Abstract
Magnetic resonance imaging (MRI) is used to evaluate gastrointestinal (GI) structure and functions in humans. Despite filling the viscus lumen with a contrast agent, visualization of the viscus wall is limited. To overcome this limitation, we de novo synthesized a conjugate that covalently combines a Gd-based MRI contrast agent, encaged with a chelating agent (DOTA), with pantoprazole, which is a widely used proton pump inhibitor that binds to proton pumps in the stomach and colon. The DOTA linkage was installed at a mechanism-based strategic location in the pantoprazole molecule to minimize a possible negative effect of the structural modification on the drug. It is anticipated that by defining the wall of the stomach and colon, this compound will facilitate functional MRI of the GI tract in humans.
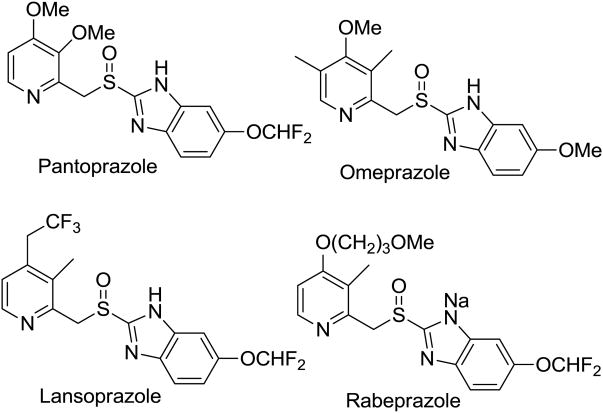
The role of membrane proteins as transporters and mediators of cellular interaction with environment make them excellent targets for drug targeting. These enzymes, located in the cellular lipid bilayer, exert their biological effects through active transport mechanisms that involve pumping specific cations through the cell.1-3 This cation flux is crucial for cellular control of electrochemical gradients and cell homeostasis. Some of these proton pump inhibitors (PPIs), such as gastric H+,K+-ATPase inhibitors, exemplified by pantoprazole, omeprazole, lansoprazole and rabeprazole–Figure 1) are used to treat peptic ulcer and gastroesophageal reflux disease.4
Figure 1. Prototypes of H+,K+-ATPase inhibitors.
In conditions such as functional dyspepsia and gastroparesis, disturbances of gastric motility can accelerate or delay gastric emptying.5 Existing techniques to assess stomach functions are limited by radiation exposure (e.g., scintigraphy), invasiveness (e.g., gastroduodenal motility), and limited spatial resolution. Research studies suggest that MRI can measure gastric volumes and motility in humans.6,7,10
Moreover, for the first time dynamic MRI identified increased gastric motility in patients with rapid gastric emptying.7
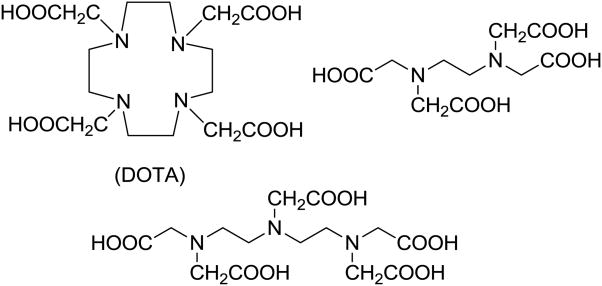
However, segmenting the stomach from surrounding structures on MR images is a manual, time-consuming, and rate-limiting process. To overcome this limitation and facilitate visualization of the gastric wall, we sought to develop a novel gadolinium conjugate, tagged to an intravenous proton-pump inhibitor approved for human use. A plausible way to encage Gd cations is to use one of the several polyamino polycarboxylate ligands that are commercially available (Figure 2). For our studies reported herein, we opted to use 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) as a Gd-entrapped linker on the Pantoprazole molecule. This linker which stably traps Gd-ion by chelation was designed after Gadoteridol/ProHance model (Bracco Diagnostics, Inc., Princeton).
Figure 2. Polyamino polycarboxylate ligands.
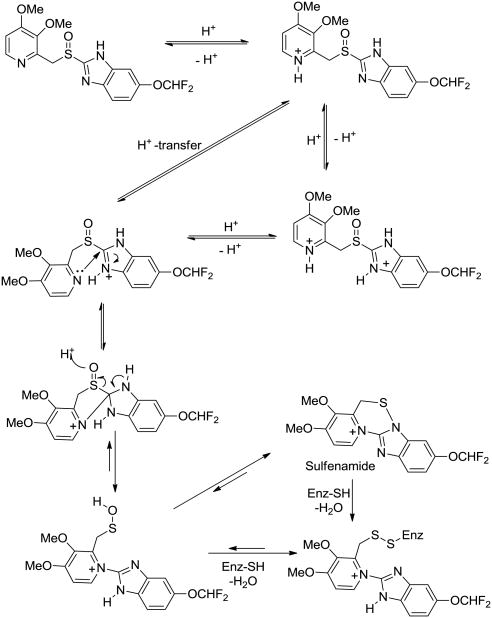
In designing a synthetic strategy for a Pantoprazole-DOTA conjugate, determination of location of an appropriate point of linker attachment in the drug molecule was needed. To this end, an apparent and practically straightforward strategy would appear to be to link a DOTA moiety through one of the nitrogen atoms of the benzimidazole ring. However, a plausible approach was to give due consideration to earlier speculations and observations regarding mechanism of PPI inhibitory action. Nissen4a and Fujisaki4b have described a mechanism of inhibition of P-type ATPase inhibitors represented by Pantoprazole structural types. This mechanism is considered to involve reversible H+-transfers between the pyridine nitrogen and the two nitrogen of the benzimidazole ring (Figure 3). Under physiological conditions, the nucleophilic pyridine nitrogen atom attacks the neighboring highly electrophilic protonated imine bond (C=N) of the benzimidazole ring and open it to generate a sulfenate moiety that can either directly establish a disulfide covalent bond with the enzyme through a cysteine residue, or it can bond with the same cysteine after ring closure to a 1,2,4-thiadiazinium ion. Thus, it appears that the DOTA linker could not be linked through any of nitrogen atoms because of their involvement in the enzyme inhibition mechanism. Since other positions on the Pantoprazole-type PPI molecules were already optimized for activity, we envisioned appending a DOPA-linked ether at the 5-position of the benzimidazole ring of Pantoprazole.
Figure 3. Proposed mechanism of pantoprazole-type PPI inhibition of ATPase enzyme.
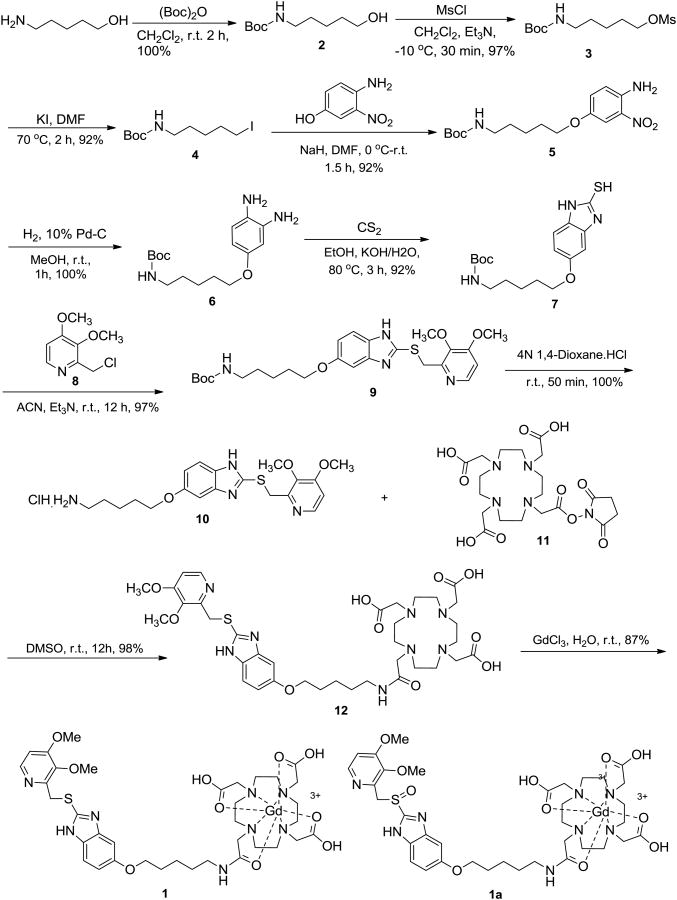
The synthesis of the Pantoprazole-DOTA conjugate (Scheme 1) started with 5-amino-1-pentanol that was protected with a tert-butyloxycarbonyl (Boc) group to give 2. After mesylation, the resulting mesylate 3 was coverted to the iodide 4. A SN2 displacement of the iodide by 3-nitro-4-aminophenol under basic conditions furnished the phenolic ether 5 in an overall yield of 95% over three steps. After the linker was installed, the nitroaniline 5 was converted to a diamine 6 by catalytic reduction. A 2-mercaptobenzimidazole ring 7 was then secured by reacting the diamine 6 with carbon disulphide under basic conditions.8,9 An SN2-displacement of chloride from the benzylic chloride 8 with the thiol 6 in acetonitrile provided the benzimidazole sulfide 9 in high yield. After removal of the Boc from 9, the resulting free amine 10 was finally coupled with the activated DOTA-succinimide (Macrocyclics, Dallas, TX) 11 in DMSO to give the conjugate 12 in quantitative yield. Simple stirring of an aqueous of 12 with GdCl3 furnished desoxy-Pantoprazole-DOTA conjugate 1 as a crude that was purified by HPLC on a RP Vydac (150 × 220 mm) column (gradient elution: 10% B-100% B in 40 min (B = 80% aq. CH3CN containing 0.1% TFA, A = H2O containing 0.1% TFA; FR = 8 mL/min; λmax = 254 nm; RT = 19.0 min).
Scheme 1. Synthesis of Pantoprazole-DOTA conjugate.
The pure desoxy-Pantoprazole-DOTA (Gd3+) 1, obtained as a yellow-brown solid in 87% yield, was fully characterized by 1H- and C-13 NMR, and ESI- based mass spectrometry which confirmed a stable trapping of Gd3+ ion. This compound, when stored at -20 °C was found to be stable for at least two months. The sulfide 1 was partially oxidized with m-chloroperoxybenzoic acid to furnish the final contrast agent Pantoprazole-DOTA (Gd3+) 1a. Purification and spectral characterization of 1a was achieved in the same way as for the sulfide 1. Unfortunately, this compound was found to rapidly degrade to unidentified products as concluded by its purity by mass spectrometry and 1H-NMR. The low temperature storage of 1a did not improve did not prevent its degradation. Since the precursor 1 was found to have a long shelf life, this was used in gastric wall imaging studies with the hope that the sulfide 1 itself, or its possibly in vivo oxidized form might serve the purpose. The successful imaging results using the PPI contrast conjugate agent 1 will be described in a separate communication (manuscript in preparation).
In conclusion, this communication reports a pump inhibitor pantoprazole that has been linked to a Gd-encaging module DOTA to make a novel conjugate. This conjugate was designed to be used as a stomach wall contrast imaging agent. In order to minimize perturbation in the normal function of the drug, a mechanism-based de novo chemical synthesis was designed and successfully executed.
Supplementary Material
Acknowledgments
This study was supported in part by USPHS NIH Grant R01 DK068055 and is gratefully acknowledged.
Footnotes
Supplementary data The spectroscopic and chromatographic data as well as synthetic procedures for all the reported intermediates, and those of the final products are available free of charge via the Internet at:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Kühlbrandt W. Nat Rev Mol Cell Biol. 2004;5:282. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 2.Apell HJ. Bioelectrochemistry. 2004;63:149. doi: 10.1016/j.bioelechem.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Post RL, Kume S, Tobin T, Orcutt B, Sen AK. J Gen Physiol. 1969;54:306. doi: 10.1085/jgp.54.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Yatime L, Buch-Pedersen MJ, Musgaard M, Morth P, Winther AML, Pedersen BP, Olesen C, Andersen JP, Vilsen B, Schiøtt B, Palmgren MG, Møller JV, Nissen P, Fedosova N. Biochim Biophys Acta. 2009;1787:207. doi: 10.1016/j.bbabio.2008.12.019. and references cited therein. [DOI] [PubMed] [Google Scholar]; (b) Fujisaki H, Shibata H, Oketani K, Murakami M, Fujimoto M, Wakabayashi T, Yamatsu I, Yamaguchi M, Sakai H, Takeguchi N. Biochem pharmacol. 1991;42:321. doi: 10.1016/0006-2952(91)90719-l. [DOI] [PubMed] [Google Scholar]
- 5.Parkman HP, Camilleri M, Farrugia G, et al. Neurogastroenterol Motil. 2010;22:113. doi: 10.1111/j.1365-2982.2009.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidler J, Bharucha AE, Camilleri M, et al. Neurogastroenterol Motil. 2009;21:42. doi: 10.1111/j.1365-2982.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharucha AE, Manduca A, Lake DS, et al. Neurogastroenterol Motil. 2010;23:617. doi: 10.1111/j.1365-2982.2011.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida M, Chihiro M, Morita S, Yamashita H, Yamasaki K, Kanbe T, Yabuuchi Y, Nakagawa K. Chem Pharm Bull. 1990;38:1575. doi: 10.1248/cpb.38.1575. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DJ. The Upjohn Company; USA: US patent 4,873,346. Substituted Benzothiazoles, Benzimidazoles, and Benzoxazoles. 1989 Oct 10;
- 10.Kean DM, Smith MA. Magnetic resonance imaging: principles and applications. Williams & Wilkins; Baltimore U.S.A.: 1986. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.