Abstract
Polybrominated diphenyl ethers (PBDEs) are widely used flame retardant compounds. Brominated diphenyl ether (BDE)-47 is one of the most prevalent PBDE congeners found in human breast milk, serum and placenta. Despite the presence of PBDEs in human placenta, effects of PBDEs on placental cell function are poorly understood. The present study investigated BDE-47-induced reactive oxygen species (ROS) formation and its role in BDE-47-stimulated proinflammatory cytokine release in a first trimester human extravillous trophoblast cell line, HTR-8/SVneo. Exposure of HTR-8/SVneo cells for 4 h to 20 μM BDE-47 increased ROS generation 1.7 fold as measured by the dichlorofluorescein (DCF) assay. Likewise, superoxide anion production increased approximately 5 fold at 10 and 15 μM and 9 fold at 20 μM BDE-47 with a 1-h exposure, as measured by cytochrome c reduction. BDE-47 (10, 15 and 20 μM) decreased the mitochondrial membrane potential by 47–64.5% at 4, 8 and 24 h as assessed with the fluorescent probe Rh123. Treatment with 15 and 20 μM BDE-47 stimulated cellular release and mRNA expression of IL-6 and IL-8 after 12 and 24 h exposures: the greatest increases were a 35-fold increased mRNA expression at 12 h and a 12-fold increased protein concentration at 24 h for IL-6. Antioxidant treatments (deferoxamine mesylate, (±)α-tocopherol, or tempol) suppressed BDE-47-stimulated IL-6 release by 54.1%, 56.3% and 37.7%, respectively, implicating a role for ROS in regulation of inflammatory pathways in HTR-8/SVneo cells. Solvent (DMSO) controls exhibited statistically significantly decreased responses compared with non-treated controls for IL-6 release and IL-8 mRNA expression, but these responses were not consistent across experiments and times. Nonetheless, it is possible that DMSO (used to dissolve BDE-47) may have attenuated the stimulatory actions of BDE-47 on cytokine responses. Because abnormal activation of proinflammatory responses can disrupt trophoblast functions necessary for placental development and successful pregnancy, further investigation is warranted of the impact of ROS and BDE-47 on trophoblast cytokine responses.
Keywords: Polybrominated diphenyl ethers (PBDEs), reactive oxygen species, oxidative stress, HTR-8/SVneo cells, human placental cells, cytokines
INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) are synthetic flame-retardants widely used in polyurethane foam, textiles, plastics, building materials and insulation (Hites, 2004). Among the 209 PBDE congeners, BDE-47 (2,2′,4,4′-tetra-BDE) is one of the most prevalent congers found in human tissues and environmental samples (Hites, 2004). Because of PBDEs’ environmental persistence and toxicity, the US EPA has identified PBDEs as a priority human health concern (U.S. Environmental Protection Agency, 2006). Limited studies reported possible reproductive toxicity of PBDEs during pregnancy. Rabbits orally exposed to PBDEs show decreased gestation length (Breslin et al., 1989). Elevated levels of PBDEs in human umbilical cord blood have been correlated with preterm birth, low birth weight or stillbirth (Wu et al., 2010). Although these studies suggest the association between PBDE exposure and adverse birth outcomes, and PBDEs distribute to human placenta (Frederiksen et al., 2009), extraplacental membranes (Miller et al., 2009), amniotic fluid (Miller et al., 2012), and umbilical cord blood (Frederiksen et al., 2009), studies of mechanisms by which PBDEs act on gestational tissues during pregnancy are limited.
It is suggested that cytokine dysregulation alters extravillous trophoblast (EVT) processes, leading to placental dysfunction that may compromise pregnancy (Anton et al., 2012). For example, increased levels of inflammatory mediators such as cytokines and C-reactive protein are associated with the pathophysiology of preeclampsia and intrauterine growth restriction (IUGR), possibly contributing to abnormal placental function (Tjoa et al., 2003; Vince et al., 1995). Also, women who delivered preterm had higher rates of placental ischemia and abnormal placentation than controls (Germain et al., 1999; Kim et al., 2003), with high levels of interleukin (IL)-8 and IL-6 in cervical fluid, amniotic fluid and maternal serum (Goldenberg et al., 2005). Although these studies suggest that inflammation occurring at the maternal–fetal interface during pregnancy could contribute to abnormal placental function associated with adverse obstetrical outcomes, a recent report on PBDE-stimulated cytokine release in placenta, using second trimester human placental explant cultures (Peltier et al., 2012), showed that pre-exposure of placental explants to a PBDE mixture of congers 47, 99 and 100 enhanced placental proinflammatory response to heat-killed E. Coli. However, to our knowledge, there are no previous reports of BDE-47 directly altering inflammatory pathways in human placental cells.
Oxidative stress is defined as the imbalance between pro-oxidants and antioxidants resulting in increase of reactive oxygen species (ROS). Oxidative stress in placenta has been associated with pathologies of pregnancy, including preterm labor, preeclampsia, and IUGR (Agarwal et al., 2012). A growing body of literature indicates that oxidative stress can activate a variety of transcription factors, including nuclear factor kappa B (NF-κB), activator protein 1(AP-1), and nuclear factor like 2 (Nrf2), leading to altered expression of genes for inflammatory cytokines, chemokines, and anti-inflammatory molecules (Reuter et al., 2010). Moreover, N-acetylcysteine, which can act as an antioxidant by increasing cellular concentrations of glutathione, prevents lipopolysaccharides (LPS)-stimulated parturition, fetal death in mice, and LPS-induced release of pro-inflammatory cytokines from human extraplacental membranes in vitro (Buhimschi et al., 2003; Cindrova-Davies et al., 2007). Together, these findings implicate interplay between cytokines and oxidative stress in the etiology of adverse pregnancy outcomes.
A few studies suggest that PBDEs induce generation of ROS in mammalian cells. He et al. (2008) showed that PBDEs induce lipid peroxidation and DNA damage in primary cultured rat hippocampal neurons. Reistad and Mariussen reported that pentabrominated diphenyl ether (DE-71) and BDE-47 enhanced the production of ROS, potentially through NADPH oxidase activation in human granulocytes (Reistad and Mariussen, 2005b). It is also reported that BDE-47 induced apoptosis in Jurkat cells, possibly through ROS overproduction and mitochondrial dysfunction (Yan et al., 2011). Shao et al (2008) reported that BDE-47 induced ROS overproduction, loss of mitochondrial membrane potential and apoptosis in human fetal liver hematopoietic stem cells. These data suggest a close relationship between ROS formation and toxicity induced by PBDEs. However, there is no previous report on PBDE-stimulated ROS formation in human placental cells and tissues.
Although inappropriate activation of the innate immune response can lead to placental dysfunction and certain environmental contaminants can activate innate immune responses (Campbell, 2004; Lin et al., 2010), there is a paucity of reports on PBDE-stimulated inflammation in first trimester placenta. Moreover, increased oxidative stress in placenta has been observed in pathological pregnancies, and ROS have been implicated in the activation of inflammatory responses in gestational compartments (Buhimschi et al., 2003; Cindrova-Davies et al., 2007). The present study examines the hypothesis that BDE-47 stimulates pro-inflammatory cytokine production via a ROS-mediated mechanism in the first trimester EVT human placental cell line HTR-8/SVneo.
MATERIALS AND METHODS
Chemicals and assay kits
BDE-47 was purchased from AccuStardard (New Haven, CT, USA). DMSO, deferoxamine mesylate (DFO), tert-butyl hydroperoxide (TBHP), cytochrome c from bovine heart, superoxide dismutase (SOD) from bovine erythrocytes, N-ethylmaleimide and rhodamine (Rh) 123, 4-hydroxy-TEMPO (tempol), and (±)-α-tocopherol were purchased from Sigma Aldrich (St. Louis, MO, USA). The 6-carboxy-dichlorodihydrofluorescein diacetate (carboxy-H2DCF-DA), CellMask™ Deep Red plasma membrane stain C10046, RPMI medium 1640, fetal bovine serum (FBS), OptiMem 1 reduced-serum medium, Hank’s balanced salt solution (HBSS), 0.25% trypsin/EDTA solution and penicillin/streptomycin (P/S) were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Recombinant IL-1β and sandwich enzyme-linked immunosorbent assay (ELISA) kits for human IL-6, IL-8 were purchased from R & D systems (Minneapolis, MN, USA). The MultiTox-Glo Multiplex cytotoxicity assay kit was purchased from Promega (Madison, WI, USA). QIAshredder, RNeasy mini plus kit, RT2 First Strand kit for reverse transcriptase reaction, RT2 qPCR SYBR Green/ROX Master Mix and primers for human β-microglobulin, IL-6, and IL-8 were purchased from Qiagen (Valencia, CA, USA). BDE-47 was prepared in dimethyl sulfoxide (DMSO) as a 50 mM stock solution. (±)-α-tocopherol was prepared in DMSO as a 100 mM stock solution. Rh123 was prepared in DMSO as a 2μg/ml stock solution. Carboxy-H2DCF-DA was prepared in DMSO as 50 mg/ml stock solution. Other chemicals were applied directly into media.
Cell Culture and treatment
The human first trimester extravillous trophoblast cell line HTR-8/SVneo was kindly provided by Dr. Charles S. Graham (Queen’s University, Kingston, ON, Canada). Cells between passages 71 and 84 were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a 5% CO2 humidified atmosphere. Cells were grown to a confluence of 70–90% before treatment. Cells were washed with OptiMem 1 containing 1% FBS and 1% P/S twice and acclimated with the medium for 1 h at 37 °C. From solutions of 5, 10, 15 and 20 mM BDE-47 in DMSO, exposure media containing 5, 10, 15 and 20 μM BDE-47 were made in OptiMem 1 containing 1% FBS and 1% P/S immediately prior to initiating the experiment. The final concentration of DMSO in medium was 0.7 % (v/v).
Viability and Cytotoxicity Assays
Cells were seeded in a white 96-well plate at a density of 1 × 104 cells per well and incubated for 24 h at 37 °C. Cells were exposed to DMSO (solvent control) or BDE-47 (5, 10, 15 or 20 μM) and incubated for 24 h. After the 24-h incubation with BDE-47, cell viability and cytotoxicity were measured by the MultiTox-Glo Multiplex cytotoxicity assay kit. Briefly, this assay is based on two protease activities: one is a live-cell protease, and the other is a dead-cell protease, which is released from cells. Fluorescence is proportional to live cells while luminescence is proportional to dead cells. The assay was performed according to the manufacturer’s instructions. Digitonin (300 μg/ml) was used as a positive control.
Dichlorofluorescein assay
Stimulation of ROS generation was assessed using the dichlorofluorescein (DCF) assay. Because artifactual results can occur in the DCF assay due to interactions with toxicants (Tetz et al., 2013), we confirmed that there was no increased DCF fluorescence by BDE-47 in cell free medium (data not shown). The HTR-8/SVneo cells were seeded at a density of 2.4 × 105 cells per well in a 6-well plate and cultured for 24 h at 37 °C. Cells were pre-incubated in the presence or absence of 1 mM DFO for 1 h. Cells were washed once with Optimem1 medium containing 10 % FBS and 1% P/S, and then exposed to 5, 10, 15 or 20 μM BDE-47 for 4 h. Treatment with 100 μM tert-butyl hydroperoxide (TBHP) was included as a positive control. After removal of the treatment and rinsing with HBSS, cultures were incubated for an additional 1 h with 100 μM carboxy-H2DCF-DA in HBSS. After removal of the dye solution and rinsing with HBSS, cells were counterstained with 5 μg/ml CellMask™ Deep Red plasma membrane stain for 5 min. After washing with HBSS and adding fresh HBSS back to the cultures, intracellular DCF fluorescence was visualized at 470 nm excitation and 525 nm emission, and Deep Red stain was visualized at 530 nm excitation and 593 nm emission using an EVOS digital inverted fluorescence microscope. Five images per treatment were taken: one image in each of the four quadrants and one in the center of the well. Equivalent adjustments for brightness and contrast were applied to each image in ImageJ software (National Institutes of Health). Additionally, fluorescence intensity was quantified using the method of He et al. (2008) with a few modifications. Cells exposed to BDE-47 were collected by treatment with 0.25% trypsin/EDTA solution for 2 min and washed twice with HBSS by centrifugation at 1200 rpm for 3 min, then re-suspended in HBSS. After 1-h incubation with 100 μM carboxy-H2DCF-DA in HBSS, the fluorescence intensity of 200,000 cells in a 96-well, black, clear-bottomed plate was measured using the Molecular Devices SpectraMax Gemini M2e at an excitation wavelength of 492 nm and emission wavelength of 522 nm.
Cytochrome c reduction assay
Superoxide production in HTR-8/SVneo cells exposed to BDE-47 was quantified by the cytochrome C reduction assay based on the method of Boota et al. (1996) with a few modifications. Superoxide production in HTR-/SVneo cells exposed to BDE-47 was quantified by the cytochrome C reduction assay. HTR-8/SVneo cells were seeded at a density of 1 × 104 cells per well in a white, clear bottomed, 96-well plate, and incubated for 24 h at 37 °C. Cells were exposed to 5, 10, 15 or 20 μM BDE-47 for 1 h. Pyrogallol (100 μM) was included as a positive control. Reaction buffer was prepared in HBSS with 70 μM ferricytochrome c with or without 80 μg/ml of SOD. After treatment with BDE-47, cells were washed once with HBSS and a 100 μl-aliquot of the reaction buffer solution was added to each well of the plates. Cytochrome c reduction was measured at 550 nm after 10, 30, 60 and 90 min incubation with reaction buffer: results are shown for the 90-min time point because this was the reaction time that yielded maximal response. Superoxide production was determined based on the difference in cytochrome c reduction with or without SOD. An extinction coefficient of 28.0 mM−1cm−1 was used for calculations. Results were expressed as nmoles superoxide released per 1×104 cells.
Determination of the mitochondrial membrane potential (MMP)
Rh123, which can bind specifically to mitochondria, was used to estimate MMP based on the methods of Yan et al. (2011) with few modifications. HTR-8/SVneo cells were seeded at a density of 3 × 104 cells per well in a black, clear bottomed, 96-well plate, and incubated for 24 h at 37 °C. Cells were exposed to 5, 10, 15 or 20 μM BDE-47 for 4, 8 or 24 h. Treated cells were washed once with HBSS and incubated with Rh123 (2.5 μg/ml) in HBSS for 60 min in the dark at 37 °C. After replacing Rh123 with fresh HBSS, the fluorescence was measured with a fluorescence spectrophotometer using 507 nm Ex and 529 nm Em filter settings.
Measurement of cytokine release
The HTR-8/SVneo cells were seeded at a density of 2.4 × 105 cells per well in a 6-well plate and cultured for 24 h at 37 °C. Cells were washed once with OptiMem1 medium containing 10 % FBS and 1% P/S and exposed to 5, 10, 15 or 20 μM BDE-47 for 4, 8 or 24 h. After incubation with BDE-47, culture medium was collected and centrifuged to remove any residual cell lysates. The concentration of IL-6 and IL-8 in the supernatant was measured by sandwich ELISA following the manufacturer’s protocols. To determine oxidative stress-mediated activation of inflammatory pathways by BDE-47, HTR-8/SVneo cells were pretreated with 1 mM DFO for 1 h prior to BDE-47 treatment for 24 h, or co-treated either with 20 μM (±)-α-tocopherol, a peroxyl radical scavenger, or with 1mM tempol, a membrane-permeable SOD mimetic for 24 h. Concentrations of IL-6 and IL-8 in the medium was analyzed by ELISA as described above. Release of cytokines was expressed as pg/ml.
RNA extraction and Quantitative real-time polymerase chain reaction
After 4, 12 or 24-h incubation with BDE-47, cell lysates were collected and homogenized using QIA shredder. Total RNA was extracted from homogenized lysates using RNeasy mini plus kit and cDNA was synthesized from 1μg of total RNA using RT2 First Strand Kit. The procedures were performed according to the instructions of the manufacturer. Quantitative real-time polymerase chain reaction (qPCR) was performed in a total volume of 25 μL containing 4 μL of cDNA template, 1 μL of a gene-specific primer (IL-6, IL-8, TNF-α, or IL-10), 12.5 μL of RT2 SYBR Green qPCR Master Mix and 7.5 μL of nuclease-free H2O using CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). A housekeeping gene, β-microglobulin, was co-amplified as an internal control. qRT-PCR was performed with an initial denaturation step of 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 5 s at 60°C. At the end of each cycle, the fluorescence emitted by the SYBR Green was measured. After completion of the cycling process, samples were subjected to a temperature ramp (from 65°C to 95°C at 0.5°C/s) with continuous fluorescence monitoring for melting curve analysis. Signal intensities of target genes were quantified and normalized to the signal of β-microglobulin using Bio-Rad CFX manager software. The level of mRNA expression was presented as fold change compared to solvent controls.
Statistical analysis
Statistical analysis was performed with Sigma Plot 11.0 software (Systat Software Inc., San Jose, CA, USA). Data were analyzed either by one-way analysis of variance (ANOVA) or repeated measured two-way ANOVA. If significant effects are detected, the ANOVA will be followed by Tukey post-hoc comparison of means. A P <0.05 was considered statistically different. Data were expressed as means ± SEM. All experiments were repeated at least three times and all treatments were performed at least in triplicate in each experiment.
RESULTS
Cytotoxicity of BDE-47
To investigate the cytotoxic effect of BDE-47 on HTR-8/SVneo cells, protease-based viability and cytotoxicity assays were performed. Exposure to BDE-47 with concentrations up to 20 μM for 24 h did not result in a significant loss of cell viability in HTR-8/SVneo 8 cells as measured by cellular retention of proteases and indicated by sustained cellular fluorescence (Supplementary Figure 1A). In contrast, the loss of cell viability was clearly evident in cells treated with digitonin, included as a positive control. Similarly, cytotoxic effects were not significant with concentrations of BDE-47 up to 20 μM, as measured by increased luminescence due to protease release from dead cells, although digitonin-mediated cytotoxicity of HTR-8/SVneo cells was apparent (Supplementary Figure 1B; P<0.05).
Effect of BDE-47 on ROS production
Treatment of HTR-8/SVneo cells with 20 μM BDE-47 for 4 h increased DCF fluorescence compared with solvent controls as visualized with epifluorescence microscopy, indicating increased carboxy-H2DCF-DA oxidation to the fluorescent DCF moiety, an indication of cellular reactive species generation (Figure 1A). Pretreatment with the iron-chelating antioxidant DFO decreased BDE-47-stimulated DCF fluorescence (Figure 1A). There were no differences in fluorescence comparing cells from control cultures incubated in HBSS alone, HBSS with 0.7% DMSO, or HBSS with 1 mM DFO (data not shown). Quantification of the fluorescence intensity using a spectrophotometer showed that treatment with 20 μM BDE-47 induced 1.7-fold increase in the DCF fluorescence in the HTR-8/SVneo cells (Table 1; P<0.05), which was inhibited by DFO pretreatment to the equivalent level of the solvent control (Table 2; P<0.05). There were no statistically significant differences between non-treated controls and solvent controls (Table 1 and 2).
Figure 1.
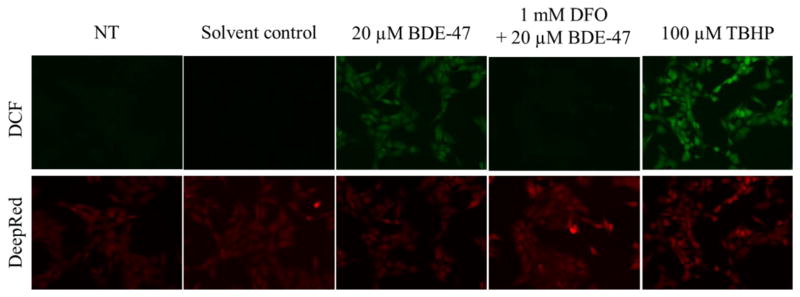
Fluorescence microscopy visualization of BDE-47-stimulated dichlorofluorescein (DCF) fluorescence, an index of reactive oxygen species production, in a first trimester human extravillous trophoblast cell line, HTR-8/SVneo. HTR-8/SVneo cells were pretreated for 1 h with or without 1 mM deferoxamine mesylate (DFO), and then received no further treatment (NT, non-treated control) or were exposed to DMSO (solvent control), 20 μM BDE-47 or 100 μM tert-butyl hydroperoxide (TBHP; positive control) for 4 h. Subsequently, the cells were loaded with the non-fluorescent pro-dye carboxy-H2DCF-DA for 1 h, counterstained with CellMask™ Deep Red plasma membrane dye, and photographed using an epifluorescence microscope. The top panel shows representative images of intracellular DCF fluorescence, and the bottom panel show corresponding Deep Red membrane staining. Representative images of 3 independent experiments.
TABLE 1.
Quantification of BDE-47-Stimulated ROS Production in HTR-8 cellsa
| Treatment | DCF fluorescence intensity |
|---|---|
|
| |
| NT | 174.60 ± 8.17 |
| Solvent control | 183.10 ± 12.36 |
| 5 μM BDE-47 | 257.50 ± 23.77 |
| 10 μM BDE-47 | 260.60 ± 25.88 |
| 15 μM BDE-47 | 247.40 ± 27.36 |
| 20 μM BDE-47 | 309.10 ± 16.72* |
| 100 μM TBHP | 555.00 ± 109.40* |
HTR-8 cells were non-treated (NT; control), or were treated with DMSO (solvent control), BDE-47 or tert-butyl hydroperoxide (TBHP, positive control) for 4 h, then loaded with carboxy-H2DCF-DA for 1 h. Values represent the means ± SE of 3 independent experiments containing 4 replicates each.
P<0.05, significant compared to solvent control.
TABLE 2.
Inhibition of BDE-47-Stimulated ROS Production by Antioxidant Pretreatment in HTR-8 cellsb
| Treatment | DCF Fluorescence Intensity
|
|
|---|---|---|
| No DFO | 1mM DFO | |
|
| ||
| NT | 216.30 ± 7.71 | 218.80 ± 5.71 |
| Solvent control | 216.70 ± 7.53 | 215.60 ± 6.96 |
| 20 μM BDE-47 | 279.60 ± 8.98* | 215.40 ± 6.98# |
HTR-8 cells were pretreated for 1 h with or without deferoxamine mesylate (DFO) prior to exposure to non-treated control (NT), DMSO (solvent control) or BDE-47 for 4 h, and then loaded with carboxy-H2DCF-DA for 1 h. Values represent the means of 3 independent experiments containing 4 replicates each ± SE.
P<0.05, significant compared to solvent control.
P<0.05, significantly different compared to non-DFO treated group.
Effect of BDE-47 on superoxide production
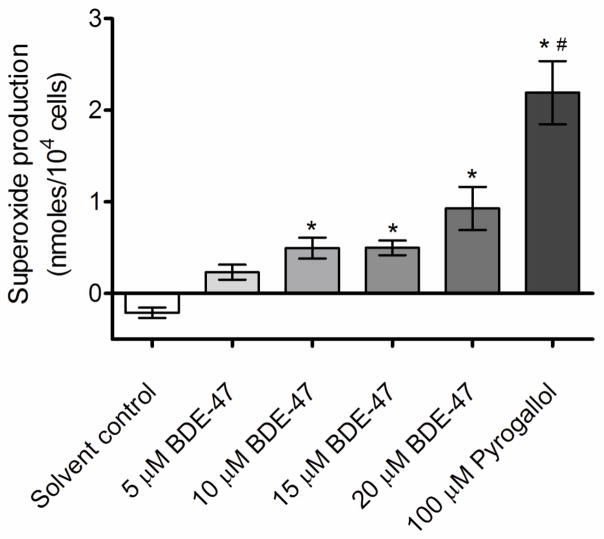
BDE-47 treatment increased production of superoxide in HTR-8/SVneo cell cultures as measured with the cytochrome c reduction assay (Figure 2; P<0.05). Specifically, superoxide production was 0.49, 0.5 and 0.93 nmoles/104 cells in cells exposed to 10, 15, and 20 μM BDE-47 for 1 h, significantly increased compared to the solvent control (Figure 2; P<0.05). Treatment with 100 μM pyrogallol, included as a positive control, increased ferricytochrome c reduction to 2.19 nmoles/104 cells, a significant increase compared to the solvent control and BDE-47-treated groups (Figure 2; P<0.05). The negative values detected in solvent controls with the cytochrome c reduction assay suggest oxidation of ferricytochrome c under our basal experimental conditions, as observed by others (Arthur et al., 1987).
Figure 2.
BDE-47-induced cytochrome c reduction, an index of superoxide anion production, in HTR-8/SVneo cells. HTR-8/SVneo cells were treated for 1 h with DMSO (solvent control) or BDE-47, then incubated with cytochrome c reaction buffer with or without SOD for 90 min. Bars represent the means of 3 independent experiments containing 6 replicates each ±SE. *P<0.05, significant compared to solvent control. #P<0.05, significant compared to 5, 10, 15, and 20 μM BDE-47.
Changes in mitochondrial membrane potential (MMP) by BDE-47 treatment
Because mitochondria are potential sources of cell-generated ROS, MMP was assessed by measuring fluorescence of Rh123, a dye specifically taken up by mitochondria in the normal polarized state. Treatment with 10, 15 and 20 μM BDE-47 significantly decreased Rh123 fluorescence compared to solvent controls at 4, 8 and 24 h (Figure 3; P<0.05), indicating decreased MMP. Reduction in Rh123 fluorescence ranged from 47% to 65%, but was neither concentration-dependent nor time-dependent. The decrease of MMP was significant compared to non-treated control, also, at 4 h with 20 μM BDE-47 and at 8 h with 10, 15, and 20 μM BDE-47 (Figure 3; P<0.05). Treatment with 5 μM BDE-47 did not significantly change Rh123 fluorescence at any time point up to 24 h. There were no statistically significant differences between non-treated controls and solvent controls at any time points.
Figure 3.
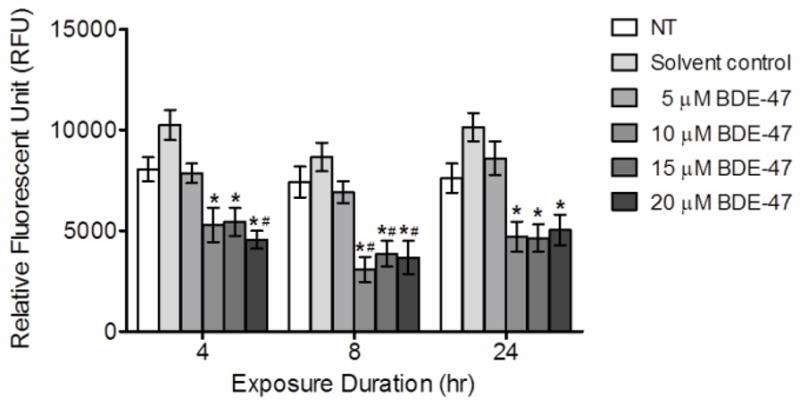
BDE-47 effects in HTR-8/SVneo cells on mitochondrial membrane potential (MMP). HTR-8/SVneo cells were treated for 4, 8 or 24 h with non-treated control (NT), DMSO (solvent control) or BDE-47, and then loaded with Rh123 for 1 h. Bars represent the means of 6 independent experiments containing 4 replicates each ±SE. *P<0.05, significant compared to solvent control within same time point.
Effect of BDE-47 on cytokine production
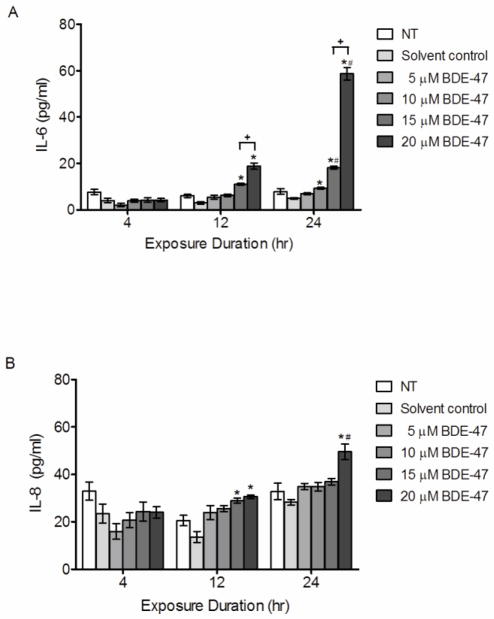
Because cytokines play critical roles in pregnancy (Keelan et al., 2003; Orsi, 2008), we investigated the effect of BDE-47 on IL-6 and IL-8 production in HTR-8/SVneo cells. BDE-47 treatment for 12 and 24 h stimulated concentration-dependent and time-dependent increases in IL-6 (Figure 4A; P<0.05) and time-dependent increases in IL-8 release (Figure 4B; P<0.05). Treatment with 15 or 20 μM BDE-47 significantly increased IL-6 3.7- fold and 6.3- fold at 12 h, and 3.7-fold and 12-fold at 24 h, respectively, relative to the solvent control (Figure 4A; P<0.05). After 24 h, the lower concentration of 10 μM BDE-47 also induced a significant 1.9-fold increase of IL-6 compared to the solvent control (Figure 4A; P<0.05). Moreover, 15 and 20 μM BDE-47 treatment increased IL-6 release in a time-dependent manner from 12 h to 24 h (Figure 4A; P<0.05). No statistically significant changes in IL-6 concentrations were observed with 4 h treatment at any BDE-47 concentration examined or with the lowest concentration evaluated, 5 μM BDE-47, at any time point. Pro-inflammatory chemokine IL-8 concentrations in the medium was significantly increased after a 12-h treatment with 15 and 20 μM BDE-47 by 2.1-fold and 2.3-fold, respectively, compared with solvent control (Figure 4B; P<0.05). In addition, 24-h treatment with 20 μM BDE-47 increased IL-8 release 1.8-fold compared with solvent control, to an average concentration that was significantly increased compared the average IL-8 concentration observed after 12 h of exposure to 20 μM BDE-47 (Figure 4B; P<0.05). No statistically significant changes in IL-8 concentrations were observed with 4-h treatment at any BDE-47 concentration examined or with 5 and 10 μM BDE-47 at any time point. There were no statistically significant differences between non-treated controls and solvent controls at any time point.
Figure 4.
BDE-47-induced pro-inflammatory cytokine production in HTR-8/SVneo cells. HTR-8/SVneo cells were untreated (NT, non-treated control), or treated for 4, 8 or 24 h with DMSO (solvent control) or BDE-47, and then concentrations of IL-6 (A) and IL-8 (B) in culture medium were quantified by EIA. Bars represent the means ± SE of 3 independent experiments containing 3 replicates each. *P<0.05, significant compared to solvent control within same time point. #P<0.05, significant compared to same treatment at 12 h. +P<0.05, significantly different from next lowest concentration within time point.
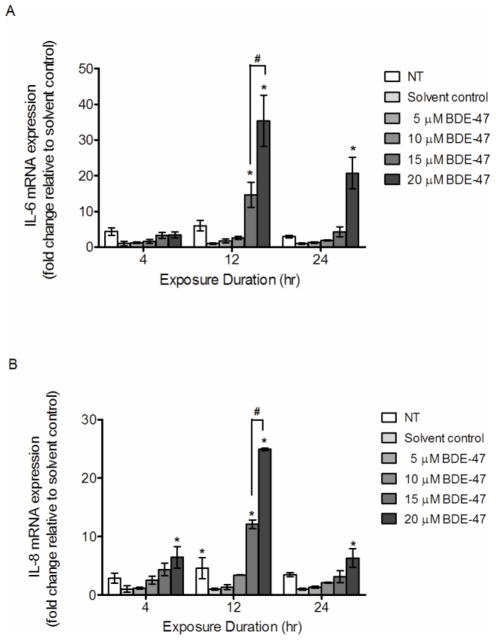
Effect of BDE-47 on mRNA expression of cytokines in HTR-8/SVneo cells
Expression of inflammatory cytokine genes in HTR-8/SVneo cells exposed to BDE-47 was quantified using real time qRT-PCR. Treatment with 15 and 20 μM BDE-47 for 12 h increased IL-6 mRNA expression by 14.7 fold and 35.4 fold, respectively, compared to the solvent control (Figure 5A; P<0.05). Treatment with 20 μM BDE-47 for 24 h increased IL-6 mRNA 20.1 fold compared to the solvent control (Figure 5A; P<0.05). Likewise, IL-8 expression increased with 20 μM BDE-47 after 4, 12 and 24-h exposures compared to solvent control (Figure 5B; P<0.05). IL-8 mRNA expression increased with 12-h exposure to 15 and 20 μM BDE-47 by 12.1 fold and 24.9 fold, respectively, compared with solvent control (Figure 5B; P<0.05). For both IL-6 and IL-8, concentration-dependent increases in mRNA expression were observed with 15 and 20 μM at 12 h only. Treatment with 5 and 10 BDE-47 did not result in significant changes in IL-6 and IL-8 mRNA expression at any time point. Treatment with DMSO (solvent control) suppressed IL-8 mRNA expression at 12 h compared to non-treated control (Figure 5B, P<0.05). There were no other statistically significant differences between non-treated controls and solvent controls for IL-6 and IL-8 mRNA expression.
Figure 5.
BDE-47 effects on mRNA expression of inflammatory cytokine genes in HTR-8/SVneo cells. HTR-8/SVneo cells were treated for 4, 8 or 24 h with non-treated control (NT), DMSO (solvent control) or BDE-47. The mRNA expression of IL-6 (A) and IL-8 (B) was quantified by qRT-PCR. Bars represent the means ± SE of 3 independent experiments containing 3 replicates each. *P<0.05, significant compared to solvent control within same time point. #P<0.05, significantly different from next lowest concentration within time point.
Effect of Antioxidant treatment on BDE-47-stimulated cytokine production in HTR-8/SVneo cells
To investigate the role of reactive oxygen species on BDE-47-mediated cytokine release in HTR-8/SVneo cells, cells were pretreated with 1 mM DFO for 1 h prior to BDE-47 treatment for 24 h, or co-treated with 20 μM (±)α-tocopherol for 24 h. As shown in Figure 6A, DFO pretreatment inhibited IL-6 release stimulated by 20 μM BDE-47 in HTR-8/SVneo cells, reducing IL-6 release by 54.1% compared to cultures exposed to BDE-47 without DFO pretreatment (Figure 6A; P<0.05). Although IL-6 concentrations in cultures pretreated with DFO prior to exposure to 15 μM BDE-47 were not significantly reduced compared with cultures exposed to 15 μM BDE-47 without DFO pretreatment, they also were not statistically significantly different from the non-treated or solvent controls. Similar to DFO, (±)α-tocopherol and tempol co-treatment for 24 h resulted in 56.3% (relative to No Vehicle Control group, Figure 6B; P<0.05) and 37.7% (Figure 6C; P<0.05) reduction in BDE-47-mediated IL-6 production in HTR-8/SVneo cells. In the absence of (±)α-tocopherol cotreatment, a significant reduction in IL-6 release was observed for cultures exposed to DMSO (0.7 % v/v) as the solvent control group for BDE-47 treatments compared to non-treated controls (Fig. 6B; P<0.05); however, there were no statistically significant differences between (DMSO) solvent and non-treated controls that also received the vehicle (DMSO 0.02% v/v) used with (±)α-tocopherol cotreatment (Fig. 6B). There were no significant changes in IL-8 production from HTR-8/SVneo cells with DFO pretreatment, (±)α-tocopherol co-treatment, or tempol co-treatment (data not shown).
Figure 6.
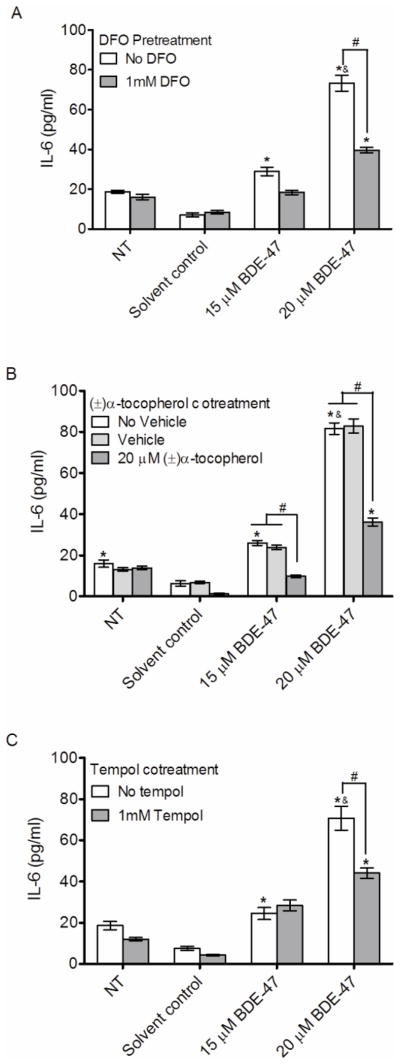
Effects of antioxidant treatments on BDE-47-stimulated IL-6 release in HTR-8/SVneo cells. HTR-8/SVneo cells receiving antioxidant treatments were pretreated with DFO for prior to exposure to had no further treatment (non-treated control, NT), or were exposed to DMSO (solvent control), 15 μM BDE-47 or 20 μM BDE-47, or were co-treated with (±)-α-tocopherol or tempol. A) Effects of 1-h pretreatment with DFO on BDE-47-stimulated IL-6 release. B) Effects of (±)-α-tocopherol cotreatment on BDE-47-stimulated IL-6 release; vehicle controls were exposed to DMSO (used to deliver (±)-α-tocopherol, 0.02% v/v) and additional controls received no vehicle. C) Effects of tempol cotreatment on BDE-47-stimulated IL-6 release. Bars represent the means ± SE of 3 independent experiments containing 3 replicates each. *P<0.05, significant compared to respective solvent controls. &P<0.05, significant compared to 15 μM BDE-47-treated group. #P<0.05, significantly different from each other.
DISCUSSION
Due to widespread use as flame-retardants in household and commercial products, human exposure to PBDEs increased exponentially over recent decades (Hites, 2004). Analysis of 2,062 human serum samples from the NHANES 2003–2004 detected BDE-47 in nearly all participants, with BDE-47 having the highest concentration of the PBDE congeners measured (Sjodin et al., 2008). Despite BDE-47 presence in human placental tissues (Frederiksen et al., 2009; Miller et al., 2009) and the importance of cytokine regulation in placental development during early pregnancy (Anton et al., 2012), there are no previous reports of BDE-47-stimulated effects on inflammatory pathways in human first trimester placental cells. Moreover, we identified only a single previous report on PBDE-stimulated cytokine release in placenta with results showing that pre-exposure of second trimester human placental explant cultures to a PBDE mixture of congers 47, 99 and 100 enhanced placental proinflammatory response to heat-killed E. Coli (Peltier et al., 2012). The present study is distinct from the latter study in that we showed direct stimulation of proinflammatory cytokines in the absence of pathogen exposure in a first trimester human extravillous trophoblast cell line treated with a single BDE congener.
Oxidative stress has been suggested to play a role in human pregnancy-related disorders, such as preterm labor, preeclampsia, and IUGR (Agarwal et al., 2012). The present study provides new information that BDE-47 increased ROS generation in the human trophoblast cell line HTR-8/SVneo. Moreover, BDE-47 decreased mitochondrial membrane potential, indicating mitochondrial dysfunction (Brand and Nicholls, 2011). Because mitochondrial defects can lead to enhanced mitochondrial production of ROS and superoxide is a major type of ROS generated by mitochondrial respiration (Sohal et al., 1995), we suggest that the BDE-47-stimulated superoxide production in HTR-8/SVneo cells may have originated from mitochondria. Superoxide also acts as a precursor for formation of other types of ROS (Al-Gubory et al., 2010). As such, the increased DCF fluorescence observed with BDE-47 could be explained by subsequent formation of peroxyl, hydroxyl radical and other ROS from mitochondrial superoxide. However, our findings are correlative only and require further research to confirm the present findings and to elucidate our mechanistic hypothesis.
Similar to our results with HTR-8/SVneo cells, BDE-47-stimulates intracellular ROS formation in rat neuronal cells (He et al., 2008), Jurkat cells (Yan et al., 2011), human fetal liver hematopoietic stem cells (Shao et al., 2008), and human neutrophil granulocytes (Reistad and Mariussen, 2005a). In contrast to previously reported studies that BDE-47 induces apoptosis in Jurkat cells (Yan et al., 2011) and human fetal liver hematopoietic stem cells (Shao et al., 2008), we observed no significant loss of cell viability in HTR-8/SVneo cells after a 24-h treatment with BDE-47 (Supplementary Figure 1). A possible explanation for these inconsistencies is that the range of BDE-47 concentrations used in our study (5–20 μM) was much lower than concentration ranges used in studies reporting BDE-47-induced cytotoxicity and/or a mitochondrial membrane potential reduction: 25–100 μM (Yan et al., 2011) and 50 μM (Shao et al., 2008). Moreover, different types of cells (human trophoblasts versus rat neuronal cells, Jurkat cells, human fetal liver hematopoietic stem cells, or human neutrophil granulocytes) and experimental conditions (media, serum concentration, exposure duration, cell density, etc.) may generate divergent responses to the same chemical. Notably, our study provides the first evidence that BDE-47 decreases mitochondrial membrane potential at lower concentrations of BDE-47 (10, 15 and 20 μM) than reported previously.
Treatment with BDE-47 reduced mitochondrial membrane potential, though the observed reduction was not concentration-dependent for the BDE-47 concentration range examined (10, 15, and 20 μM). The lack of a concentration-dependent reduction in mitochondrial membrane potential could be explained by the narrow range of BDE-47 concentrations in our studies, especially if the sensitivity of Rh123 may not be sufficient to detect the differences in mitochondrial membrane potential within this range of concentrations. Similar to our findings, Yan et al. (2011) did not show a concentration-dependent decrease in mitochondrial membrane potential in BDE-47-treated Jurkat cells with 5, 10 and 25 μM BDE-47 using Rh123, although a decreasing trend was observed as concentrations increased up to 100 μM. Shao et al.2008 also reported a decrease in mitochondrial membrane potential in human fetal liver hematopoietic stem cells at 12.5 and 50 μM using another mitochondria-specific dye, JC-1, but did not show a significant concentration-dependent reduction, either. Further study using an expanded range of BDE-47 concentrations will be needed to clarify reasons for the lack of a concentration-dependent effect on the mitochondrial membrane response.
Little is known about interactions of BDE-47 with the innate immune response. It was recently reported that BDE-47-pretreatment of peripheral blood mononuclear cells from children with autism spectrum disorders exhibit divergent LPS-stimulated innate cytokine responses compared with age-matched controls (Ashwood et al., 2009). Peltier et al. (2012) reported that pre-exposure of placental explants to a PBDE mixture of congers 47, 99 and 100 enhanced placental proinflammatory response to heat-killed E. Coli, with increased IL-1β and reduced IL-10 production. In the latter study by Peltier et al.2012, however, PBDE treatment alone did not stimulate proinflammatory cytokine production in placental explant cultures. We observed that BDE-47-stimulated cytokine mRNA expression increased for IL-8 as early as 4 h with 20 μM exposure, then peaked 12 h after initiating exposure, the only time point in our study where we observed significantly increased IL-6 and IL-8 mRNA expression at the lower concentration of 15 μM, also. Compared with mRNA expression, cytokine protein release lagged temporally, with initial increases after 12 h and further increases after 24 of exposure to BDE-47. The lowest effective concentration for cytokine response was observed with 10 μM BDE-47-stimulated increase of IL-6 protein after 24 h of exposure. As such, our study is the first to report direct effect of BDE-47 on regulation of cytokine production in human placental cells, showing that BDE-47 increased proinflammatory IL-8 and IL-6 in HTR-8/SVneo cells at the transcriptional and protein levels. Moreover, the BDE-47-stimulated increased mRNA levels of IL-6 and IL-8, suggesting that activation of transcription contributes to the overall increased cytokine expression. Because the fold change in cytokine protein production was modest compared to the fold change in cytokine mRNA expression, additional post-transcriptional mechanisms may modulate protein expression (Griesinger et al., 2001; Wang et al., 2011; Ye et al., 2011).
Inflammation within the gestational compartment may lead to impaired trophoblast cellular function, contributing to the placental dysfunction seen in pregnancy-related disorders. Histologic examination found evidence of localized inflammation (histologic chorioamnionitis) in 85% of placentae from spontaneous preterm births delivered at 28 weeks gestation (Yoon et al., 2000) with higher rates of placental ischemia and abnormal placentation compared with controls at term (Germain et al., 1999; Kim et al., 2003). A recent study reported that LPS increases production of IL-8 and IL-6 and decreases invasion activity in HTR-8/SVneo cells (Anton et al., 2012). Our study showed that exposure to BDE-47 stimulated pro-inflammatory IL-6 and IL-8 production in HTR-8/SVneo cells, suggesting that BDE-47 could potentially impair normal trophoblast cellular function and invasion. However, we did not measure BDE-47 effects on trophoblast invasion, and further investigation is needed to ascertain the potential relevance of BDE-47 exposure to placental function and pregnancy.
It has been reported that ROS can regulate signal transduction pathways in mammalian cells as second messengers (Khan and Wilson, 1995). Our study clearly showed the novel finding that ROS play a role in activation of BDE-47-mediated inflammatory response in HTR-8/SVneo cells. Three different antioxidant treatments suppressed BDE-47-stimulated IL-6 production in HTR-8/SVneo cells. Although mechanisms of regulation of inflammatory response by ROS are not fully understood, involvement of the redox-sensitive transcription factor NF-κB has been implicated (Reuter et al., 2010). NF-κB plays a crucial role in immune and inflammatory response, regulating gene expression of a large number of genes, including cytokines, growth factors, adhesion molecules, immunoreceptors, and acute-phase proteins (Blackwell and Christman, 1997). Several lines of evidence suggest a role for ROS in activation of NF-κB, and antioxidants inhibit NF-κB activation in vitro and in vivo (Blackwell and Christman, 1997). Moreover, NF-κB plays a crucial role in the transcription of IL-6 and IL-8 (Blackwell and Christman, 1997; Reuter et al., 2010). Although we did not assess BDE-47-induced NF-κB activation in HTR-8/SVneo cells, we speculate that NF-κB may be involved in BDE-47-stimulated cytokine production in HTR-8/SVneo cells because both cytokines were notably increased with BDE-47 treatment.
The mechanisms of PBDE toxicity have not been fully resolved. Because of the similar chemical structures of PBDEs and their metabolites to thyroid hormones, polychlorinated biphenyls (PCBs), and 2,3,7,8-tetra- chlorodibenzo-p-dioxin (TCDD) (Ren and Guo, 2013), other studies have focused on PBDEs’ toxic effects through nuclear hormone receptor (NR) mediated pathways involving thyroid hormone receptor (TR), estrogen receptor (ER), aryl hydrocarbon receptor (AhR), androgen receptor (AR) and progesterone receptor (PR) (Ibhazehiebo et al., 2011; Kojima et al., 2009; Mercado-Feliciano and Bigsby, 2008) (Liu et al., 2011; Ren and Guo, 2013; Stoker et al., 2005). For example, hydroxylated BDE-47 metabolites showed modest binding potency with rat TR (Kitamura et al., 2008) whereas BDE-199, 153, 154, 209 and DE-71 (a commercial PBDE mixture) showed antagonistic activity for TRβ in CV-1 monkey fibroblast-derived cells (Ibhazehiebo et al., 2011). BDE-47 showed agonistic activities in the ERα and ERβ by the ER-CALUX assay using Chinese hamster ovary cells (Kojima et al., 2009). Chen et al. (2001) reported that 18 PBDE congeners and 3 commercial mixtures bound to rat hepatic AhR with affinities 10−2 to 10−5 times that of TCDD. Anti-androgenic activity of PBDEs also has been observed (Kojima et al., 2009; Liu et al., 2011). Although these studies suggest potential roles of NRs on endocrine disruption by PBDEs, the contribution of NR activation to BDE-47-stimulated inflammatory responses in the present study is not clear. It was recently suggested that NRs regulate inflammatory pathways by altering the turnover or recruitment of co-repressors and co-activators in a gene-specific manner. These NR-dependent trans-repression pathways may play roles in controlling the initiation, magnitude and duration of pro-inflammatory gene expression (Glass and Saijo, 2010; Huang and Glass, 2010). However, further study on the biological mechanisms of action of PBDEs is warranted to investigate roles of potential receptors and mediators in the activation and regulation of BDE-47 mediated inflammation in human placental cells.
In the present study, we used DMSO at a final concentration of 0.7% to deliver BDE-47 to the cell cultures. Although previous reports used lower DMSO concentrations to deliver similar or higher concentrations of BDE-47 to cell cultures(Shao et al., 2008; Yan et al., 2011), we found that BDE-47 precipitated out over time in cultures at final DMSO concentrations below 0.7% in our laboratory. DMSO is widely used as a vehicle for hydrophobic pharmaceutical agents in biomedical research. In preliminary experiments, we evaluated 1,4-dioxane as an alternative vehicle for BDE-47, but decided on using DMSO because BDE-47 had better solubility in DMSO and because DMSO is more commonly used in cell culture studies with PBDEs. However, DMSO’s reported antioxidant and anti-inflammatory properties raise cautions when interpreting results from studies using DMSO as a solvent. It has been reported that DMSO (≥1% v/v) is a strong antioxidant that scavenges hydroxyl free radicals (Bektasoglu et al., 2006; Halliwell et al., 1987; Panganamala et al., 1976), and reduces production of hydroxyl radicals, lipid peroxidation, and protein carbonyl formation (Sanmartin-Suarez et al., 2011). DMSO also exhibits anti-inflammatory properties by inhibiting secretion and/or mRNA expression of pro-inflammatory mediators such as TNF-α, IL-6, and IL-8, by decreasing prostaglandin E2 production related to COX-2 activity, and by reducing NF-κB activation in in vivo and in vitro (Chang et al., 1999; DeForge et al., 1992; Hollebeeck et al., 2011; Kelly et al., 1994; Nakamuta et al., 2001). Although means of solvent controls and non-treated controls had divergent values in most experiments in the present study, statistically significant differences between these control groups were detected only in experiments measuring IL-8 mRNA expression and IL-6 protein. Preliminary data prior to this study showed that treatment of HTR-8/SVneo cells with higher DMSO concentrations (0.75–1%) suppressed BDE-47-stimulated IL-6 release in a concentration-dependent manner (Supplementary Figure 2). Because DMSO effects were in the opposite direction than the observed BDE-47-stimulated effects, it is likely that any confounding by DMSO on IL-6 release would result in muting the IL-6 response, making it more difficult to observe significant increases. We clearly show that BDE-47 stimulated ROS overproduction and inflammatory responses in HTR-8/SVneo cells, outweighing possible opposing DMSO effects. We suggest that the DMSO effects on cytokine production are due to its radical scavenger or anti-inflammatory properties. However, it is out of the scope of the present work to clarify the mode of actions of DMSO in cytokine production because DMSO was used purely as a vehicle control in this investigation.
A limitation of our study is that overproduction of IL-6 and IL-8 alone may not accurately represent the proinflammatory response and the possible impact of BDE-47 exposure on trophoblast cellular function in vivo. Although changes in cytokine levels by an activated immune response play an important role in regulating trophoblast function, there are complex interactions between cytokines and trophoblast invasion involving other pro- and anti-invasive factors, such as other cytokines/chemokines, integrins, and adhesion and proteolytic molecules (Anton et al., 2012).
The concentrations of BDE-47 in this study are several orders of magnitude higher (100-fold or more) than the median concentrations reported previously in utero and placenta: 337 – 21842 pg/ml in amnionic fluid (Miller et al., 2012), 0.11 – 3000 ng/g lipid in placentae (Doucet et al., 2009; Frederiksen et al., 2009), and 0.46 to 504 ng/g lipid in umbilical cord blood (Frederiksen et al., 2009; Guvenius et al., 2003; Wu et al., 2010). However, PBDE concentrations vary markedly among samples and can differ by three orders of magnitude, possibly due to factors such as proximity to the source of contamination, length of exposure, individual life style, occupation, nutritional status, absorption, metabolism, and excretion (Athanasiadou et al., 2008; Doucet et al., 2009; Gill et al., 2004; Stapleton et al., 2008). Taking this variability of exposure into consideration and assuming that the lipid content in placentae is 1.31%, the concentrations of PBDEs in placentae can be as high as ~8 μM (Doucet et al., 2009). Moreover, correcting for adsorption onto plastic, estimated at 73% (Barber et al., 2006; Mundy et al., 2004), the corrected concentrations of BDE-47 in culture medium in this study range from 1.34 to 5.4 μM. In addition, we have not examined the effect of multiple or chronic exposures to PBDEs, and it is unlikely for people to be exposed to a single PBDE congener like BDE-47 (Shao et al., 2008). Moreover, some PBDE congeners showed the ability to act synergistically when combined (Tagliaferri et al., 2010) or with other toxicants (Fischer et al., 2008; Pellacani et al., 2012). Ongoing research in our laboratory on the effects of other prevalent PBDE congeners, such as BDE-49 and 99, will lead us toward a better understanding of the mechanisms and relevant risks associated with PBDE exposures in gestational compartments. Moreover, ongoing analysis of gene expression array data is expected to guide us in future experiments to elucidate the mechanisms of BDE-47-stimulated ROS formation and cytokine production.
In summary, BDE-47, a predominant flame retardant chemical found in human tissues, activates proinflammatory responses in human first trimester EVTs. Our results provide evidence of altered mitochondrial membrane potential, enhanced production of ROS, and enhanced production of the proinflammatory cytokines IL-6 and IL-8 stimulated by BDE-47 in human placental cells. The inhibition of stimulated release of IL-6 by a variety of antioxidant treatments implicates the involvement of ROS in regulation of cytokine production in HTR-8/SVneo cells. This is the first study to show that BDE-47 activates proinflammatory pathways in human first trimester EVTs and to link PBDE-stimulated pro-inflammatory responses with ROS. Because proper trophoblast function is necessary for placental development and successful pregnancy, further investigation of the impact of BDE-47 on trophoblast function is warranted.
Supplementary Material
Supplementary Figure 1. BDE-47 effects in HTR-8/SVneo cells on cytotoxicity and cell viability.
Supplementary Figure 2. DMSO effects in HTR-8/SVneo cells on BDE-47-stimulated IL-6 release.
Highlights.
BDE-47 induced ROS overproduction and mitochondrial dysfunction.
BDE-47 stimulated production of proinflammatory cytokines.
Antioxidant treatment reduced BDE-47-stimulated ROS generation and cytokine release.
Acknowledgments
We thank the University of Michigan’s Immunology Core for its assistance with cytokine ELISA analysis. This work was supported by a grant to RLC (R01 ES014860) and the Center for Lifestage Exposure and Adult Disease (P30 ES017885) from the National Institute of Environmental Health Sciences (NIEHS).
Footnotes
Conflict of interest statement
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42(10):1634–50. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Anton L, Brown AG, Parry S, Elovitz MA. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: Possible mechanisms of first trimester placental dysfunction. Hum Reprod. 2012;27(1):61–72. doi: 10.1093/humrep/der362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur MJ, Kowalski-Saunders P, Gurney S, Tolcher R, Bull FG, Wright R. Reduction of ferricytochrome c may underestimate superoxide production by monocytes. J Immunol Methods. 1987;98(1):63–9. doi: 10.1016/0022-1759(87)90436-4. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Schauer J, Pessah IN, Van de Water J. Preliminary evidence of the in vitro effects of bde-47 on innate immune responses in children with autism spectrum disorders. Journal of neuroimmunology. 2009;208(1–2):130–5. doi: 10.1016/j.jneuroim.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadou M, Cuadra SN, Marsh G, Bergman A, Jakobsson K. Polybrominated diphenyl ethers (pbdes) and bioaccumulative hydroxylated pbde metabolites in young humans from managua, nicaragua. Environ Health Perspect. 2008;116(3):400–8. doi: 10.1289/ehp.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber JL, Walsh MJ, Hewitt R, Jones KC, Martin FL. Low-dose treatment with polybrominated diphenyl ethers (pbdes) induce altered characteristics in mcf-7 cells. Mutagenesis. 2006;21(5):351–60. doi: 10.1093/mutage/gel038. [DOI] [PubMed] [Google Scholar]
- Bektasoglu B, Esin Celik S, Ozyurek M, Guclu K, Apak R. Novel hydroxyl radical scavenging antioxidant activity assay for water-soluble antioxidants using a modified cuprac method. Biochem Biophys Res Commun. 2006;345(3):1194–200. doi: 10.1016/j.bbrc.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Christman JW. The role of nuclear factor-kappa b in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17(1):3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- Boota A, Zar H, Kim YM, Johnson B, Pitt B, Davies P. Il-1 beta stimulates superoxide and delayed peroxynitrite production by pulmonary vascular smooth muscle cells. Am J Physiol. 1996;271(6 Pt 1):L932–8. doi: 10.1152/ajplung.1996.271.6.L932. [DOI] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435(2):297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin WJ, Kirk HD, Zimmer MA. Teratogenic evaluation of a polybromodiphenyl oxide mixture in new zealand white rabbits following oral exposure. Fundamental and applied toxicology: official journal of the Society of Toxicology. 1989;12(1):151–7. doi: 10.1016/0272-0590(89)90070-5. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of n-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. American journal of obstetrics and gynecology. 2003;188(1):203–8. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- Campbell A. Inflammation, neurodegenerative diseases, and environmental exposures. Ann N Y Acad Sci. 2004;1035:117–32. doi: 10.1196/annals.1332.008. [DOI] [PubMed] [Google Scholar]
- Chang CK, Llanes S, Schumer W. Inhibitory effect of dimethyl sulfoxide on nuclear factor-kappa b activation and intercellular adhesion molecule 1 gene expression in septic rats. J Surg Res. 1999;82(2):294–9. doi: 10.1006/jsre.1998.5527. [DOI] [PubMed] [Google Scholar]
- Chen G, Konstantinov AD, Chittim BG, Joyce EM, Bols NC, Bunce NJ. Synthesis of polybrominated diphenyl ethers and their capacity to induce cyp1a by the ah receptor mediated pathway. Environmental science & technology. 2001;35(18):3749–56. doi: 10.1021/es0107475. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, Burton GJ, Charnock-Jones DS. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. The American journal of pathology. 2007;171(4):1168–79. doi: 10.2353/ajpath.2007.070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge LE, Fantone JC, Kenney JS, Remick DG. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest. 1992;90(5):2123–9. doi: 10.1172/JCI116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet J, Tague B, Arnold DL, Cooke GM, Hayward S, Goodyer CG. Persistent organic pollutant residues in human fetal liver and placenta from greater montreal, quebec: A longitudinal study from 1998 through 2006. Environ Health Perspect. 2009;117(4):605–10. doi: 10.1289/ehp.0800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Fredriksson A, Eriksson P. Coexposure of neonatal mice to a flame retardant pbde 99 (2,2′,4,4′,5-pentabromodiphenyl ether) and methyl mercury enhances developmental neurotoxic defects. Toxicol Sci. 2008;101(2):275–85. doi: 10.1093/toxsci/kfm271. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to pbdes--a review of levels and sources. Int J Hyg Environ Health. 2009;212(2):109–34. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: Placental pathology and clinical correlation. Obstet Gynecol. 1999;94(2):284–9. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- Gill U, Chu I, Ryan JJ, Feeley M. Polybrominated diphenyl ethers: Human tissue levels and toxicology. Rev Environ Contam Toxicol. 2004;183:55–97. doi: 10.1007/978-1-4419-9100-3_3. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and t cells. Nat Rev Immunol. 2010;10(5):365–76. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Goepfert AR, Ramsey PS. Biochemical markers for the prediction of preterm birth. American journal of obstetrics and gynecology. 2005;192(5 Suppl):S36–46. doi: 10.1016/j.ajog.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Griesinger G, Saleh L, Bauer S, Husslein P, Knofler M. Production of pro- and anti-inflammatory cytokines of human placental trophoblasts in response to pathogenic bacteria. Journal of the Society for Gynecologic Investigation. 2001;8(6):334–40. [PubMed] [Google Scholar]
- Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111(9):1235–41. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165(1):215–9. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- He P, He W, Wang A, Xia T, Xu B, Zhang M, Chen X. Pbde-47-induced oxidative stress, DNA damage and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology. 2008;29(1):124–9. doi: 10.1016/j.neuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environmental science & technology. 2004;38(4):945–56. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Hollebeeck S, Raas T, Piront N, Schneider YJ, Toussaint O, Larondelle Y, During A. Dimethyl sulfoxide (dmso) attenuates the inflammatory response in the in vitro intestinal caco-2 cell model. Toxicol Lett. 2011;206(3):268–75. doi: 10.1016/j.toxlet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Huang W, Glass CK. Nuclear receptors and inflammation control: Molecular mechanisms and pathophysiological relevance. Arterioscler Thromb Vasc Biol. 2010;30(8):1542–9. doi: 10.1161/ATVBAHA.109.191189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, Koibuchi N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect. 2011;119(2):168–75. doi: 10.1289/ehp.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Hill MR, Youkhana K, Wanker F, Gimble JM. Dimethyl sulfoxide modulates nf-kappa b and cytokine activation in lipopolysaccharide-treated murine macrophages. Infect Immun. 1994;62(8):3122–8. doi: 10.1128/iai.62.8.3122-3128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AU, Wilson T. Reactive oxygen species as cellular messengers. Chem Biol. 1995;2(7):437–45. doi: 10.1016/1074-5521(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189(4):1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Shinohara S, Iwase E, Sugihara K, Uramaru N, Shigematsu H, Fujimoto N, Ohta S. Affinity for thyroid hormone and estrogen receptors of hydroxylated polybrominated diphenyl ethers. Journal of Health Science. 2008;54(5):607–614. [Google Scholar]
- Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using chinese hamster ovary cells. Environ Health Perspect. 2009;117(8):1210–8. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Lee IT, Yang YL, Lee CW, Kou YR, Yang CM. Induction of cox-2/pge(2)/il-6 is crucial for cigarette smoke extract-induced airway inflammation: Role of tlr4-dependent nadph oxidase activation. Free Radic Biol Med. 2010;48(2):240–54. doi: 10.1016/j.freeradbiomed.2009.10.047. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu W, Sun H, Shen O, Wang X, Lam MH, Giesy JP, Zhang X, Yu H. In vitro profiling of endocrine disrupting potency of 2,2′,4,4′-tetrabromodiphenyl ether (bde47) and related hydroxylated analogs (ho-pbdes) Mar Pollut Bull. 2011;63(5–12):287–96. doi: 10.1016/j.marpolbul.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Mercado-Feliciano M, Bigsby RM. Hydroxylated metabolites of the polybrominated diphenyl ether mixture de-71 are weak estrogen receptor-alpha ligands. Environ Health Perspect. 2008;116(10):1315–21. doi: 10.1289/ehp.11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Batterman S, Loch-Caruso R. Polybrominated diphenyl ethers in human gestational membranes from women in southeast michigan. Environmental science & technology. 2009;43(9):3042–6. doi: 10.1021/es8032764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Chernyak SM, Domino SE, Batterman SA, Loch-Caruso R. Concentrations and speciation of polybrominated diphenyl ethers in human amniotic fluid. Sci Total Environ. 2012;417–418:294–8. doi: 10.1016/j.scitotenv.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy WR, Freudenrich TM, Crofton KM, DeVito MJ. Accumulation of pbde-47 in primary cultures of rat neocortical cells. Toxicol Sci. 2004;82(1):164–9. doi: 10.1093/toxsci/kfh239. [DOI] [PubMed] [Google Scholar]
- Nakamuta M, Ohta S, Tada S, Tsuruta S, Sugimoto R, Kotoh K, Kato M, Nakashima Y, Enjoji M, Nawata H. Dimethyl sulfoxide inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Int J Mol Med. 2001;8(5):553–60. doi: 10.3892/ijmm.8.5.553. [DOI] [PubMed] [Google Scholar]
- Orsi NM. Cytokine networks in the establishment and maintenance of pregnancy. Hum Fertil (Camb) 2008;11(4):222–30. doi: 10.1080/14647270802206879. [DOI] [PubMed] [Google Scholar]
- Panganamala RV, Sharma HM, Heikkila RE, Geer JC, Cornwell DG. Role of hydroxyl radical scavengers dimethyl sulfoxide, alcohols and methional in the inhibition of prostaglandin biosynthesis. Prostaglandins. 1976;11(4):599–607. doi: 10.1016/0090-6980(76)90063-0. [DOI] [PubMed] [Google Scholar]
- Pellacani C, Tagliaferri S, Caglieri A, Goldoni M, Giordano G, Mutti A, Costa LG. Synergistic interactions between pbdes and pcbs in human neuroblastoma cells. Environ Toxicol. 2012 doi: 10.1002/tox.21768. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Klimova NG, Arita Y, Gurzenda EM, Murthy A, Chawala K, Lerner V, Richardson J, Hanna N. Polybrominated diphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta. 2012;33(9):745–9. doi: 10.1016/j.placenta.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reistad T, Mariussen E. A commercial mixture of the brominated flame retardant pentabrominated diphenyl ether (de-71) induces respiratory burst in human neutrophil granulocytes in vitro. Toxicol Sci. 2005a;87(1):57–65. doi: 10.1093/toxsci/kfi222. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E. A commercial mixture of the brominated flame retardant pentabrominated diphenyl ether (de-71) induces respiratory burst in human neutrophil granulocytes in vitro. Toxicological sciences: an official journal of the Society of Toxicology. 2005b;87(1):57–65. doi: 10.1093/toxsci/kfi222. [DOI] [PubMed] [Google Scholar]
- Ren XM, Guo LH. Molecular toxicology of polybrominated diphenyl ethers: Nuclear hormone receptor mediated pathways. Environ Sci Process Impacts. 2013;15(4):702–8. doi: 10.1039/c3em00023k. [DOI] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49(11):1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartin-Suarez C, Soto-Otero R, Sanchez-Sellero I, Mendez-Alvarez E. Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. J Pharmacol Toxicol Methods. 2011;63(2):209–15. doi: 10.1016/j.vascn.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Shao J, White CC, Dabrowski MJ, Kavanagh TJ, Eckert ML, Gallagher EP. The role of mitochondrial and oxidative injury in bde 47 toxicity to human fetal liver hematopoietic stem cells. Toxicol Sci. 2008;101(1):81–90. doi: 10.1093/toxsci/kfm256. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG., Jr Serum concentrations of polybrominated diphenyl ethers (pbdes) and polybrominated biphenyl (pbb) in the united states population: 2003–2004. Environmental science & technology. 2008;42(4):1377–84. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH, Orr WC. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative damage, and longevity in different species of flies. Free Radic Biol Med. 1995;19(4):499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Sjodin A, Jones RS, Niehuser S, Zhang Y, Patterson DG., Jr Serum levels of polybrominated diphenyl ethers (pbdes) in foam recyclers and carpet installers working in the united states. Environmental science & technology. 2008;42(9):3453–8. doi: 10.1021/es7028813. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of de-71, a commercial polybrominated diphenyl ether (pbde) mixture. Toxicol Appl Pharmacol. 2005;207(1):78–88. doi: 10.1016/j.taap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Tagliaferri S, Caglieri A, Goldoni M, Pinelli S, Alinovi R, Poli D, Pellacani C, Giordano G, Mutti A, Costa LG. Low concentrations of the brominated flame retardants bde-47 and bde-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicol In Vitro. 2010;24(1):116–22. doi: 10.1016/j.tiv.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Tetz LM, Kamau PW, Cheng AA, Meeker JD, Loch-Caruso R. Troubleshooting the dichlorofluorescein assay to avoid artifacts in measurement of toxicant-stimulated cellular production of reactive oxidant species. J Pharmacol Toxicol Methods. 2013;67(2):56–60. doi: 10.1016/j.vascn.2013.01.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. Elevated c-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59(1):29–37. doi: 10.1016/s0165-0378(02)00085-2. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency; Office of Pollution Prevention & Toxics, editor Polybrominated diphenyl ethers (pbdes) project plan. 2006. [Google Scholar]
- Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102(1):20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- Wang F, Liu W, Jin Y, Dai J, Zhao H, Xie Q, Liu X, Yu W, Ma J. Interaction of pfos and bde-47 co-exposure on thyroid hormone levels and th-related gene and protein expression in developing rat brains. Toxicol Sci. 2011;121(2):279–91. doi: 10.1093/toxsci/kfr068. [DOI] [PubMed] [Google Scholar]
- Wu K, Xu X, Liu J, Guo Y, Li Y, Huo X. Polybrominated diphenyl ethers in umbilical cord blood and relevant factors in neonates from guiyu, china. Environmental science & technology. 2010;44(2):813–9. doi: 10.1021/es9024518. [DOI] [PubMed] [Google Scholar]
- Yan C, Huang D, Zhang Y. The involvement of ros overproduction and mitochondrial dysfunction in pbde-47-induced apoptosis on jurkat cells. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie. 2011;63(5):413–7. doi: 10.1016/j.etp.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Ye RR, Lei EN, Lam MH, Chan AK, Bo J, van de Merwe JP, Fong AC, Yang MM, Lee JS, Segner HE, Wong CK, Wu RS, Au DW. Gender-specific modulation of immune system complement gene expression in marine medaka oryzias melastigma following dietary exposure of bde-47. Environ Sci Pollut Res Int. 2011;19(7):2477–87. doi: 10.1007/s11356-012-0887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Park JS, Kim MS, Oh SY, Kim CJ, Jun J. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. American journal of obstetrics and gynecology. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. BDE-47 effects in HTR-8/SVneo cells on cytotoxicity and cell viability.
Supplementary Figure 2. DMSO effects in HTR-8/SVneo cells on BDE-47-stimulated IL-6 release.