health. MnP was prepared as previously described (74) and used at 10 mg/kg in all in vivo experiments.
In vivo 7 day treatment
BDC-2.5.TCR.Tg mice were treated with MnP (10 mg/kg) or HBSS intraperitoneally for 7 days. On day 8, spleens were collected for further analysis, described below. Equal amounts of cells and mgs of protein were used in each experiment. Splenocytes were immediately used for experimentation following isolation or, in the case of aconitase/ATP/lactate assays, spleens were snap frozen until use.
Adoptive transfer of diabetogenic splenocytes
Following 7 day in vivo treatment, splenocytes were isolated from control or MnP-treated mice. A total of 1×107 splenocytes was then adoptively transferred intravenously into NOD.scid mice. Diabetes was monitored starting at 10 days post-transfer. Overt diabetes was measured by a positive glucosuria test followed by two consecutive blood glucose readings of ≥300 mg/dL.
In vitro T cell assay and enzyme-linked immunosorbent assay
After 7 days of in vivo treatment, 5×105 BDC-2.5.TCR.Tg splenocytes were seeded in 96-well round-bottom plates with 0.5 μM of BDC-2.5 mimotope (M) (EKAHRPIWARMDAKK) in supplemented Dulbecco's minimal essential medium (DMEM) (74) (Invitrogen Life Technologies, Grand Island, NY). At 48–96 h post-stimulation, supernatants were harvested for IFN-γ enzyme-linked immunosorbent assay (ELISA). ELISAs were performed according to the manufacturer's instructions (BD Biosciences, San Jose, CA). All ELISAs were read on a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA), and data were analyzed using SoftMax Pro v5.4.2 (Molecular Devices).
Splenocyte respiration rates
On day 8 post-MnP or HBSS treatment, splenocytes were harvested, and 2×107 cells were measured by an Oroboros High Resolution Respirometer (Innsbruck, Austria) in a stirred 2 ml chamber in supplemented DMEM with 20 mM glucose as a substrate for oxidation. Oxygen sensor was calibrated at each experiment according to the manufacturer's instructions. Calculations of respiratory rates were performed by software built into the instrument. Basal oxygen consumption was measured followed by (i) oligomycin (1 μM), to inhibit ATP production by blocking complex V (ATPase) of the electron transport chain; (ii) at least 8 additions of 1 μM FCCP, to uncouple oxidative phosphorylation; and (iii) rotenone (1 μM), to measure non-mitochondrial oxygen consumption via inhibition of complex I in the electron transport chain.
Preparation of cell lysates and western blotting
BDC-2.5.TCR.Tg splenocytes were harvested after 7 days of in vivo treatment. Whole cell lysates were prepared as described (16). Protein concentration of all lysates was determined by bicinchoninic protein assay (BCA) protein assay (Thermo Fisher, Rockford, IL). Protein lysates were separated on 4%–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels. Western blots were performed as described (73) (with the exception of no boiling, 150 mAmp transfer for 1.5 h, and blocking O/N in 5% non-fat dry milk in PBS for those samples probed with MitoOXPHOS antibody) with antibodies MitoOXPHOS (1:250) (Santa Cruz Biotechnology, Santa Cruz, CA), Glut1 (1 μg/ml) (Abcam, Cambridge, MA), mTOR (1:1000), phospho-mTOR (1:1000), phospho-S6K (1:1000), phospho-PHAS (1:1000), G6PD (1:1000), c-Myc (1:1000) (Cell Signaling, Danvers, MA), and β-actin (1:10,000) (Sigma-Aldrich, St. Louis, MO). All primary antibodies were diluted in 5% bovine serum albumin (BSA) in TBST, except for MitoOXPHOS in 1% non-fat dry milk in PBS. Secondary antibodies were from Jackson ImmunoResearch, West Grove, PA. Chemiluminescence was detected using ECL Plus reagent (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Blots were analyzed using Fujifilm LAS-4000 imager and Multi Gauge software (Fujifilm Life Science, Tokyo, Japan).
mRNA quantification
BDC-2.5.TCR.Tg splenocytes were isolated after 7 days of in vivo treatment. Cells were collected, pelleted, and used for RNA isolation. RNA was isolated via the RNAeasy Kit (Quiagen, Valencia, CA), followed by complementary deoxyribonucleic acid (cDNA) synthesis using the SuperScript III First-Strand Synthesis System (Invitrogen Life Technologies). The qRT-PCR was performed using the following primer pairs: PCG-1 (forward) 5′-ACCCACAGGATCAGAACAAACCCT-3′, (reverse) 5′-TGGTGTGAGGAGGGTCATCGTTT-3′; TFAM (forward) 5′-AGTCTTGGGAAGAGCAGATGGCT-3′, (reverse) 5′-AGACCTAACTGGTTTCTTGGGCCT-3′; Nrf1 (forward) 5′-AACGGAAACGGCCTCATGTGTTTG-3′, (reverse) 5′-GAGTACAATCGCTTGCTGTCCCA-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward) 5′-GCATCCTGCACCACCAACT-3′, (reverse) 5′-CTGGCATGGCCTTCCGTGTT-3′. Quantitative RT-PCR was performed using a Light Cycler 2.0 (Roche, Indianapolis, IN). The reaction mixtures containing SYBR Green were generated following the manufacturer's protocol. The cycling program was as follows: initial denaturation at 95°C for 10 min, 40 cycles of amplification with a denaturation step at 95°C for 5 s, an annealing temperature of 60°C for 15 s, and an extension step at 72°C for 20 s. All samples were normalized to GAPDH, and MnP-treated samples were compared to control samples arbitrarily set to 1.
Glucose and palmitate uptake assay
BDC-2.5.TCR.Tg splenocytes were isolated after 7 days of in vivo MnP treatment. Glucose oxidation was determined as described previously (50). Briefly, 1 μCi of D-[6-14C] glucose and 2.5 μM cold glucose in 0.2% BSA-HBSS were added to splenocytes. Tubes were incubated at 37°C in water bath with rotation for 1 h. To terminate metabolic reactions, 200 μl 2 N HCl was added and 500 μl Hyamine (PerkinElmer Life Sciences, Waltham, MA) was added. 14CO2 generated was then detected using a β counter. Palmitate oxidation was measured as described previously (43). Briefly, splenocytes were resuspended in sucrose/Tris/EDTA buffer and incubated for 1 h in reaction mixture (pH 8.0) containing [1-14C] palmitic acid. Measurements of acid-soluble metabolites and trapped CO2 were then detected.
ATP determination
ATP determination kit was used following the manufacturer's instructions (Invitrogen Life Technologies). Spleens were collected on day 8 following 7 days of in vivo treatment, weighed, homogenized in 1×reaction buffer, and centrifuged. 1 ml boiling water was added to each cell pellet, vortexed, centrifuged at 12,000 rpm for 5 min at 4°C, and supernatants were utilized in the assay. Background was measured for each standard and sample and subtracted accordingly. Luciferase was measured on a Victor3 Multilabel Counter 1420 (PerkinElmer) and ATP values were normalized to spleen weight.
Aconitase activity assay
Aconitase Assay Kit was used following the manufacturer's instructions (Cayman Chemical Co, Ann Arbor, MI). Spleens were collected on day 8 following 7 days of in vivo treatment, homogenized, and adjusted to 1000 μg/ml total protein. Aconitase activity was measured kinetically at an optical density 340 nm for 15 min at 37°C. All samples were normalized to a blank. Samples±aconitase inhibitor were measured and used to calculate final aconitase activity (nmol/min/ml).
Lactate assay
L-Lactate Assay Kit was used following the manufacturer's instructions (Abcam). Spleens were collected on day 8 following 7 days of in vivo treatment, weighed, sonicated in lactate assay buffer, and diluted 1:5 in buffer prior to assay.
Statistical analysis
The difference between mean values was assessed by Student's t-test, with p<0.05 considered significant. All experiments were performed at least three times. Data are mean±standard error of the mean. Survival analysis was done using the product-limit (Kaplan–Meier) method with the endpoint defined as disease. Data on animals that did not develop type 1 diabetes were censored. The p-values were determined by Log-Rank test.
Abbreviations Used
- μCi
micro Curie
- Akt
protein kinase B
- APCs
antigen-presenting cells
- ATP
adenosine triphosphate
- BCA
bicinchoninic protein assay
- BDC
Barbara Davis Center
- BSA
bovine serum albumin
- C
carbon
- CD
cluster of differentiation
- cDNA
complementary deoxyribonucleic acid
- CO2
carbon dioxide
- DMEM
Dulbecco's minimal essential medium
- EAE
experimental autoimmune encephalomyelitis
- EDTA
ethylenediaminetetraacetic acid
- eIF-4E
eukaryotic initiation factor-4E
- ELISA
enzyme-linked immunosorbent assay
- FCCP
carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- G6PD
glucose-6-phosphate dehydrogenase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Glut1
glucose transporter 1
- HBSS
Hank's balanced salt solution
- HCl
hydrochloric acid
- HIF
hypoxia-inducible factor
- i.p.
intraperitoneal
- IFN
interferon
- LAG-3
lymphocyte activation gene 3
- M
BDC-2.5 mimotope
- MnTE-2-PyP5+, MnP
Mn(42) meso-tetrakis(N-alkylpyridinium-2-yl)porphyrin
- mTOR
mammalian target of rapamycin
- NAD+/NADH
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa B
- NOD
nonobese diabetic
- Nrf1
nuclear respiratory factor 1
- Oligo
oligomycin
- p70S6K
S6 kinase
- PGC-1
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PHAS-1 (4E-BP1)
eukaryotic initiation factor 4E-binding protein
- PI3K
phosphatidylinositide 3-kinases
- pmol/(s*Mill)
picomoles/second/million cells
- PPP
pentose phosphate pathway
- qRT-PCR
quantitative real-time–polymerase chain reaction
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- Rot
rotenone
- Ser
serine
- SOD
superoxide dismutase
- TCA
tricarboxylic acid
- TFAM
transcription factor A, mitochondrial
- Tg
transgenic
- TH
T helper
- Treg
regulatory T
Acknowledgment
The authors thank Ivan Spasojevic for his insightful discussion.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Batinic-Haberle I. Benov L. Spasojevic I. Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 2.Batinic-Haberle I. Reboucas JS. Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batinic-Haberle I. Spasojevic I. Tse HM. Tovmasyan A. Rajic Z. St Clair DK. Vujaskovic Z. Dewhirst MW. Piganelli JD. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids. 2012;42:95–113. doi: 10.1007/s00726-010-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battaglia M. Stabilini A. Migliavacca B. Horejs-Hoeck J. Kaupper T. Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 5.Biswas M. Chan JY. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol Appl Pharmacol. 2010;244:16–20. doi: 10.1016/j.taap.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borregaard N. Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottino R. Balamurugan AN. Bertera S. Pietropaolo M. Trucco M. Piganelli JD. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes. 2002;51:2561–2567. doi: 10.2337/diabetes.51.8.2561. [DOI] [PubMed] [Google Scholar]
- 8.Bottino R. Balamurugan AN. Tse H. Thirunavukkarasu C. Ge X. Profozich J. Milton M. Ziegenfuss A. Trucco M. Piganelli JD. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 9.Bouzakri K. Austin R. Rune A. Lassman ME. Garcia-Roves PM. Berger JP. Krook A. Chibalin AV. Zhang BB. Zierath JR. Malonyl CoenzymeA decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes. 2008;57:1508–1516. doi: 10.2337/db07-0583. [DOI] [PubMed] [Google Scholar]
- 10.Buckley AF. Kuo CT. Leiden JM. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc-dependent pathway. Nat Immunol. 2001;2:698–704. doi: 10.1038/90633. [DOI] [PubMed] [Google Scholar]
- 11.Cam H. Balciunaite E. Blais A. Spektor A. Scarpulla RC. Young R. Kluger Y. Dynlacht BD. A common set of gene regulatory networks links metabolism and growth inhibition. Mol Cell. 2004;16:399–411. doi: 10.1016/j.molcel.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 12.Case AJ. McGill JL. Tygrett LT. Shirasawa T. Spitz DR. Waldschmidt TJ. Legge KL. Domann FE. Elevated mitochondrial superoxide disrupts normal T cell development, impairing adaptive immune responses to an influenza challenge. Free Radic Biol Med. 2011;50:448–458. doi: 10.1016/j.freeradbiomed.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cham CM. Driessens G. O'Keefe JP. Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charbonnier LM. Le Moine A. Rapamycin as immunosuppressant in murine transplantation model. Methods Mol Biol. 2012;821:435–445. doi: 10.1007/978-1-61779-430-8_28. [DOI] [PubMed] [Google Scholar]
- 15.Corcoran A. Cotter TG. Redox regulation of protein kinases. FEBS J. 2013;280:1944–1965. doi: 10.1111/febs.12224. [DOI] [PubMed] [Google Scholar]
- 16.Coudriet GM. He J. Trucco M. Mars WM. Piganelli JD. Hepatocyte growth factor modulates interleukin-6 production in bone marrow derived macrophages: implications for inflammatory mediated diseases. PLoS One. 2010;5:e15384. doi: 10.1371/journal.pone.0015384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Culvenor JG. Weidemann MJ. Phytohaemagglutinin stimulation of rat thymus lymphocytes glycolysis. Biochim Biophys Acta. 1976;437:354–363. doi: 10.1016/0304-4165(76)90005-2. [DOI] [PubMed] [Google Scholar]
- 18.Dang CV. Le A. Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang EV. Barbi J. Yang HY. Jinasena D. Yu H. Zheng Y. Bordman Z. Fu J. Kim Y. Yen HR. Luo W. Zeller K. Shimoda L. Topalian SL. Semenza GL. Dang CV. Pardoll DM. Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day BJ. Batinic-Haberle I. Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic Biol Med. 1999;26:730–736. doi: 10.1016/s0891-5849(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 21.DeBerardinis RJ. Lum JJ. Hatzivassiliou G. Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Delgoffe GM. Pollizzi KN. Waickman AT. Heikamp E. Meyers DJ. Horton MR. Xiao B. Worley PF. Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delmastro MM. Piganelli JD. Oxidative stress and redox modulation potential in type 1 diabetes. Clin Dev Immunol. 2011;2011:593863. doi: 10.1155/2011/593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delmastro MM. Styche AJ. Trucco MM. Workman CJ. Vignali DA. Piganelli JD. Modulation of redox balance leaves murine diabetogenic TH1 T cells “LAG-3-ing” behind. Diabetes. 2012;61:1760–1768. doi: 10.2337/db11-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Delmastro-Greenwood MM. Tse HM. Piganelli JD. Effects of metalloporphyrins on reducing inflammation and autoimmunity. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5257. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Dobbs C. Haskins K. Comparison of a T cell clone and of T cells from a TCR transgenic mouse: TCR transgenic T cells specific for self-antigen are atypical. J Immunol. 2001;166:2495–2504. doi: 10.4049/jimmunol.166.4.2495. [DOI] [PubMed] [Google Scholar]
- 26.Essa MM. Vijayan RK. Castellano-Gonzalez G. Memon MA. Braidy N. Guillemin GJ. Neuroprotective effect of natural products against Alzheimer's disease. Neurochem Res. 2012;37:1829–1842. doi: 10.1007/s11064-012-0799-9. [DOI] [PubMed] [Google Scholar]
- 27.Fan Y. Rudert WA. Grupillo M. He J. Sisino G. Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;28:2812–2824. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Field CJ. Wu G. Metroz-Dayer MD. Montambault M. Marliss EB. Lactate production is the major metabolic fate of glucose in splenocytes and is altered in spontaneously diabetic BB rats. Biochem J. 1990;272:445–452. doi: 10.1042/bj2720445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox CJ. Hammerman PS. Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 30.Frauwirth KA. Thompson CB. Regulation of T lymphocyte metabolism. J Immunol. 2004;172:4661–4665. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- 31.Ganapathy-Kanniappan S. Vali M. Kunjithapatham R. Buijs M. Syed LH. Rao PP. Ota S. Kwak BK. Loffroy R. Geschwind JF. 3-bromopyruvate: a new targeted antiglycolytic agent and a promise for cancer therapy. Curr Pharm Biotechnol. 2010;11:510–517. doi: 10.2174/138920110791591427. [DOI] [PubMed] [Google Scholar]
- 32.Gardner PR. Nguyen DD. White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci U S A. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guppy M. Greiner E. Brand K. The role of the Crabtree effect and an endogenous fuel in the energy metabolism of resting and proliferating thymocytes. Eur J Biochem. 1993;212:95–99. doi: 10.1111/j.1432-1033.1993.tb17637.x. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton NM. Dawson M. Fairweather EE. Hamilton NS. Hitchin JR. James DI. Jones SD. Jordan AM. Lyons AJ. Small HF. Thomson GJ. Waddell ID. Ogilvie DJ. Novel steroid inhibitors of glucose 6-phosphate dehydrogenase. J Med Chem. 2012;55:4431–4445. doi: 10.1021/jm300317k. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto T. Hussien R. Oommen S. Gohil K. Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 36.Hay N. Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 37.Hennessy BT. Smith DL. Ram PT. Lu Y. Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 38.Howell JJ. Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iritani BM. Delrow J. Grandori C. Gomez I. Klacking M. Carlos LS. Eisenman RN. Modulation of T-lymphocyte development, growth and cell size by the Myc antagonist and transcriptional repressor Mad1. EMBO J. 2002;21:4820–4830. doi: 10.1093/emboj/cdf492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaramillo MC. Briehl MM. Crapo JD. Batinic-Haberle I. Tome ME. Manganese porphyrin, MnTE-2-PyP5+, Acts as a pro-oxidant to potentiate glucocorticoid-induced apoptosis in lymphoma cells. Free Radic Biol Med. 2012;52:1272–1284. doi: 10.1016/j.freeradbiomed.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaramillo MC. Frye JB. Crapo JD. Briehl MM. Tome ME. Increased manganese superoxide dismutase expression or treatment with manganese porphyrin potentiates dexamethasone-induced apoptosis in lymphoma cells. Cancer Res. 2009;69:5450–5457. doi: 10.1158/0008-5472.CAN-08-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnsen Lind A. Helge Johnsen B. Hill LK. Sollers Iii JJ. Thayer JF. A user-friendly application for the extraction of kubios hrv output to an optimal format for statistical analysis - biomed 2011. Biomed Sci Instrum. 2011;47:35–40. [PubMed] [Google Scholar]
- 43.Kim JY. Hickner RC. Cortright RL. Dohm GL. Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 44.Krawczyk CM. Holowka T. Sun J. Blagih J. Amiel E. DeBerardinis RJ. Cross JR. Jung E. Thompson CB. Jones RG. Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruger NJ. von Schaewen A. The oxidative pentose phosphate pathway: structure and organisation. Curr Opin Plant Biol. 2003;6:236–246. doi: 10.1016/s1369-5266(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 46.Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008;36:343–347. doi: 10.1042/BST0360343. [DOI] [PubMed] [Google Scholar]
- 47.Lin CY. Loven J. Rahl PB. Paranal RM. Burge CB. Bradner JE. Lee TI. Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin MS. Tse HM. Delmastro MM. Bertera S. Wong CT. Lakomy R. He J. Sklavos MM. Coudriet GM. Pietropaolo M. Trucco MM. Piganelli JD. A multivalent vaccine for type 1 diabetes skews T cell subsets to Th2 phenotype in NOD mice. Immunol Res. 2011;50:213–220. doi: 10.1007/s12026-011-8215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin TA. Kong X. Haystead TA. Pause A. Belsham G. Sonenberg N. Lawrence JC., Jr. PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science. 1994;266:653–656. doi: 10.1126/science.7939721. [DOI] [PubMed] [Google Scholar]
- 50.Liu TF. Vachharajani VT. Yoza BK. McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem. 2012;287:25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lunt SY. Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 52.Maciver NJ. Jacobs SR. Wieman HL. Wofford JA. Coloff JL. Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84:949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manganelli G. Fico A. Masullo U. Pizzolongo F. Cimmino A. Filosa S. Modulation of the pentose phosphate pathway induces endodermal differentiation in embryonic stem cells. PLoS One. 2012;7:e29321. doi: 10.1371/journal.pone.0029321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Moeller BJ. Batinic-Haberle I. Spasojevic I. Rabbani ZN. Anscher MS. Vujaskovic Z. Dewhirst MW. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Radiat Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 55.Nave BT. Ouwens M. Withers DJ. Alessi DR. Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427–431. [PMC free article] [PubMed] [Google Scholar]
- 56.Osthus RC. Shim H. Kim S. Li Q. Reddy R. Mukherjee M. Xu Y. Wonsey D. Lee LA. Dang CV. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 57.Palm NW. Medzhitov R. Not so fast: adaptive suppression of innate immunity. Nat Med. 2007;13:1142–1144. doi: 10.1038/nm1007-1142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfeiffer T. Schuster S. Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 60.Pierotti MA. Berrino F. Gariboldi M. Melani C. Mogavero A. Negri T. Pasanisi P. Pilotti S. Targeting metabolism for cancer treatment and prevention: metformin, an old drug with multi-faceted effects. Oncogene. 2013;32:1475–1487. doi: 10.1038/onc.2012.181. [DOI] [PubMed] [Google Scholar]
- 61. This reference has been deleted.
- 62.Piganelli JD. Flores SC. Cruz C. Koepp J. Batinic-Haberle I. Crapo J. Day B. Kachadourian R. Young R. Bradley B. Haskins K. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 63.Rabinovitch A. Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 64.Raza H. Prabu SK. John A. Avadhani NG. Impaired mitochondrial respiratory functions and oxidative stress in streptozotocin-induced diabetic rats. Int J Mol Sci. 2011;12:3133–3147. doi: 10.3390/ijms12053133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scagliotti GV. Selvaggi G. Antimetabolites and cancer: emerging data with a focus on antifolates. Expert Opin Ther Pat. 2006;16:189–200. doi: 10.1517/13543776.16.2.189. [DOI] [PubMed] [Google Scholar]
- 66.Schmid D. Burmester GR. Tripmacher R. Kuhnke A. Buttgereit F. Bioenergetics of human peripheral blood mononuclear cell metabolism in quiescent, activated, and glucocorticoid-treated states. Biosci Rep. 2000;20:289–302. doi: 10.1023/a:1026445108136. [DOI] [PubMed] [Google Scholar]
- 67.Sekulic A. Hudson CC. Homme JL. Yin P. Otterness DM. Karnitz LM. Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 68.Selman C. Tullet JM. Wieser D. Irvine E. Lingard SJ. Choudhury AI. Claret M. Al-Qassab H. Carmignac D. Ramadani F. Woods A. Robinson IC. Schuster E. Batterham RL. Kozma SC. Thomas G. Carling D. Okkenhaug K. Thornton JM. Partridge L. Gems D. Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma R. Vinayak M. Antioxidant alpha-tocopherol checks lymphoma promotion via regulation of expression of protein kinase C-alpha and c-Myc genes and glycolytic metabolism. Leuk Lymphoma. 2012;53:1203–1210. doi: 10.3109/10428194.2011.637213. [DOI] [PubMed] [Google Scholar]
- 70.Shriver LP. Manchester M. Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep. 2011;1:79. doi: 10.1038/srep00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sklavos MM. Bertera S. Tse HM. Bottino R. He J. Beilke JN. Coulombe MG. Gill RG. Crapo JD. Trucco M. Piganelli JD. Redox modulation protects islets from transplant-related injury. Diabetes. 2010;59:1731–1738. doi: 10.2337/db09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sklavos MM. Tse HM. Piganelli JD. Redox modulation inhibits CD8 T cell effector function. Free Radic Biol Med. 2008;45:1477–1486. doi: 10.1016/j.freeradbiomed.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 73.Tse HM. Milton MJ. Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 74.Tse HM. Milton MJ. Schreiner S. Profozich JL. Trucco M. Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- 75.van Stipdonk MJ. Hardenberg G. Bijker MS. Lemmens EE. Droin NM. Green DR. Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 76.Vander Heiden MG. Cantley LC. Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang J. Ren Z. Xu Y. Xiao S. Meydani SN. Wu D. Epigallocatechin-3-gallate ameliorates experimental autoimmune encephalomyelitis by altering balance among CD4+T-cell subsets. Am J Pathol. 2012;180:221–234. doi: 10.1016/j.ajpath.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xuzhu G. Komai-Koma M. Leung BP. Howe HS. McSharry C. McInnes IB. Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012;71:129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- 79.Yan LJ. Levine RL. Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci U S A. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y. Chaiswing L. Oberley TD. Batinic-Haberle I. St Clair W. Epstein CJ. St. Clair D. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 81.Zheng Y. Collins SL. Lutz MA. Allen AN. Kole TP. Zarek PE. Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]