Abstract
Although several mechanisms have been proposed to explain the activity of thalidomide, lenalidomide and pomalidomide in multiple myeloma (MM), including demonstrable anti-angiogenic, anti-proliferative and immunomodulatory effects, the precise cellular targets and molecular mechanisms have only recently become clear. A landmark study recently identified cereblon (CRBN) as a primary target of thalidomide teratogenicity. Subsequently it was demonstrated that CRBN is also required for the anti-myeloma activity of thalidomide and related drugs, the so-called immune-modulatory drugs (IMiDs). Low CRBN expression was found to correlate with drug resistance in MM cell lines and primary MM cells. One of the downstream targets of CRBN identified is interferon regulatory factor 4 (IRF4), which is critical for myeloma cell survival and is down-regulated by IMiD treatment. CRBN is also implicated in several effects of IMiDs, such as down-regulation of tumor necrosis factor-α (TNF-α) and T cell immunomodulatory activity, demonstrating that the pleotropic actions of the IMiDs are initiated by binding to CRBN. Future dissection of CRBN downstream signaling will help to delineate the underlying mechanisms for IMiD action and eventually lead to development of new drugs with more specific anti-myeloma activities. It may also provide a biomarker to predict IMiD response and resistance.
Keywords: Myeloma, IMiDs, cereblon
History of thalidomide and immune-modulatory drugs in the treatment of multiple myeloma
As the first of the immune-modulatory drug (IMiD) class, thalidomide was introduced as a sedative used to prevent nausea during pregnancy in the late 1950s. In 1961, it was withdrawn due to teratogenicity and neuropathy [1], In 1965, thalidomide was shown to be effective in erythema nodosum leprosum [2], and in 1999 effectiveness against multiple myeloma was reported [3]. Thalidomide significantly improves the response rate and survival of patients with multiple myeloma (MM) [4–7]. The second generation of IMiDs include lenalidomide (CC-5013) and pomalidomide (CC4047), and both of them demonstrated more potent anti-MM, anti-inflammatory and immunomodulatory activities than thalidomide [8,9]. Interestingly the single-agent response rate to IMiDs is about 30%, indicating that many patients have primary resistance. A number of studies have, however, demonstrated the efficacy of retreatment with thalidomide and lenalidomide in patients receiving IMiDs for initial therapy of newly diagnosed MM [10-12], suggesting that resistance to this class of drug may be reversible perhaps due to the re-emergence of drug sensitive clones. Recent clinical studies of pomalidomide also indicated that pomalidomide is active in the treatment of myeloma, which is refractory to lenalidomide [13].
Immune-modulatory drugs have multiple cellular and molecular effects
Anti-myeloma activity of IMiDs is mediated through direct and indirect mechanisms [14,15]. Unlike thalidomide, which showed little effect on myeloma cell proliferation and survival, the second generation of IMiDs can induce cell cycle arrest and apoptosis directly in MM cells. Previous studies revealed several downstream changes after IMiD treatment which may associate with direct anti-myeloma activity of IMiDs, such as up-regulation of P21wafl expression [16], inactivation of nuclear factor-κB (NF-κB) [17], down-regulation of C/EBPβ [18] and activation of caspase 8 [19].
Indirect anti-myeloma activity of IMiDs is postulated to be mediated by alteration of the interaction between MM cells and non-myeloma cells in the bone marrow (BM) microenvironment including BM stromal cells (BMSCs), osteoclasts (OCs) and immune cells. The interaction of MM cells with the BM microenvironment enhances MM cell growth, survival, migration and drug resistance. This results in the activation of important signaling cascades (such as NF-κB) in both the myeloma cells and BMSCs and leads to increased production of several growth factors for myeloma [20-22], such as interleukin-6 (IL-6), insulin-like growth factor 1 (IGF-1) and vascular endothelial growth factor (VBGF). IMiDs block interaction of MM cells and BMSCs through inhibition of expression of surface adhesion molecules on both MM cells and BMSCs [23–25]. MM cells stimulate osteoclastogenesis, and osteoclasts (OCs) in turn enhance MM growth and drug resistance. IMiDs inhibit MM bone lesions by either directly inhibiting OC maturation [26] or indirectly by reducing MM tumor burden. Further IMiDs down-regulate transcription factor PU.1 [27], which is important in the formation of OC precursors. IMiDs also inhibit angiogenesis. Thalidomide significantly reduces microvascular density in bone marrows of responding patients with myeloma, but not in non-responders [28].
Immunomodulatory activity of IMiDs distinguishes this drug family from other anti-myeloma drugs. IMiDs enhance CD4+ and CD8+ T cell costimulation [29,30]. Both lenalidomide and pomalidomide are more potent than thalidomide in inducing T cell proliferation and enhancing IL-2 and interferon γ (IFNγ) production. Second-generation IMiDs also inhibit regulatory T cells (Tregs) [31]. Tregs are a group of immunosuppressive T cells that play an important role in self-tolerance and immune response against tumor cells [32]. Elevated Tregs recently have been reported to be associated with shorter survival of patients with MM [33].
IMiDs enhance natural killer (NK) and NKT (T lymphocytes which bear NK cell surface markers) cells [30,34]. They increase NK cell proliferation in patients with MM responding to therapy. Lenalidomide and pomalidomide also enhance both NK cell mediated cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) induced by triggering IL-2 production from T cells [35].
Finally, IMiDs have been shown to inhibit production of proinflammatory cytokines tumor necrosis factor-α (TNF-α), IL-1, IL-6 and IL-12 from human peripheral blood mononuclear cells (PBMCs) [8].
Molecular targets for thalidomide mediated teratogenicity
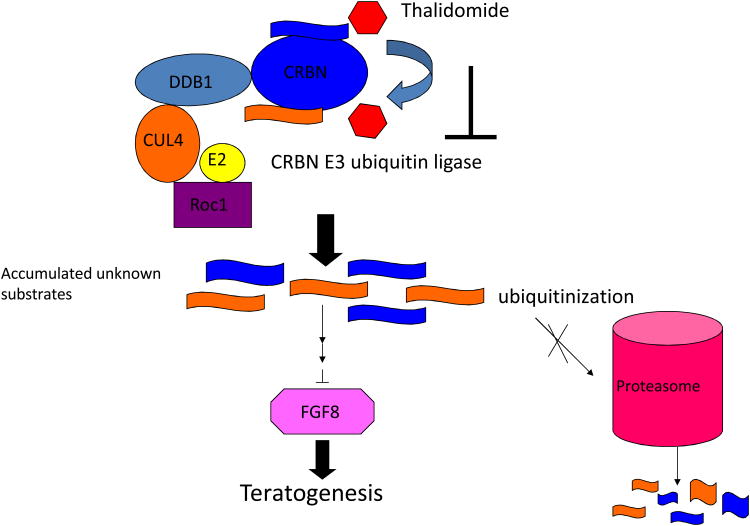
IMiDs have teratogenic properties [36]. Thalidomide in particular is infamous for its teratogenicity. Many hypotheses were proposed to explain this side effect, and the most two popular theories include oxidative stress and anti-angiogenesis [37–42]. However, none of the existing hypotheses are satisfactory in explaining how these processes are initiated and proceed after drug treatment. A landmark article has recently been published [43]. In this article cereblon (CRBN: cerebral protein with Ion protease), a protein previously reported to associate with autosomal recessive non-syndromic mental retardation [44], was identified as a primary target of thalidomide teratogenicity. This study demonstrated that CRBN directly binds DNA damage-binding protein 1 (DDB1) in a DCX (DDB1-CUL4-X-Box) E3 ubiquitin ligase complex. The interaction of thalidomide and CRBN inhibits the function of CRBN associated E3 ubiquitin ligase, which may prevent still-unidentified proteins from degradation by the proteasome, and eventually affects downstream molecules linked to teratogenicity, most likely through the down-regulation of Fgf8 (Figure 1) [43]. CRBN was suggested to function as a substrate receptor in its associated E3 ubiquitin ligase [45]. So far, apart from CRBN itself, the substrates of CRBN E3 ligase are still unknown.
Figure 1.
Schematic model of CRBN E3 ubiquitin ligase in thalidomide-induced teratogenicity. Thalidomide binds to CRBN and inhibits its associated E3 ubiquitin ligase activity. This inhibition results in unknown substrates accumulating and eventually cause developmental defects, partially via downregulation of FGF8 (Ito, et al, 2010).
CRBN is essential for IMiD mediated direct anti-myeloma activity
In order to know whether CRBN is also involved in anti-myeloma activity of IMiDs, we recently conducted research to study the role of CRBN in IMiD induced response in MM cells [46]. Using an shRNA lentiviral expression system, we investigated the effects of silencing CRBN in MM cells. Knockdown of CRBN in five different MM cell lines resulted in a significant reduction in MM cell viability (reduced 65–78%), implying that CRBN associated downstream signaling is important for MM survival, Approximately 5–to 30% MM cells survived CRBN silencing in different MM cell lines. Those surviving MM cells with stable CRBN silencing have been further demonstrated to acquire resistance to both lenalidomide and pomalidomide when compared with non-targeting control virus infected cells, but retained sensitivity to melphalan, dexamethasone and bortezomib. Gene expression changes induced by lenalidomide were dramatically suppressed in CRBN depleted cells; specifically, OPM2 cells showed only 30 down-regulated genes (3% of control) and 150 up-regulated genes (24% of control) after lenalidomide treatment, demonstrating that CRBN is absolutely required for IMiD response.
CRBN expression level and IMiD response
In order to know whether CRBN expression level correlates with IMiD response, gene expression profile (GEP) data were analyzed [46]. Human MM cell lines (HMCLs) generally have lower CRBN expression than primary MM cells, which may explain why HMCLs usually have a lower response to IMiDs. IMiD resistant MM cell lines, such as OCI-My5 and OPM1, expressed lower CRBN at both transcriptional and protein levels when contrasted with relatively sensitive cell lines such as OPM2 and MM1.S. To further confirm that the CRBN level affects the sensitivity to IMiDs in MM cells, we recently overex-pressed CRBN in two myeloma cell lines which have low CRBN expression and are resistant to IMiDs. We found that the sensitivity to lenalidomide was increased in these cell lines after the introduction of wild-type CRBN. However, transduction of CRBN with point mutations at the thalidomide binding site did not affect lenalidomide sensitivity. The results suggest that CRBN expression level is associated with IMiD sensitivity and it also indicates indirectly that lenalidomide binds CRBN at the same site as thalidomide. A recent study demonstrated that both lenalidomide and pomalidomide directly bind CRBN, but with higher affinities compared with thalidomide [36].
The correlation between CRBN expression levels and IMiD response has also been demonstrated in IMiD resistant MM cells. In an isogenic resistant MM1.S cell line, generated by culturing MM1.S cells in a gradually increasing dose of lenalidomide for several months [47], we found that CRBN expression is greatly reduced compared with its parental MM1.S cell line [46]. A recent study also found that CRBN expression decreases concomitantly with acquisition of lenalidomide resistance in H929 myeloma cells [36]. Using quantitative polymerase chain reaction (qPCR), we examined nine patients with MM who had become resistant to lenalidomide therapy. The CRBN expression level in five of nine patient samples had a significant reduction at the time of drug resistance, suggesting CRBN expression as a potential biomarker to predict IMiD response [46]. Another recent study reported that high CRBN expression levels correlated with improved clinical response in MM treated with lenalidomide and dexamethasone [48]. Using comparative genomic hybridization (CGH) to analyze CRBN status in HMCLs and primary MM samples we found that genomic deletion and mutation affecting this gene appear to be uncommon events, suggesting that CRBN expression is likely regulated at transcriptional or post-transcriptional level. A recent study by comprehensive profiling of microRNA and mRNA in lenalidomide treated patients with MM speculated that expression of CRBN might be regulated by microRNA [49]. Notably, some clearly resistant patients have normal CRBN levels, indicating that while the CRBN level could affect IMiD responses, multiple resistance mechanisms are likely present, A recent study showed that up-regulation of Wnt signaling was associated with IMiD resistance [47]. We assume that any genomic and expression changes in the downstream targets of CRBN can potentially affect the sensitivity to IMiDs.
Downstream targets of CRBN include interferon regulatory factor 4
In order to identify downstream molecules of CRBN that may associate with IMiD induced MM cytotoxicity, we compared the gene expression profiles after CRBN silencing and IMiD treatment in MM cell lines [46]. A panel of gene changes shared by knockdown CRBN and lenalidomide treatment was identified. Some of those changes have been reported previously after IMiD treatment, such as down-regulation of Myb and up-regulation of PTEN. One of the genes down-regulated after CRBN silencing was interferon regulatory factor 4 (IRF4). IRF4 was recently identified as a critical factor for MM cell survival [50]. IRF4 inhibition is toxic to myeloma cell lines, regardless of the transforming oncogenic mechanism. The direct targets of IRF4 include several important genes for cell proliferation and survival, such as Myc, CDK6 and CASP3 [50]. Both lenalidomide and pomalidomide were demonstrated to inhibit IRF4 gene expression [18,51]. One study demonstrated that IMiD compounds down-regulate CCAAT/enhancer-binding protein-β (C/EBPβ), which further regulates the transcription of IRF4 [18]. In our study, IRF4 expression was reduced in MM cells after the introduction of CRBN shRNA for 72 h, Interestingly, the IRF4 level in IMiD resistant cells which survived CRBN silencing was back to normal, as in control cells. A recent study reported that induction of P21wafl expression and inhibition of IRF4 by IMiD treatment were prevented or impaired in the absence of CRBN expression [36]. Both P21wafl and IRF4 are targets of NF-κB, suggesting that IMiD induced inactivation of NF-κB may involve IRF4 and P21wafl regulation. Further investigations are being undertaken to understand how CRBN affects IRF4 expression and whether down-regulation of IRF4 is critical for IMiD induced anti-myeloma activity, Lenalidomide is an active agent in the activated B cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL). A recent study demonstrated that the lenalidomide-induced tumoricidal effect on ABC-DLBCL is mediated by augmenting IFNβ production through a CRBN-dependent down-regulation of IRF4 and SPIB, CRBN was found to be required to maintain IRF4 and SPIB levels in ABC-DLBCL [52].
CR8N and other functions of IMiDs
To determine whether CRBN is essential for other effects of IMiDs, we recently studied the effects of knockdown CRBN on IMiD induced inhibition of TNF-α production in a monocyte cell line after lipopolysaccharide (LPS) stimulation. Pre-treatment of these cells with IMiDs significantly prevented TNF-α production. Pomalidomide is the most potent in inhibition of TNF-α production. Knockdown of CRBN was shown to mimic the effects of lenalidomide and pomalidomide treatment on control cells, and the inhibitory effect of IMiDs on TNF-α production was also impaired upon CRBN silencing. Using a similar CRBN silencing technology others also demonstrated that some immunomodulatory effects of IMiDS on T cells are also mediated via CRBN [36].
Based on the data above, we believe that the actions of IMiDs in different cell types are all likely to be initiated by binding to CRBN. Although probable, to date there is no direct evidence that IMiD induced anti-myeloma activity is mediated through inhibiting CRBN associated E3 ubiquitin ligase function. In addition to binding DDB1, CRBN interacts with other proteins such as KCNT1 [53,54] and AMP-activated protein kinase (AMPK) [55]. KCNT1 encodes a component of large-conductance calcium-activated potassium [BK(Ca)] channels, which play important roles in Ca2+ dependent signaling. CRBN regulates the assembly and surface expression of BK(Ca) channels in the brain region involved in memory via its interaction with the BK(Ca) channel alpha subunit KCNT1. AMPK is a key regulator of cellular energy homeostasis. CRBN physically interacts with AMPK alpha and this interaction results in inhibition of AMPK activity [55]. The AMPK activators induce inhibition of proliferation of myeloma cell lines [56]. It is interesting to speculate whether or not anti-myeloma activity of IMiDs is associated with the interaction between CRBN and AMPK. Both KCNT1 and AMPK are potential substrates of CRBN E3 ligase, but they may not be associated with downstream signaling that leads to anti-myeloma activity of IMiDs. It is possible that CRBN associated substrates and downstream signaling in different cell types are unique, which accounts for the diverse effects of IMiDs. In myeloma cells the downstream molecules, such as Myc, IRF4 and P21wafl, are associated with myeloma cell growth and survival. We found that fibroblast growth factor 8 (FGF8), a CRBN downstream molecule linked to thalidomide induced teratogenicity, is unlikely to be involved in IMiD induced anti-myeloma activity. Although CRBN appears essential for IMiD response, it has been suggested that some IMiDs may bind other proteins in addition to CRBN. Identification of these proteins and their relationship with CRBN associated signaling is also important for fully understanding the action of this family of drugs.
Summary.
The anti-myeloma activity of IMiDs is dependent on the presence of CRBN, which was identified as a primary target of thalidomide teratogenicity. Low CRBN expression is associated with IMiD resistance in some IMiD resistant cell lines and primary MM samples. Downstream targets of CRBN include MM survival factors such as IRF4. Future study should focus on identifying CRBN E3 ligase substrates, and dissecting CRBN downstream signaling that is associated with the diverse effects of IMiDs.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Speirs AL. Thalidomide and congenital abnormalities. Lancet. 1962;1:303–305. doi: 10.1016/s0140-6736(62)91248-5. [DOI] [PubMed] [Google Scholar]
- 2.Sheskin J. Thalidomide in the treatment of lepra reactions. Clin Pharmacol Ther. 1965;6:303–306. doi: 10.1002/cpt196563303. [DOI] [PubMed] [Google Scholar]
- 3.Singhal S, Mehta J, Desikan R, et al. Antitumoractivity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 4.Juliusson G, Celsing F, Turesson I, et al. Frequent good partial remissions from thalidomide including best response ever in patients with advanced refractory and relapsed myeloma. Br J Haematol. 2000;109:89–96. doi: 10.1046/j.1365-2141.2000.01983.x. [DOI] [PubMed] [Google Scholar]
- 5.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 6.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 7.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112:3107–3114. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 8.Muller GW, Corral LG, Shire MG, et al. Structural modifications of thalidomide produce analogs with enhanced tumor necrosis factor inhibitory activity. J Med Chem. 1996;39:3238–3240. doi: 10.1021/jm9603328. [DOI] [PubMed] [Google Scholar]
- 9.Marriott JB, Muller G, Stirling D, et al. Immunotherapeutic and antitumour potential of thalidomide analogues. Expert Opin Biol Ther. 2001;1:675–682. doi: 10.1517/14712598.1.4.675. [DOI] [PubMed] [Google Scholar]
- 10.Madan S, Lacy MQ, Dispenzieri A, et al. Efficacy of retreatment with immunomodulatory drugs (IMiDs) in patients receiving IMiDs for initial therapy of newly diagnosed multiple myeloma. Blood. 118:1763–1765. doi: 10.1182/blood-2011-04-350009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–4451. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 12.Guglielmelli T, Bringhen S, Rrodhe S, et al. Previous thalidomide therapy may not affect lenalidomide response and outcome in relapse or refractory multiple myeloma patients. Eur J Cancer. 2011;47:814–818. doi: 10.1016/j.ejca.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Lacy MQ, Tefferi A. Pomalidomide therapy for multiple myeloma and myelofibrosis. Leuk Lymphoma. 2011;52:560–566. doi: 10.3109/10428194.2011.552139. [DOI] [PubMed] [Google Scholar]
- 14.Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36. doi: 10.1186/1756-8722-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escoubet-Lozach L, Lin IL, Jensen-Pergakes K, et al. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69:7347–7356. doi: 10.1158/0008-5472.CAN-08-4898. [DOI] [PubMed] [Google Scholar]
- 17.Keifer JA, Guttridge DC, Ashburner BP, et al. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem. 2001;276:22382–22387. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Pal R, Monaghan SA, et al. IMiD immunomodulatory compounds block C/EBP{beta} translation through eIF4E down-regulation resulting in inhibition of MM. Blood. 2011;117:5157–5165. doi: 10.1182/blood-2010-10-314278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 20.Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 21.Dankbar B, Padro T, Leo R, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000;95:2630–2636. [PubMed] [Google Scholar]
- 22.Gupta D, Treon SP, Shima Y, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 23.Hideshima T, Chauhan D, Schlossman R, et al. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–4527. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 24.Geitz H, Handt S, Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology. 1996;31:213–221. doi: 10.1016/0162-3109(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 25.Hideshima T, Bergsagel PL, Kuehl WM, et al. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 26.Breitkreutz I, Raab MS, Vallet S, et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia. 2008;22:1925–1932. doi: 10.1038/leu.2008.174. [DOI] [PubMed] [Google Scholar]
- 27.Anderson G, Gries M, Kurihara N, et al. Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood. 2006;107:3098–3105. doi: 10.1182/blood-2005-08-3450. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Witzig TE, Dispenzieri A, et al. Effect of thalidomide therapy on bone marrow angiogenesis in multiple myeloma. Leukemia. 2004;18:624–627. doi: 10.1038/sj.leu.2403285. [DOI] [PubMed] [Google Scholar]
- 29.Haslett PA, Corral LG, Albert M, et al. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187:1885–1892. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies FE, Raje N, Hideshima T, et al. Thalidomide and Immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 31.Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braga WM, Atanackovic D, Colleoni GW. The role of regulatory T cells and TH17 cells in multiple myeloma. Clin Dev Immunol. 2012;2012:293479. doi: 10.1155/2012/293479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannopoulos K, Kaminska W, Hus I, et al. The frequency of T regulatory cells modulates the survival of multiple myeloma patients: detailed characterisation of immune status in multiple myeloma. Br J Cancer. 2012;106:546–552. doi: 10.1038/bjc.2011.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu D, Corral LG, Fleming YW, et al. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother. 2008;57:1849–1859. doi: 10.1007/s00262-008-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu L, Adams M, Carter T, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012 May 3; doi: 10.1038/leu.2012.119. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med. 1999;5:582–585. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- 38.Knobloch J, Shaughnessy JD, Jr, Ruther U. Thalidomide induces limb deformities by perturbing the Bmp/Dkk1/Wnt signaling pathway. FASEB J. 2007;21:1410–1421. doi: 10.1096/fj.06-7603com. [DOI] [PubMed] [Google Scholar]
- 39.Hansen JM, Gong SG, Philbert M, et al. Misregulation of gene expression in the redox-sensitive NF-kappab-dependent limb outgrowth pathway by thalidomide. Dev Dyn. 2002;225:186–194. doi: 10.1002/dvdy.10150. [DOI] [PubMed] [Google Scholar]
- 40.Hansen JM, Harris C. A novel hypothesis for thalidomide-induced limb teratogenesis: redox misregulation of the NF-kappaB pathway. Antioxid Redox Signal. 2004;6:1–14. doi: 10.1089/152308604771978291. [DOI] [PubMed] [Google Scholar]
- 41.D'Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Therapontos C, Erskine L, Gardner ER, et al. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc Natl Acad Sci USA. 2009;106:8573–8578. doi: 10.1073/pnas.0901505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 44.Higgins JJ, Pucilowska J, Lombardi RQ, et al. A mutation in a novel ATP-dependent Lon protease gene in a kindred with mild mental retardation. Neurology. 2004;63:1927–1931. doi: 10.1212/01.wnl.0000146196.01316.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angers S, Li T, Yi X, et al. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- 46.Zhu YX, Braggio E, Shi CX, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjorklund CC, Ma W, Wang ZQ, et al. Evidence of a role for activation of Wnt/{beta]-catenin signaling in the resistance of plasma cells to lenalidomide. J Biol Chem. 2011;286:11009–11020. doi: 10.1074/jbc.M110.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heintel D, Bolomsky A, Schreder M, et al. High expression of the thalidomide-binding protein cereblon (CRBN) is associated with improved clinical response in patients with multiple myeloma treated with lenalidomide and dexamethasone. Blood. 2011;118(Suppl. 1) doi: 10.1111/bjh.12338. Abstract 2879. [DOI] [PubMed] [Google Scholar]
- 49.Neri P, Belch AR, Johnson J, et al. A miRNA risk score for the prediction of response to lenalidomide in multiple myeloma (MM) patients. Blood. 2011;118(Suppl. 1) Abstract 987. [Google Scholar]
- 50.Shaffer AL, Emre NC, Lamy L, et al. IRF4 addiction in multiple myeloma. Nature. 2008;454:226–231. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Girona A, Heintel D, Zhang LH, et al. Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br J Haematol. 2011;154:325–336. doi: 10.1111/j.1365-2141.2011.08689.x. [DOI] [PubMed] [Google Scholar]
- 52.Yang Y, Shaffer AL, 3rd, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell. 2012;21:723–737. doi: 10.1016/j.ccr.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higgins JJ, Hao J, Kosofsky BE, et al. Dysregulation of large-conductance Ca2+-activated K+ channel expression in nonsyndromal mental retardation due to a cereblon p.R419X mutation. Neurogenetics. 2008;9:219–223. doi: 10.1007/s10048-008-0128-2. [DOI] [PubMed] [Google Scholar]
- 54.Jo S, Lee KH, Song S, et al. Identification and functional characterization of cereblon as a binding protein for large-conductance calcium-activated potassium channel in rat brain. J Neurochem. 2005;94:1212–1224. doi: 10.1111/j.1471-4159.2005.03344.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee KM, Jo S, Kim H, et al. Functional modulation of AMP-activated protein kinase by cereblon. Biochim Biophys Acta. 2011;1813:448–455. doi: 10.1016/j.bbamcr.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Baumann P, Mandl-Weber S, Emmerich B, et al. Activation of adenosine monophosphate activated protein kinase inhibits growth of multiple myeloma cells. Exp Cell Res. 2007;313:3592–3603. doi: 10.1016/j.yexcr.2007.06.020. [DOI] [PubMed] [Google Scholar]