Abstract
Substantial developments have occurred in the past 5–10 years in clinical translational research of thyroid cancer. Diagnostic molecular markers, such as RET-PTC, RAS, and BRAFV600E mutations; galectin 3; and a new gene expression classifier, are outstanding examples that have improved diagnosis of thyroid nodules. BRAF mutation is a prognostic genetic marker that has improved risk stratification and hence tailored management of patients with thyroid cancer, including those with conventionally low risks. Novel molecular-targeted treatments hold great promise for radioiodine-refractory and surgically inoperable thyroid cancers as shown in clinical trials; such treatments are likely to become a component of the standard treatment regimen for patients with thyroid cancer in the near future. These novel molecular-based management strategies for thyroid nodules and thyroid cancer are the most exciting developments in this unprecedented era of molecular thyroid-cancer medicine.
Introduction
Epithelial follicular-cell-derived thyroid cancer is the most common endocrine malignancy with a rapid worldwide rise in incidence in the past few decades.1–4 Age-standardised incidence of thyroid cancer is estimated to be 9·1 per 100 000 females and 2·9 per 100 000 males in developed countries.5
This rapid rise in incidence of thyroid cancer parallels the increase in incidence of diagnosed thyroid nodules, which have an overall malignant risk of about 5–10%. The prevalence of thyroid nodules is about 5–10% in adults on physical palpation of the thyroid gland; it is much higher on thyroid ultrasonography—up to 50–70% in people older than 60 years.6,7 The main goal in the assessment of patients with thyroid nodules is to distinguish thyroid cancer from benign nodules. Although this goal can be achieved in most patients with conventional diagnostic techniques, including ultra sonography and fine needle aspiration biopsy (FNAB), conventional diagnostic methods cannot provide definitive diagnoses in many cases.8
Several histological types of thyroid cancer exist, including papillary thyroid cancer, follicular thyroid cancer, poorly differentiated thyroid cancer, and anaplastic thyroid cancer. Papillary thyroid cancer and follicular thyroid cancer are differentiated thyroid cancers, which account for more than 90% of all thyroid malignancies. Differentiated thyroid cancer is generally associated with an indolent disease course and is usually curable. Anaplastic thyroid cancer is rare but associated with high mortality.9 Poorly differentiated thyroid cancer has a disease course that is between those of differentiated thyroid cancer and anaplastic thyroid cancer. The classic treatment of thyroid cancer is total thyroidectomy, followed by, in some cases, radioiodine treatment. Surgically inoperable and radioiodine-refractory differentiated thyroid cancers, poorly differentiated thyroid cancer, and anaplastic thyroid cancer are currently the major causes of deaths related to thyroid cancer and do not have effective treatments. Although differentiated thyroid cancer is associated with low mortality, disease recurrence is high, at 20–30%, or even higher in some subgroups of patients.10,11 In most patients with differentiated thyroid cancer, however, occurrence of recurrence is low as discussed in the accompanying review by Donald McLeod and collegues.12 Overcoming the challenges of accurate assessment of the risk of individual patients is important so that they can be appropriately treated for the best outcomes. A core issue is how to balance treatment-associated benefits against treatment-associated harms.
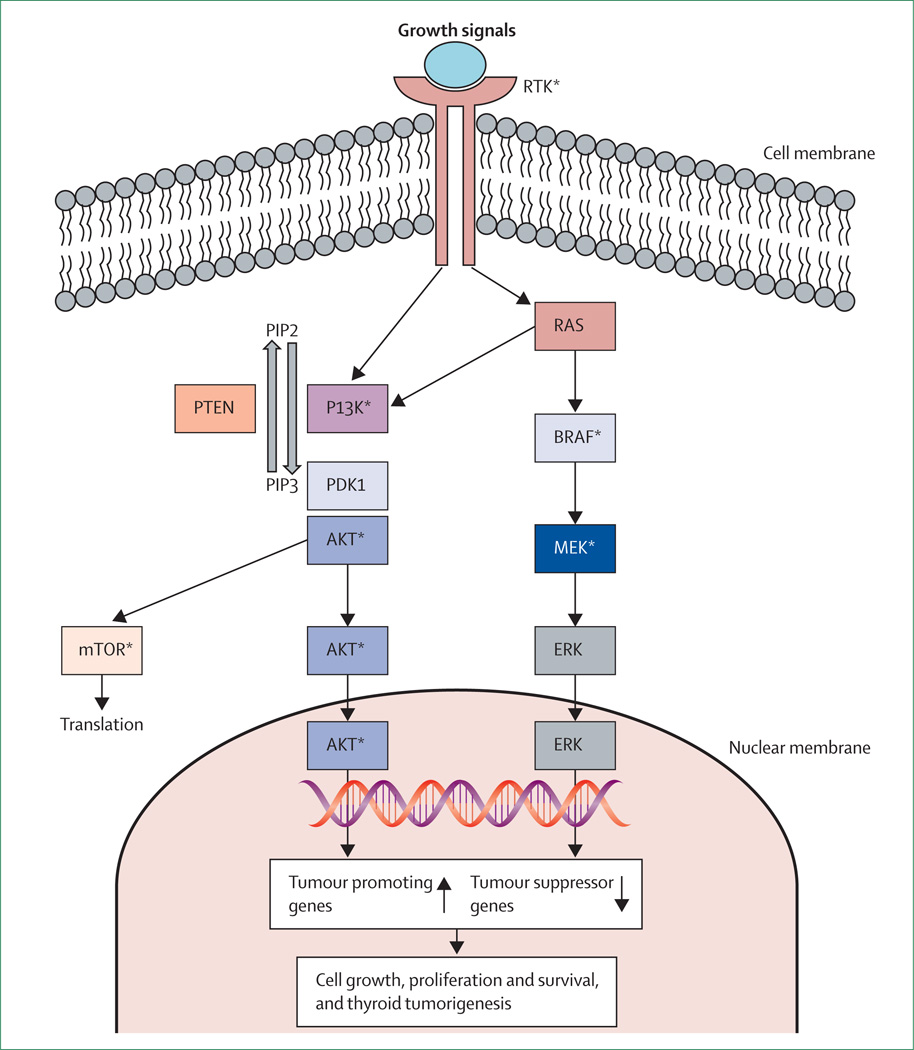
Much progress has been made in understanding the molecular mechanisms of thyroid cancer in the past 5–10 years.13 This progress is best represented by the elucidation of the MAPK and PI3KCA/AKT pathways and related molecular pathogenesis in thyroid cancer (figure 1). This provides an unprecedented opportunity for the identification of novel diagnostic and prognostic molecular markers as well as novel therapeutic targets, on the basis of which more effective management strategies for thyroid cancer are being developed. In this review, we discuss this exciting area of modern thyroidcancer medicine from a clinical perspective.
Figure 1. MAPK and PI3K-AKT-MTOR pathways—genetic alterations and therapeutic targets in thyroid cancer.
Right side shows the MAPK pathway; left side shows the PI3K-AKT-MTOR pathway. The two classic signalling pathways are coupled to the receptor thyrosine kinase (RTK) at the cell membrane which transduces extracellular growth signals into intracellular signalling downstream of the two pathways. RAS can couple the signalling from RTK to both pathways. PTEN terminates the PI3K signalling. Genetic RTK amplifications are common. Common activating mutations in the MAPK pathway include RET-PTC rearrangement, RAS mutation, and BRAF mutation. Common genetic alterations in the PI3K pathway include RAS mutation, PTEN mutation or deletion, PIK3CA mutation or amplification, and AKT1 mutation. The two pathways, driven by these genetic alterations, have a fundamental role in thyroid tumorigenesis. Amplifications of RTK genes are also common. *Denotes therapeutic targets in the two pathways that are currently being actively tested clinically.
Molecular diagnostics
Cytology
FNAB and cytological assessment have been a cornerstone of diagnostic thyroid nodule management since the 1980s and this basic preoperative assessment has substantially reduced the number of patients sent for diagnostic surgery for nodules that ultimately prove to be benign. A meta-review of 11 large studies from the USA, published between 2002 and 2010, showed that a median of 72% (range 62–85%) of FNAB undertaken were benign, 5% (1–8%) were malignant, 17% (10–26%) were indeterminate, and 6% (1–11%) were non-diagnostic.14 A median of 34% (range 14–48%) of patients with indeterminate cytology who underwent surgery had a malignancy. This occurrence is too high to recommend watchful waiting. The United States National Cancer Institute (NCI) sponsored a State of the Science Conference in 2007, in Bethesda, MD, to review diagnostic terminology and morphological criteria for cytological diagnosis of thyroid lesions. The investigators proposed a Bethesda classification scheme that divided indeterminate FNAB results into three subcategories: atypia of undetermined significance or follicular lesion of undetermined significance, with malignancy in 5–10% of cases; follicular neoplasm or suspicious for follicular neoplasm, with malignancy in 20–30% of cases; and suspicious for malignancy, with malignancy in 50–75% of cases.15 Large FNAB studies applying the NCI Bethesda classification scheme have confirmed the malignancy rates for follicular neoplasm or suspicious for follicular neoplasm and suspicious for malignancy cited at the NCI Conference, but occurence of malignancy for patients undergoing diagnostic surgery for atypia of undetermined significance or follicular lesion of undetermined significance was much more variable (7–48%), suggesting that watchful waiting might not be the best approach.16–20 Ideally, FNAB and cytological interpretation should be done by specialists with much experience of the procedures to reduce the variability noted in the atypia of undetermined significance or follicular lesion of undetermined significance category, but because of the many patients with thyroid nodules undergoing assessment, this recommendation might not be practical.
Most patients with an FNAB specimen suspicious for malignancy should undergo surgery and the question is what type of diagnostic or therapeutic surgery should be done. Diagnostic surgery, a lobectomy, or total thyroidectomy is used to establish whether a thyroid nodule is benign or malignant, whereas therapeutic thyroid surgery is done to decrease the risk of cancer recurrence and mortality. Patients with atypia of undetermined significance or follicular lesion of undetermined significance or follicular neoplasm or suspicious for follicular neoplasm FNAB cytology have a lower risk of malignancy than do those with nodules suspicious for malignancy, but, as discussed for the indeterminate cytology overall, this risk might be too high for watchful waiting in most patients. The question for these patients is whether other preoperative diagnostic instruments are available to help confidently avoid diagnostic surgery in those who do not need it.
Molecular markers
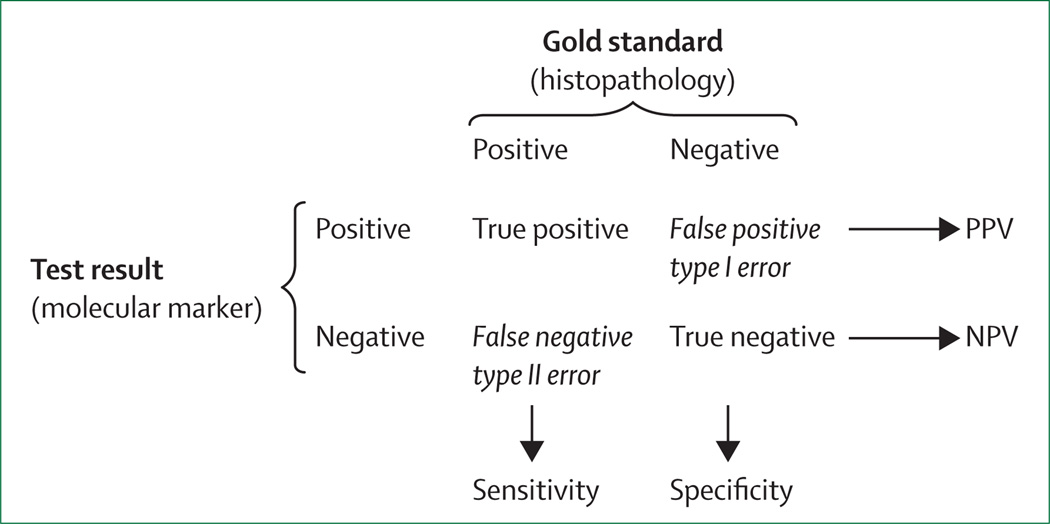
Identification of suitable molecular markers to guide surgery or watchful waiting for patients with indeterminate FNAB of thyroid nodules has been the so-called holy grail of thyroid nodule research for more than 20 years. Many potential markers and combinations of markers have been studied in thyroid tissues and FNAB specimens.21–23 Here, we focus on large studies assessing these molecular markers in FNAB specimens with indeterminate cytology, which is where the need for such directly compare the potential usefulness of varied markers from different studies, uniform statistical measures need to be used. The most applicable measures for these purposes are sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV; figure 2). Sensitivity and NPV are complementary, and these measures are indicative of the confidence that a negative molecular-marker test allows clinicians to defer surgery for patients with an indeterminate FNAB cytology. Specificity and PPV are complementary, and these measures are indicative of the confidence that a positive molecular marker test allows clinicians to pursue therapeutic cancer surgery for patients with an indeterminate FNAB cytology.
Figure 2. Sensitivity, specificity, PPV, and NPV.
Histopathology diagnosis is the reference (ie, gold standard). PPV=positive predictive value. NPV=negative predictive value.
Several studies have assessed the usefulness of different molecular-marker tests to predict final histopathological findings in many patients with preoperative indeterminate FNAB cytology (table 119,20,24–37). The molecular markers with the highest sensitivity and NPV are quite varied and include immunocytochemistry combinations of protein markers, a set of four microRNAs, and a complex gene expression classifier consisting of 142 mRNAs. The molecular markers with the highest specificity and PPV were the genetic markers (mutations and rearrangements) that are believed to be the drivers behind many of the thyroid cancers.
Table 1.
Summary of studies of diagnostic molecular markers on thyroid FNAB specimens with indeterminate cytology
| n* | Malignant (%)† |
Markers | Prospective | Multicentre | Blinded | Sensitivity | NPV | Specificity | PPV | |
|---|---|---|---|---|---|---|---|---|---|---|
| Faroux et al, 199728 | 69 | 13% | A | NA | No | NA | 89% | 97% | 58% | 24% |
| Umbricht et al, 200429 | 100 | 48% | B | No | Yes | NA | 90% | 87% | 65% | 70% |
| Saggiorato et al, 200530 | 125 | 60% | C | No | No | Yes | 100% | 100% | 82% | 78% |
| Bartolazzi et al, 200825 | 432 | 30% | D | Yes | Yes | Yes | 78% | 91% | 93% | 82% |
| Franco et al, 200931 | 138 | 51% | E | Yes | No | NA | 95% | 92% | 76% | 83% |
| Nikiforov et al, 200919 | 52 | 40% | F | Yes | Yes | Yes | 71% | 84% | 100% | 100% |
| Moses et al, 201027 | 137 | 31% | F | Yes | No | Yes | 48% | 80% | 94% | 78% |
| Milas et al, 201024 | 61 | 75% | G | No | No | No | 59% | 80% | 90% | 39% |
| Samija et al, 201132 | 142 | 20% | H | Yes | No | Yes | 79% | 91% | 53% | 28% |
| Fadda et al, 201133 | 119 | 45% | I | NA | No | NA | 89% | 85% | 64% | 71% |
| Nikiforov et al, 201120 | 513 | 24% | J | Yes | No | No | 61% | 89% | 98% | 89% |
| Shen et al, 201234 | 68 | 65% | K | No | No | Yes | 89% | 79% | 79% | 89% |
| Keutgen et al, 201235 | 72 | 31% | L | Yes | Yes | Yes | 100% | 100% | 86% | 73% |
| Agretti et al, 201236 | 53 | 28% | M | Yes | No | Yes | 60% | 78% | 58% | 39% |
| Rossi et al, 201237 | 123 | 36% | N | Yes | No | NA | 32% | 73% | 100% | 100% |
| Alexander et al, 201226 | 265 | 32% | O | Yes | Yes | Yes | 92% | 93% | 52% | 47% |
FNAB=fine needle aspiration biopsy. NPV=negative predictive value. PPV=positive predictive value. NA=not available. A=TPO immunocytochemistry (positive for malignancy <80% cells). B=human telomerase mRNA. C=galectin 3 plus KRT19 plus HBME-1 immunocytochemistry. D=galectin 3 immunohistochemistry (cell blocks). E=galectin 3 plus HBME-1 immunohistochemistry (cell blocks), either marker positive (>10% cells staining). F=BRAF and RAS mutations, RET-PTC and PAX8-PPAR rearrangements. G=peripheral blood (non-serum, non-erythrocyte) thyroid-stimulating hormone mRNA (>1 ng/µg RNA is positive). H=galectin 3 mRNA RT-PCR (visual band on gel). I=galectin 3 plus HBME-1 immunocytochemistry (> 50% cell staining, either marker positive). J=BRAF and RAS mutations, RET-PTC and PAX8-PPAR rearrangements. K=four microRNA set (miR-30d, miR-146b, miR-187, miR-221) linear discrimination analysis. L=four microRNA set (miR-21, miR-197, miR-222, miR-328) support vector machine-radial basis kernel model. M=three microRNA set (miR-146b, miR-155, miR-221) decision-tree analysis. N=BRAFV600E mutation. O=gene expression classifier (mRNA expression levels of 142 genes).
Number of indeterminate FNAB with histopathology correlation.
Percentage malignancy among indeterminate FNAB nodules.
Table 2 lists the basic advantages and disadvantages of the major types of molecular testing. Immunocytochemistry on cytological smears and immunohistochemistry on cytological cell blocks are well-standardised and can use existing cytological material that is visually representative of the nodule. However, these methods do not easily quantitate protein expression and immunocytochemistry staining can be quite variable. The genetic markers, particularly the DNA-based point mutations, have a sound biological rationale and DNA is stable. These markers also generally have a very high specificity and PPV. Currently, however, about 30–40% of differentiated thyroid cancers do not have any of these known molecular mutations, making the sensitivity of these markers unacceptably low. Gene expression markers, including mRNA and microRNA, can be used in large combinations to provide a genomic fingerprint of the tumours that can be trained to pre operatively predict which nodules are ultimately benign or malignant. This approach is very powerful, but is dependent on the method used and the breadth of the training set. RNA stability can be a substantial limitation of genomic testing. Both genetic and genomic tests might require additional FNAB. Peripheral blood RNA markers, such as the blood-based thyroid-stimulating hormone mRNA assay, do not require additional thyroid nodule FNAB and might be complementary to FNAB-based testing.24
Table 2.
Comparison of different molecular diagnostic approaches to FNAB with indeterminate cytology
| Advantages | Disadvantages | |
|---|---|---|
| Nodule specimens | ||
| Protein (IHC/ICC) | Done on existing material Visualisation of cells of interest (representative) Relatively inexpensive | Semi-quantitative Can be subjective ICC staining can be variable |
| Genetic | DNA stability High specificity and PPV | 30–40% of cancers do not have mutations (lower sensitivity) Additional biopsy required Representative of lesion? Expensive |
| Gene expression mRNA, microRNA | Simultaneous measure of many mRNAs or microRNAs (expression fingerprint of the nodule) | RNA instability Additional biopsy required Representative of lesion? Expensive |
| Peripheral blood | ||
| mRNA | No need to sample nodule | Variable RNA recovery from blood |
FNAB=fine needle aspiration biopsy. IHC=immunohistochemistry. ICC=immunocytochemistry. PPV=positive predictive value.
The three largest studies so far are Nikiforov and colleagues’ study20 (513 indeterminate nodules), which used genetic tests of BRAFV600E (termed BRAF mutation in this review) and RAS mutation as well as the RET-PTC and PAX8-PPARγ rearrangements; Bartolazzi and colleagues’ study25 (432 indeterminate nodules), which used galectin-3 immunocytochemistry; and Alexander and colleagues’ study (265 indeterminate nodules), which used the 142 mRNA gene expression classifier.26 The gene expression classifier was iteratively trained against known histopathological and cytopatho logical samples and was then tested against unknown samples, producing a receiver operator characteristics curve with an area under curve of 0·95.38 The gene expression classifier had the highest sensitivity and NPV, the genetic test (BRAF mutation, RAS mutation, RET-PTC, and PAX8-PPARγ) had the highest specificity and PPV, and the galectin 3 immunocytochemistry test had inter mediate sensitivity, specificity, NPV, and PPV compared with the other two tests. Notably, some of these genetic events occur in benign thyroid nodules with a fairly common prevalence (6–13%) in some studies, potentially compromising their diagnostic specificity.27,39,40 However, BRAF mutation has a very high specificity and PPV for thyroid cancer. The gene expression classifier did best on the atypia of undetermined significance or follicular lesion of undetermined significance and follicular neoplasm or suspicious for follicular neoplasm lesions (sensitivity 90%, NPV 94–95%), whereas the NPV was lower for the suspicious for malignancy lesions (85%), which have a higher prevalence of malignancy. The overall NPV for indeterminate cytology was 93% (table 1). Some researchers have therefore recommended use of the gene expression classifier for patients with atypia of undetermined significance or follicular lesion of undetermined significance and follicular neoplasm or suspicious for follicular neoplasm lesions to rule out malignancy, but suggest that this test should not be used for those with suspicious for malignancy cytology.26 The specificity (52%) and PPV (47%) were low for the gene expression classifier applied to indeterminate cytology, hence it is not an optimum test to rule in (ie, confirm the possibilty of) malignancy. With the highest specificity and PPV, the genetic test is an excellent test to rule in malignancy. The galectin-3 immunocytochemistry test also has good specificity and PPV and could therefore also be used as a rule-in test.
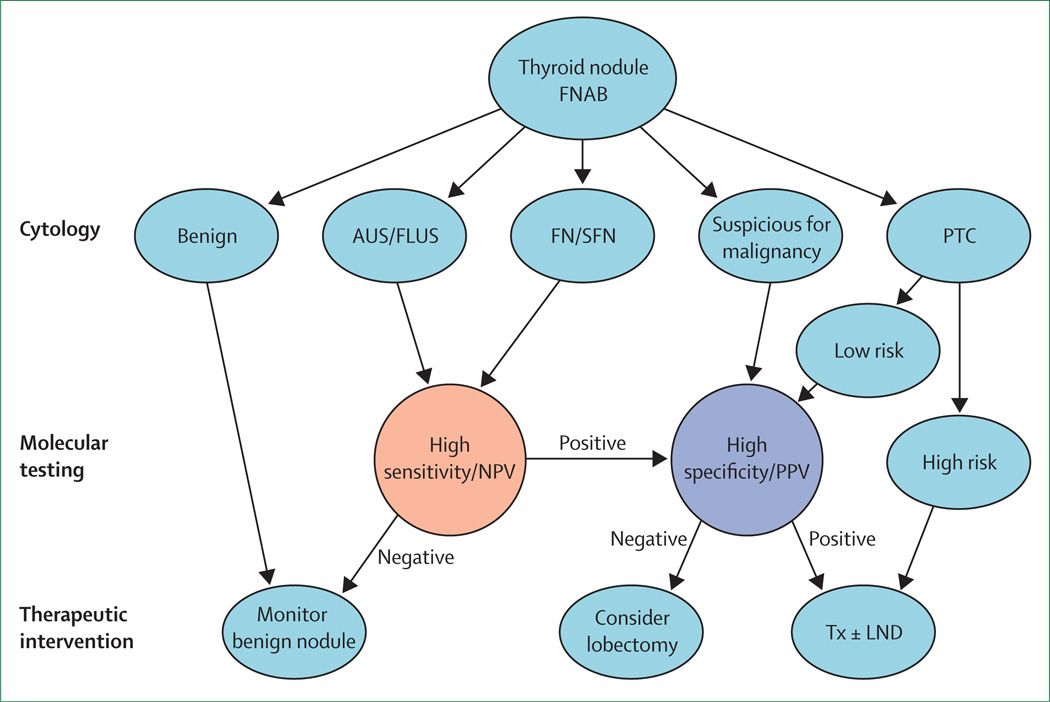
We suggest an algorithm that incorporates cytology and molecular testing in the management of patients with thyroid nodules (figure 3). The goals are to limit unnecessary surgery, use the least aggressive surgery to achieve diagnostic or therapeutic goals, and to make the first surgery the last surgery. On the basis of available data, we believe that patients with atypia of undetermined significance or follicular lesion of undetermined significance or follicular neoplasm or suspicious for follicular neoplasm cytology (lower and variable risk of malignancy) should be considered for molecular testing with high sensitivity and NPV, and those with negative or benign results can be monitored without surgery. Patients with positive or suspicious molecular results should be considered for surgery and we suggest the consideration of genetic testing, which has high specificity and PPV to help guide the extent of surgery. Patients with indeterminate FNAB results that are suspicious for malignancy have a higher risk of malignancy than those in other categories and should be referred for surgery. We suggest that molecular analysis be considered with high specificity and PPV on these patients to help guide the extent of surgery. Cost-effectiveness analyses have been done for the gene expression classifier41 and genetic tests42 and both have shown a favourable cost-effectiveness profile, although both are dependent of the cost of the test. However, these cost analyses were done in the USA and might not be applicable in other countries. We are not aware of a cost-effectiveness analysis of the galectin-3 immunocytochemistry test, but it is generally less expensive than the genetic and genomic tests (table 2). Galectin-3 immunocytochemistry might thus have a potential use particularly in low-income countries. Whether the algorithm proposed in figure 3 is cost-effective also remains to be systematically analysed.
Figure 3. Algorithm for management of thyroid nodules on the basis of FNAB and molecular marker tests.
Depending on the cytology categories, molecular tests with high sensitivity and NPV (eg, gene expression classifier) or high specificity and PPV (eg, BRAF mutation) are chosen. Extent of surgery should be decided on the basis of the combined assessment of clinical, imaging, cytological, and molecular marker data. FNAB=fine needle aspiration biopsy. AUS/FLUS=atypia of undetermined significance/follicular lesion of undetermined significance. FN/SFN=follicular neoplasm/suspicious for follicular neoplasm. PTC=papillary thyroid cancer. NPV=negative predictive value. PPV=positive predictive value. Tx=total/near total thyroidectomy. LND=lymph node dissection.
Molecular prognostication
Several prognostic molecular markers in thyroid cancer, particularly genetic markers—including mutations in RAS, PIK3CA, PTEN, P53, ALK, and BRAF genes—show promise. Some of them occur only in poorly differentiated thyroid cancer or anaplastic thyroid cancer, such as mutations in P5343,44 and ALK.45 Some, such as AKT1 mutations, were reported only in metastatic lesions but not in primary thyroid-cancer tissue.46 These mutations could be markers for thyroid cancer aggressiveness. RAS, PIK3CA, and PTEN mutations in creasingly occur and coexist in thyroid tumours from low-grade to highgrade.13,47 Their coexistence could thus favour and predict progression of thyroid cancer. Genetic patterns, particularly coexistence of these and other genetic alterations (eg, amplifications of RTK genes and BRAF mutation) that could dually activate the MAPK and PI3K-AKT pathways (figure 1) increasingly occur along the progression of thyroid tumour from low-grade to high-grade.13,48 This finding suggests that such genetic patterns are drivers of thyroid tumour progression and might potentially be markers of poor prognosis of thyroid cancer. RAS mutations, particularly NRAS mutations, are associated with increased aggressiveness of poorly differentiated thyroid cancer and follicular thyroid cancer and even decreased survival of patients.49–51 The prognostic usefulness of these genetic markers is promising but remains to be defined.
The best defined prognostic marker is the BRAF mutation, which, by constitutively activating the BRAF kinase in the MAPK pathway (figure 1), promotes aggressive ness of papillary thyroid cancer.52–57 We will focus our discussion on the clinical prognostic usefulness of this mutation.
BRAF mutation and aggressiveness of papillary thyroid cancer
Results of a multicenter study53 showed a strong association of BRAF mutation with lymph node meta stasis, extrathyroidal extension, advanced disease stages III and IV, and disease recurrence. These findings have been confirmed in most subsequent studies although some inconsistencies exist, perhaps because of technical variations.52–57 This association is also com monly noted in conventionally low-risk papillary thyroid cancer.53,58–60 The initial finding of the association of BRAF mutation with papillary thyroid cancer recur rence53 is particularly relevant clinically, which has been confirmed in many studies.59–68 These studies showed odds ratios of around 3–5 for BRAF-mutation-associated recurrence of papillary thyroid cancer, with PPV and NPV of around 30% and 90%, respectively, on overall analyses.55 BRAF mutation was previously also shown to be associated with loss of radioiodine avidity of recurrent papillary thyroid cancer, rendering the disease refractory to radioiodine treatment.53 This finding was confirmed in many other studies.64,69,70 Many studies correspondingly showed an association of BRAF mutation with decreased or loss of expression of thyroid iodide-handling genes, including SLC5A5 (also known as NIS), TSHR, SLC26A4 (also known as pendrin gene), TPO, and TG.54,55 An in-vitro study showed that induced expression of BRAF mutant could silence expression of these genes and suppression of BRAF mutation could restore expression of the genes.71 This finding has been reproduced in a transgenic mouse model.72 BRAF mutation has also been widely shown to cause over expression of many tumour-promoting molecules, such as VEGF and MET.54,55 These results provide a molecular basis for the aggressiveness and treatment failure of papillary thyroid cancer in association with BRAF mutation. These results also explain the strong association of BRAF mutation with papillary thyroid cancer mortality in an international multicentre study.73
BRAF mutation in the surgical management of papillary thyroid cancer
Most clinicians agree that, in general, patients with thyroid cancer should be treated surgically. However, for patients with low-risk differentiated thyroid cancer, debate often surrounds whether total thyroidectomy or hemithyroidectomy, or prophylactic central neck dissection (PCND) or no dissection, should be pursued in patients without preoperative or intraoperative evidence of metastatic lymph nodes.12 In view of the strong association of BRAF mutation with aggressiveness of papillary thyroid cancer and the loss of radioiodine avidity in recurrent disease, it seems to be important to surgically eradicate the mutation-positive cancer in the first place. In this context, for example, micro-papillary thyroid cancer (ie, tumours ≤1 cm), which is currently recommended by the American Thyroid Association for lobectomy,8 might better be treated with total thyroidectomy instead, if preoperative testing for BRAF mutation is positive (figure 3). Total thyroidectomy might be particularly appropriate for microcarcinomas larger than 5 mm, since microcarinomas larger than 5 mm have a significantly higher risk for recurrence than do tumours that are smaller than 5 mm.74 This approach is feasible since BRAF mutation can be easily detected on preoperative FNAB specimens with various sensitive and specific post-PCR amplification-based detecting methods, such as colori metric mutation detection assay75 and fluorescence melting curve analysis.20 However, there is, as yet, no prospective evidence that this approach will favourably change the outcome in these low-risk patients.
When a lobectomy confirms malignancy on a small cytologically indeterminate thyroid nodule, the presence of BRAF mutation alone might not be sufficient to recommend a completion thyroidectomy for patients who would not be recommended to have this surgery on the basis of traditional low-risk clinicopathological features. In this scenario, the value of BRAF mutation analysis might be obscured by the complexity of the decision making in relation to completion thyroidectomy, which takes into account other factors such as the additional risk of surgical complications and financial cost. Nevertheless, addition of BRAF mutation analysis to other risk factors might be helpful in deciding the need for completion thyroidectomy in appropriate clinical settings.
BRAF mutation is associated with increased need for surgical reoperation of recurrent papillary thyroid cancer.53,66,67 This finding is consistent with the very high prevalence of BRAF mutation in recurrent papillary thyroid cancer, ranging from 78% to 95%, usually in central neck lymph nodes (CNLN).46,57,69,76 Consideration of initial PCND is therefore reasonable to prevent occurrence of intractable recurrent BRAF-mutation-positive papillary thyroid cancer. Although sometimes inconsistent, PCND at the initial thyroidectomy is associated with decreased disease recurrence and the need for reoperation for papillary thyroid cancer in many studies, including a large prospective multicentre study.77 Thus, PCND might be considered in patients who preoperatively test positive for BRAF mutation. This idea is strongly supported by findings showing that pre operative BRAF mutation positivity on FNAB predicted lymph node metastasis, extrathyroidal extension, and advanced disease stages III and IV of papillary thyroid cancer.65,78–80 This association was noted even in patients with micro-papillary thyroid cancer.79 CNLN metastases had a substantial effect on recurrence of papillary thyroid cancer, which were both predicted by BRAF mutation.81 BRAF mutation independently predicted CNLN metastases, and preoperatively displayed PPV of 47% and NPV 91% for CNLN metastasis.82 Preoperative BRAF mutation-positivity strongly predicted occult CNLN metastases identified on PCND,78 directly sup porting the rationale of BRAF-mutation-assisted PCND. Thus, the addition of BRAF mutation to other factors seems reasonable in defining PCND for patients with conventionally low-risk to intermediate-risk papillary thyroid cancer in whom the decision about PCND might otherwise not be straightforward. PCND might also be considered, in appropriate clinical settings, for patients who are BRAF-mutation-positive and who meet the present recommendations for completion thyroidectomy. Currently insufficient data supports routine PCND on the basis of BRAF mutation status alone for very low-risk papillary thyroid cancer, such as very small (<5 mm) unifocal papillary thyroid cancer without other risk factors.
The high NPV of BRAF mutation for recurrence of papillary thyroid cancer55 is confirmed in conventionally low-risk patients.59 Thus, a negative preoperative BRAF mutation test would support the decision not to recommend PCND for conventionally low-risk patients for whom lobectomy should be sufficient. This negative BRAF mutation-based conservative approach could perhaps be applied to cancers even beyond micro-papillary thyroid cancer, such as papillary thyroid cancer of 1·0–2·0 cm, in the absence of conventional high clinicopathological risk factors.
BRAF mutation in medical management of papillary thyroid cancer
Potential adverse effects, including the risk of second primary cancer, become increasingly a concern with radioiodine treatment of thyroid cancer.83–86 Moreover, unlike in high-risk patients, the benefits of radioiodine treatments in low-risk patients for disease recurrence and mortality are questionable.87–91 Many or most of these low-risk patients might not need radioiodine. Serum TG testing is most commonly used for thyroid cancer recurrence surveillance. A retrospective study showed that in most low-risk patients, serum TG dropped to undetectable concentrations 5–7 years postoperatively in both radioiodine ablation treatment and non-treatment groups, but the TG clearance rate was significantly higher in the radioiodine treatment group.92 This finding confirmed the known usefulness of TG testing in these patients. In view of the strong association of BRAF mutation with recurrence of papillary thyroid cancer even in low-risk patients,53,59,60 effective monitoring of disease recurrence seems important in such conventionally low-risk but BRAF mutation-positive patients. Thus, clinicians might be advised to treat these patients with radioiodine to enhance the reliability of TG testing by ablating normal thyroid tissues. By contrast, the high NPV of BRAF mutation should assure the practice to spare patients who are BRAF-mutation-negative and conventionally low-risk from radioiodine ablation. For patients who are conventionally low-risk but are BRAF-mutation-positive, a low activity of 131radioiodine of 1110 MBq (30 mCi) after stimulation with recombinant human thyroid-stimulating hormone should be recommended since this activity can ablate normal thyroid tissues in low-risk patients as effectively as a higher activity of 3700 MBq (100 mCi).93,94 Also, an activity of 1110 MBq radioiodine probably has little, if any, adverse effect. However, prospective outcome data might be needed to clearly support BRAF-mutation-based radioiodine ablation for patients with low-risk papillary thyroid cancer.
How BRAF mutation status can affect other aspects of medical management of papillary thyroid cancer in appropriate clinical settings, such as the thyroid-stimulating-hormone target, the vigilance of recurrence surveillance, and the threshold of the use of fluorodeoxyglucose (FDG)-PET scan has been discussed elsewhere55 and needs further studies to define.
Molecular-targeted therapy
Radioiodine-refractory thyroid cancer
Radioiodine refractory thyroid cancer is rare, with an estimated incidence of four cases per million population (5% of patients with clinical cancer).95 It is defined in patients with advanced disease either by the presence of at least one tumour focus without any uptake of radioiodine or by progression of the disease during the year after a radioiodine treatment course; in patients with persistent disease after the administration of a cumulative activity of 22 GBq (600 mCi) radioiodine, the administration of subsequent activities of radioiodine is based on an assessment of the individual patient, taking into account benefits already identified and the likelihood of radiosensitivity of the disease. Refractory disease occurs more frequently in older patients, in those with large metastases or with poorly differentiated thyroid cancer, and in those with high FDG uptake on PET scan.96,97 Refractory disease is usually aggressive, with a median survival after the discovery of distant metastases ranging from 3 to 6 years.96 However, metastatic differentiated thyroid cancers can be asymptomatically stable for long periods of time and in such patients the benefits of novel therapies might be largely outweighed by drug toxicities. Local treatment techniques, such as radiofrequency, cryoablation, external radiation therapy, and surgery, might be useful in patients with few or symptomatic metastatic foci, and serum thyroid-simulation hormone should be maintained at a low or undetectable concentration. Progression rate can be assessed by the doubling time of TG,98 and should always be confirmed by standardised imaging that is repeated every 6 months, using Response Evaluation Criteria in Solid Tumour (RECIST). The main selection criterion for clinical trials in patients with refractory differentiated thyroid cancers was documented progression in less than 1 year in target lesions according to RECIST. Patients with a large tumour burden might require systemic treatment, including cytotoxic chemotherapy or targeted therapy, before assessment of progression, and the decision to initiate treatment might be based on high FDG uptake on PET scan and on histological characteristics of the tumour.99,100 Cytotoxic chemotherapies had low response (from 0% to 22% with the most frequently used agent, doxorubicin) in these patients and toxicity was high.101 Other treatment options are therefore needed.
Novel therapy
In most patients with differentiated thyroid cancer, an initiating oncogenic event can be identified and molecular-targeted therapy can be given with a scientific rationale.13,102 Gene rearrangements (RET-PTC and NTRK) or point mutations of the RAS and BRAF genes that are mutually exclusive in differentiated thyroid cancers are identified in two-thirds of papillary thyroid cancers, resulting in the activation of the MAPK pathway. RAS mutations are identified in 40% of follicular thyroid cancers and 25% of poorly differentiated thyroid cancers.51 Other pathways might also be activated, including the PI3K-AKT pathway in follicular thyroid cancer and poorly differentiated thyroid cancer.103,104 Activation of these pathways leads to tumour proliferation, dedifferentiation, invasion, and tumour angiogenesis. In fact, angiogenesis is activated in thyroid cancers, with an overproduction of VEGF by cancer cells and an overexpression of VEGF receptors by cancer and endothelial cells,105 and probably also by activating other angiogenic factors and pathways. The overexpression of VEGF receptors is consistent with genetic amplifications of the genes for VEGF receptors in thyroid cancers.48 Up to now, drugs used in refractory thyroid cancers are anti-angiogenic and some also target kinases in the MAPK pathway (figure 1). The relative role of the inhibition of each target or of their combined inhibition in the effects on tumours is unknown.
Anti-tumour efficacy of these agents is likely to be greater than that of earlier cytotoxic chemotherapies, with partial responses identified in phase 2 trials in 0–59% of patients and long-term stable disease in at least another third (table 3100,106–116). Even more importantly, a randomised phase 2 study of 145 patients with vandetanib—a drug that targets the KDR, RET, and EGFR kinases—versus placebo produced a significant prolongation of median progression-free survival (11·1 vs 5·9 months, hazard ratio 0·63, p=0·008),106 and a significant prolongation of progression-free survival was also shown in a phase 3 study of 417 patients with sorafenib versus placebo.116 Comparison of the outcomes among these compounds is at the present time not possible, but the response rates recently reported with pazopanib, lenvatinib, and cabozantinib (around 50%) seem higher than in previous reports, and a phase 3 trial of lenvatinib versus placebo is underway (NCT01321554). Most drugs seem more effective on metastases located in lymph nodes, liver, and lungs than in bones. Further studies of tumour samples and in experimental models are needed to correlate drug efficacy with the genetic defects present in the tumour.
Table 3.
Results of clinical trials of kinase inhibitors in patients with radioiodine-refractory differentiated thyroid cancer
| Targets | Patients (n) |
PR (%) | SD >6 months (%) |
Median PFS (months) |
Median OS (months) |
Dose reduction for toxic effects (%) |
|
|---|---|---|---|---|---|---|---|
| Vandetanib* | |||||||
| Leboulleux et al, 2012106 | KDR, FLT4, RET, EGFR | 145 | <5 | ¨ | 11 (vandetanib) vs 5·8 (placebo) | >27 | 12 |
| Sorafenib | |||||||
| Gupta-Abramson et al, 2008108 | KDR, FLT4, RET, BRAF | 30 | 23 | 53 | 20 | NE | 47 |
| Kloos et al, 2009109 | KDR, FLT4, RET, BRAF | 41 | 15 | 56 | 15 | 23 | 52 |
| Hoftijzer et al, 2009110 | KDR, FLT4, RET, BRAF | 32 | 25 | 34 | 13·5 | NE | 66 |
| Ahmed et al, 2011107 | KDR, FLT4, RET, BRAF | 19 | 18 | 82 | >24 | NE | 79 |
| Bayer HealthCare, 2013116† | KDR, FLT4, RET, BRAF | 417 | ¨ | ¨ | Improved | ¨ | ¨ |
| Motesanib | |||||||
| Sherman et al, 2008111 | FLT1, KDR, FLT4, PDGFR, KIT | 93 | 14 | 35 | 9 | NE | ¨ |
| Axitinib | |||||||
| Cohen et al, 2008112 | FLT1, KDR, FLT4 | 45 | 31 | 46 | 18·1 | >36 | 38 |
| Sunitinib | |||||||
| Carr et al, 2010100 | FLT1, KDR, FLT4, RET | 28 | 29 | 50 | 12·8 | >24 | 60 |
| Pazopanib | |||||||
| Bible et al, 2010113 | FLT1, KDR, FLT4, PDGFR, KIT | 37 | 49 | 11·7 | >24 | 43 | |
| Lenvatinib‡ | |||||||
| Sherman et al, 2011114 | FLT1, KDR, FLT4, PDGFR, FGFR | 58 | 50 | 26 | 12·6 | 28 | 66 |
| Cabozantinib | |||||||
| Cabanillas et al, 2012115 | KDR, MET, RET | 15 | 53 | 40 | Not reached | Not reached | ¨ |
PR=partial response. SD=stable disease. PFS=progression-free survival. OS=overall survival. NE=not estimated.
The vandetanib study was a randomized phase II study vs placebo with the primary endpoint being progression-free survival.
The Bayer HealthCare study is a phase 3 trial of sorafenib versus placebo.
A phase 3 study of lenvatinib is underway.
Another potential way of treating these patients is to restore the ability of radioiodine uptake in tumour cells, and then to treat with radioiodine after preparation with thyroid-stimulating hormone stimulation. This treatment strategy is strongly implicated and supported by previous in-vitro studies71,117 in which targeting of BRAF, MEK, or AKT in the MAPK and PI3K-AKT pathways could restore thyroid gene expression and radioiodine uptake, which was enhanced by thyroid-stimulating hormone in thyroid cancer cells. These findings are supported by a later in-vivo study.72 Clinical data with the MEK inhibitor selumetinib are promising, particularly in RAS mutated tumours: radioiodine (124I) uptake was increased in 12 of 20 evaluable patients and was high enough to permit radioiodine treatment in eight, among whom five had a partial response.118
Toxic effects of kinase inhibitors included fatigue, diarrhoea, hypertension, and skin toxicities, and the dose of l-thyroxine treatment had to be increased in most patients, but no unexpected toxic effects were reported. Toxic effects were significant and led to dose reduction in 11–73% of patients and to drug withdrawal in 7–25%. This finding suggests that these treatments should be initiated only in patients with significant tumour burden and with documented progressive disease and they should be managed by experienced teams. Duration of treatment is not yet validated and, for this reason, treatment is usually given as long as toxic effects remain manageable and no tumour progression occurs.
Future therapy developments
Kinase inhibitors should be used as first-line treatment in patients with refractory differentiated thyroid cancers in whom progression has been documented. No drug is currently approved for these patients, who should preferably be included in prospective trials. Even phase 1 trials that test the newest therapies should be considered for these patients, because these protocols might allow early identification of possibly effective drugs.119 In view of the commercial availability of sorafenib and sunitinib, these agents have entered into clinical use for patients with progressive refractory disease who are not suitable candidates for clinical trials. Tumour responses were identified in only a few patients and most were partial and transient.120 This finding shows the emergence of a new unmet need to treat patients who do not respond or respond but then progress so that they can maintain good performance status.
In cases of tumour progression or toxic effects with one drug, patients might benefit from another anti-angiogenic drug, but benefits of further treatments with other anti-angiogenic drugs are questionable.120 Future studies should test cross-resistance between drugs and use drugs targeted at abnormalities that are present in the tumour tissue. Combination or sequential treatments might also be sought and a report of a phase 2 trial combining temsirolimus and sorafenib is encouraging.121 Predictive biomarkers aim to allow improved selection of patients for any treatment method and should permit an early assessment of tumour response to the drug.
The search for oncogenic events that have been described in thyroid cancers has been done in several studies, either to select patients for treatment (such as BRAF mutation in refractory papillary thyroid cancer for treatment with the BRAF inhibitor vemurafenib, as in melanoma122) or for targeting of other pathways such as the PI3K-AKT pathway,123,124 or to identify other relevant targets. Results are still too preliminary to show any correlation between mutated BRAF and response to sorafenib.107–110 Other studies have shown that response to lenvatinib is more frequently identified in patients with a RAS mutation and that the increase in radioiodine uptake induced by selumitinib treatment is more frequently recorded in RAS and BRAF mutated cancers.118 However, larger series of patients are needed to define the importance of mutation screening in these patients. Many of these data have been obtained with thyroid tumour tissues that were resected long before treatment and analysis of the metastatic tumour tissue at the time of treatment would probably be more informative. This approach was suggested by one study showing that BRAF or RAS mutations found in the primary tumour were also present in the metastases, and that additional mutations (PIK3CA or AKT1) might also be present in the metastatic tissue.46
A few other biochemical markers have been studied. In patients treated with motesanib, change from base line in serum placental growth factor after 1 week of treatment correlated with best tumour response, and a decrease in soluble KDR after 3 weeks of treatment separated responders from non-responders.125 Lower baseline VEGF concentrations were associated with longer progression-free survival.125,126 Basal concentrations of other cytokine or angiogenic factors or changes in their serum concentration at 1–2 weeks have been associated with tumour response. These studies have shown the promise of using biomarkers to predict drug efficacy, which needs to be refined before they can be used in clinical practice. Comparison of FDG uptake on PET-CT at 1–2 weeks with baseline FDG uptake has produced inconsistent results and the relevance of repeated FDG-PET/CT in the management of patients with differentiated thyroid cancer during treatment with these new drugs is still unclear. During treatment with sunitinib, a decrease in FDG uptake was associated with subsequent tumour response, and an increase with subsequent tumour progression.100 However, in other studies no such relation was identified.106,109
Cytotoxic chemotherapy with new drugs should be tested since they seem to be effective in some patients.127,128 Trials done in networks of referral centres have shown that inclusion of the number of patients with thyroid cancer needed to reach statistically significant conclusions is possible in a limited period of time (about 18 months), and this finding should encourage clinicians to enrol patients in prospective trials.
Conclusions
On the basis of recently identified diagnostic and prognostic molecular markers and therapeutic targets, novel effective management strategies have been (or are being) rapidly developed for thyroid cancer. With these unprecedented achieve ments, we have now entered an exciting modern era of molecular thyroid cancer medicine.
Search strategy and selection criteria.
We searched PubMed with the key words “thyroid nodule”, “thyroid cancer”, “thyroid nodule markers”, “thyroid cancer prognostic markers”, and “molecular-targeted novel therapy of thyroid cancer” for related publications from the past 25 years, weighted toward those from the past 5–10 years, and publications in English. The last search was done on Jan 15, 2013. We also included relevant studies cited in reports identified by this search strategy and relevant work from our own scientific literature collection.
Acknowledgments
The authors’ effort for this manuscript is supported by the United States National Institutes of Health grants R01CA134225 and R01CA113507 to MX; Mary Rossick Kern and Jerome H Kern endowment for BRH; and a grant from the French National Cancer Institute to MS.
Footnotes
Contributors
All the authors participated in the conception, design, writing, and revision of the report. All the authors approved the final version of the paper for publication.
Conflicts of interest
MX is coholder of a patent related to BRAF mutation and has received research support from Ardea Biosciences; BRH has received research support from Veracyte; and MS has received research funding from Amgen, AstraZeneca, Bayer, Eisai, Exelixis, and Genzyme.
Contributor Information
Mingzhao Xing, Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology and Metabolism, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Bryan R Haugen, Division of Endocrinology, Metabolism, and Diabetes, University of Colorado School of Medicine, Aurora, CO, USA.
Martin Schlumberger, Department of Nuclear Medicine and Endocrine Oncology, Centre de Référence des Tumeurs Réfractaires de la Thyroïde, Institut Gustave Roussy and University Paris-Sud, Villejuif, France.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. [accessed Jan 15, 2013];SEER cancer statistics review, 1975–2009 (vintage 2009 populations) Data from November, 2011, posted online April, 2012. http://seer.cancer.gov/csr/1975_2009_pops09/
- 2.Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53, 856 cases of thyroid carcinoma treated in the US, 1985–1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Leenhardt L, Grosclaude P, Cherie-Challine L. Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 4.Sprague BL, Warren AS, Trentham-Dietz A. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control. 2008;19:585–593. doi: 10.1007/s10552-008-9122-0. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- 7.Guth S, Theune U, Aberle J, et al. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 9.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol) 2010;22:486–497. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown RL, de Souza JA, Cohen EE. Thyroid cancer: burden of illness and management of disease. J Cancer. 2011;2:193–199. doi: 10.7150/jca.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 12.McLeod DSA, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet. 2013;381:1046–1057. doi: 10.1016/S0140-6736(12)62205-3. [DOI] [PubMed] [Google Scholar]
- 13.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang CC, Friedman L, Kennedy GC, et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011;21:243–251. doi: 10.1089/thy.2010.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425–437. doi: 10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 16.Theoharis C, Roman S, Sosa JA. The molecular diagnosis and management of thyroid neoplasms. Curr Opin Oncol. 2012;24:35–41. doi: 10.1097/CCO.0b013e32834dcfca. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 18.Williams MD, Suliburk JW, Staerkel GA, et al. Clinical significance of distinguishing between follicular lesion and follicular neoplasm in thyroid fine-needle aspiration biopsy. Ann Surg Oncol. 2009;16:3146–3153. doi: 10.1245/s10434-009-0666-3. [DOI] [PubMed] [Google Scholar]
- 19.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 20.Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferraz C, Eszlinger M, Paschke R. Current state and future perspective of molecular diagnosis of fine-needle aspiration biopsy of thyroid nodules. J Clin Endocrinol Metab. 2011;96:2016–2026. doi: 10.1210/jc.2010-2567. [DOI] [PubMed] [Google Scholar]
- 22.de la Chapelle A, Jazdzewski K. MicroRNAs in thyroid cancer. J Clin Endocrinol Metab. 2011;96:3326–3336. doi: 10.1210/jc.2011-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouniavsky G, Zeiger MA. The quest for diagnostic molecular markers for thyroid nodules with indeterminate or suspicious cytology. J Surg Oncol. 2012;105:438–443. doi: 10.1002/jso.21935. [DOI] [PubMed] [Google Scholar]
- 24.Milas M, Shin J, Gupta M, et al. Circulating thyrotropin receptor mRNA as a novel marker of thyroid cancer: clinical applications learned from 1758 samples. Ann Surg. 2010;252:643–451. doi: 10.1097/SLA.0b013e3181f5ba51. [DOI] [PubMed] [Google Scholar]
- 25.Bartolazzi A, Orlandi F, Saggiorato E, et al. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: a prospective multicentre study. Lancet Oncol. 2008;9:543–549. doi: 10.1016/S1470-2045(08)70132-3. [DOI] [PubMed] [Google Scholar]
- 26.Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–715. doi: 10.1056/NEJMoa1203208. [DOI] [PubMed] [Google Scholar]
- 27.Moses W, Weng J, Sansano I, et al. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg. 2010;34:2589–2594. doi: 10.1007/s00268-010-0720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faroux MJ, Theobald S, Pluot M, et al. Evaluation of the monoclonal antibody antithyroperoxidase MoAb47 in the diagnostic decision of cold thyroid nodules by fine-needle aspiration. Pathol Res Pract. 1997;193:705–712. doi: 10.1016/S0344-0338(97)80030-1. [DOI] [PubMed] [Google Scholar]
- 29.Umbricht CB, Conrad GT, Clark DP, et al. Human telomerase reverse transcriptase gene expression and the surgical management of suspicious thyroid tumors. Clin Cancer Res. 2004;10:5762–5768. doi: 10.1158/1078-0432.CCR-03-0389. [DOI] [PubMed] [Google Scholar]
- 30.Saggiorato E, De Pompa R, Volante M, et al. Characterization of thyroid ‘follicular neoplasms’ in fine-needle aspiration cytological specimens using a panel of immunohistochemical markers: a proposal for clinical application. Endocr Relat Cancer. 2005;12:305–317. doi: 10.1677/erc.1.00944. [DOI] [PubMed] [Google Scholar]
- 31.Franco C, Martinez V, Allamand JP, et al. Molecular markers in thyroid fine-needle aspiration biopsy: a prospective study. Appl Immunohistochem Mol Morphol. 2009;17:211–215. doi: 10.1097/PAI.0b013e31818935a9. [DOI] [PubMed] [Google Scholar]
- 32.Samija I, Matesa N, Lukac J, et al. Galectin-3 and CD44v6 as markers for preoperative diagnosis of thyroid cancer by RT-PCR. Diagn Mol Pathol. 2011;20:233–241. doi: 10.1097/PDM.0b013e31821a59f1. [DOI] [PubMed] [Google Scholar]
- 33.Fadda G, Rossi ED, Raffaelli M, et al. Follicular thyroid neoplasms can be classified as low- and high-risk according to HBME-1 and Galectin-3 expression on liquid-based fine-needle cytology. Eur J Endocrinol. 2011;165:447–453. doi: 10.1530/EJE-11-0181. [DOI] [PubMed] [Google Scholar]
- 34.Shen R, Liyanarachchi S, Li W, et al. MicroRNA signature in thyroid fine needle aspiration cytology applied to “atypia of undetermined significance” cases. Thyroid. 2012;22:9–16. doi: 10.1089/thy.2011.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keutgen XM, Filicori F, Crowley MJ, et al. A panel of four miRNAs accurately differentiates malignant from benign indeterminate thyroid lesions on fine needle aspiration. Clin Cancer Res. 2012;18:2032–2038. doi: 10.1158/1078-0432.CCR-11-2487. [DOI] [PubMed] [Google Scholar]
- 36.Agretti P, Ferrarini E, Rago T, et al. MicroRNA expression profile helps to distinguish benign nodules from papillary thyroid carcinomas starting from cells of fine-needle aspiration. Eur J Endocrinol. 2012;167:393–400. doi: 10.1530/EJE-12-0400. [DOI] [PubMed] [Google Scholar]
- 37.Rossi M, Buratto M, Bruni S, et al. Role of ultrasonographic/clinical profile, cytology, and BRAF V600E mutation evaluation in thyroid nodule screening for malignancy: a prospective study. J Clin Endocrinol Metab. 2012;97:2354–2361. doi: 10.1210/jc.2011-3494. [DOI] [PubMed] [Google Scholar]
- 38.Chudova D, Wilde JI, Wang ET, et al. Molecular classification of thyroid nodules using high-dimensionality genomic data. J Clin Endocrinol Metab. 2010;95:5296–5304. doi: 10.1210/jc.2010-1087. [DOI] [PubMed] [Google Scholar]
- 39.Cantara S, Capezzone M, Marchisotta S, et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95:1365–1369. doi: 10.1210/jc.2009-2103. [DOI] [PubMed] [Google Scholar]
- 40.Guerra A, Sapio MR, Marotta V, et al. Prevalence of RET/PTC rearrangement in benign and malignant thyroid nodules and its clinical application. Endocr J. 2011;58:31–38. doi: 10.1507/endocrj.k10e-260. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Robinson KA, Anton B, et al. Cost-effectiveness of a novel molecular test for cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab. 2011;96:E1719–E1726. doi: 10.1210/jc.2011-0459. [DOI] [PubMed] [Google Scholar]
- 42.Yip L, Farris C, Kabaker AS, et al. Cost impact of molecular testing for indeterminate thyroid nodule fine-needle aspiration biopsies. J Clin Endocrinol Metab. 2012;97:1905–1912. doi: 10.1210/jc.2011-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fagin JA, Matsuo K, Karmakar A, et al. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179–184. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donghi R, Longoni A, Pilotti S, et al. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J Clin Invest. 1993;91:1753–60. doi: 10.1172/JCI116385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 2011;71:4403–4411. doi: 10.1158/0008-5472.CAN-10-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 49.Fukahori M, Yoshida A, Hayashi H, et al. The association between RAS gene mutations and clinical characteristics in follicular thyroid tumors: new insights from a single center and a large patient cohort. Thyroid. 2012;22:683–689. doi: 10.1089/thy.2011.0261. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Rostan G, Zhao H, Camp RL, et al. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003;21:3226–3235. doi: 10.1200/JCO.2003.10.130. [DOI] [PubMed] [Google Scholar]
- 51.Volante M, Rapa I, Gandhi M, et al. RAS mutations are the predominant molecular alteration in poorly differentiated thyroid carcinomas and bear prognostic impact. J Clin Endocrinol Metab. 2009;94:4735–4741. doi: 10.1210/jc.2009-1233. [DOI] [PubMed] [Google Scholar]
- 52.Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. 2012;118:1764–1773. doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 53.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 54.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 55.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321:86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nikiforova MN, Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid. 2009;19:1351–1361. doi: 10.1089/thy.2009.0240. [DOI] [PubMed] [Google Scholar]
- 57.Tufano RP, Bishop J, Wu G. Reoperative central compartment dissection for patients with recurrent/persistent papillary thyroid cancer: efficacy, safety, and the association of the BRAF mutation. Laryngoscope. 2012;122:1634–1640. doi: 10.1002/lary.23371. [DOI] [PubMed] [Google Scholar]
- 58.Basolo F, Torregrossa L, Giannini R, et al. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab. 2010;95:4197–4205. doi: 10.1210/jc.2010-0337. [DOI] [PubMed] [Google Scholar]
- 59.Elisei R, Viola D, Torregrossa L, et al. The BRAFV600E mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab. 2012;97:4390–4398. doi: 10.1210/jc.2012-1775. [DOI] [PubMed] [Google Scholar]
- 60.Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–470. doi: 10.1097/SLA.0b013e318148563d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–368. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]
- 62.Abubaker J, Jehan Z, Bavi P, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93:611–618. doi: 10.1210/jc.2007-1717. [DOI] [PubMed] [Google Scholar]
- 63.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 64.Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, et al. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 65.Xing M, Clark D, Guan H, et al. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol. 2009;27:2977–2982. doi: 10.1200/JCO.2008.20.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yip L, Nikiforova MN, Carty SE, et al. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery. 2009;146:1215–1223. doi: 10.1016/j.surg.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 67.O’Neill CJ, Bullock M, Chou A, et al. BRAF(V600E) mutation is associated with an increased risk of nodal recurrence requiring reoperative surgery in patients with papillary thyroid cancer. Surgery. 2010;148:1139–1145. doi: 10.1016/j.surg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 68.Prescott JD, Sadow PM, Hodin RA, et al. BRAF(V600E) status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence. Surgery. 2012;152:984–990. doi: 10.1016/j.surg.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barollo S, Pennelli G, Vianello F, et al. BRAF in primary and recurrent papillary thyroid cancers: the relationship with (131)I and 2-[(18)F]fluoro-2-deoxy-D-glucose uptake ability. Eur J Endocrinol. 2010;163:659–663. doi: 10.1530/EJE-10-0290. [DOI] [PubMed] [Google Scholar]
- 70.Mian C, Barollo S, Pennelli G, et al. Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin Endocrinol (Oxf) 2008;68:108–116. doi: 10.1111/j.1365-2265.2007.03008.x. [DOI] [PubMed] [Google Scholar]
- 71.Liu D, Hu S, Hou P, et al. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 2007;13:1341–1349. doi: 10.1158/1078-0432.CCR-06-1753. [DOI] [PubMed] [Google Scholar]
- 72.Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–4711. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. JAMA. in press doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg. 2008;32:747–753. doi: 10.1007/s00268-007-9453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xing M, Tufano RP, Tufaro AP, et al. Detection of BRAF mutation on fine needle aspiration biopsy specimens: a new diagnostic tool for papillary thyroid cancer. J Clin Endocrinol Metab. 2004;89:2867–2872. doi: 10.1210/jc.2003-032050. [DOI] [PubMed] [Google Scholar]
- 76.Henderson YC, Shellenberger TD, Williams MD, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. 2009;15:485–491. doi: 10.1158/1078-0432.CCR-08-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Popadich A, Levin O, Lee JC, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery. 2011;150:1048–1057. doi: 10.1016/j.surg.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Joo JY, Park JY, Yoon YH, et al. Prediction of occult central lymph node metastasis in papillary thyroid carcinoma by preoperative BRAF analysis using fine-needle aspiration biopsy: a prospective study. J Clin Endocrinol Metab. 2012;97:3996–4003. doi: 10.1210/jc.2012-2444. [DOI] [PubMed] [Google Scholar]
- 79.Lin KL, Wang OC, Zhang XH, et al. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol. 2010;17:3294–3300. doi: 10.1245/s10434-010-1129-6. [DOI] [PubMed] [Google Scholar]
- 80.Pelizzo MR, Boschin IM, Barollo S, et al. BRAF analysis by fine needle aspiration biopsy of thyroid nodules improves preoperative identification of papillary thyroid carcinoma and represents a prognostic factor. A mono-institutional experience. Clin Chem Lab Med. 2011;49:325–329. doi: 10.1515/CCLM.2011.031. [DOI] [PubMed] [Google Scholar]
- 81.Alzahrani AS, Xing M. Impact of lymph node metastases identified on central neck dissection (CND) on the recurrence of papillary thyroid cancer: potential role of BRAFV600E mutation in defining CND. Endocr Relat Cancer. 2012;20:13–22. doi: 10.1530/ERC-12-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Howell GM, Nikiforova MN, Carty SE, et al. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol. 2013;20:47–52. doi: 10.1245/s10434-012-2611-0. [DOI] [PubMed] [Google Scholar]
- 83.Brown AP, Chen J, Hitchcock YJ, et al. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:504–515. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 84.Fallahi B, Adabi K, Majidi M, et al. Incidence of second primary malignancies during a long-term surveillance of patients with differentiated thyroid carcinoma in relation to radioiodine treatment. Clin Nucl Med. 2011;36:277–282. doi: 10.1097/RLU.0b013e31820a9fe3. [DOI] [PubMed] [Google Scholar]
- 85.Lang BH, Wong IO, Wong KP, et al. Risk of second primary malignancy in differentiated thyroid carcinoma treated with radioactive iodine therapy. Surgery. 2012;151:844–850. doi: 10.1016/j.surg.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 86.Rubino C, de Vathaire F, Dottorini ME, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89:1638–1644. doi: 10.1038/sj.bjc.6601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–987. doi: 10.1016/j.surg.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 88.Sawka AM, Brierley JD, Tsang RW, et al. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol Metab Clin North Am. 2008;37:457–480. doi: 10.1016/j.ecl.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 89.Sacks W, Fung CH, Chang JT, et al. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: a systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid. 2010;20:1235–1245. doi: 10.1089/thy.2009.0455. [DOI] [PubMed] [Google Scholar]
- 90.Jonklaas J, Cooper DS, Ain KB, et al. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423–1424. doi: 10.1089/thy.2010.0308. [DOI] [PubMed] [Google Scholar]
- 91.Schvartz C, Bonnetain F, Dabakuyo S, et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab. 2012;97:1526–1535. doi: 10.1210/jc.2011-2512. [DOI] [PubMed] [Google Scholar]
- 92.Durante C, Montesano T, Attard M, et al. Long-term surveillance of papillary thyroid cancer patients who do not undergo postoperative radioiodine remnant ablation: is there a role for serum thyroglobulin measurement? J Clin Endocrinol Metab. 2012;97:2748–2753. doi: 10.1210/jc.2012-1123. [DOI] [PubMed] [Google Scholar]
- 93.Mallick U, Harmer C, Yap B, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–1685. doi: 10.1056/NEJMoa1109589. [DOI] [PubMed] [Google Scholar]
- 94.Schlumberger M, Catargi B, Borget I, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–1673. doi: 10.1056/NEJMoa1108586. [DOI] [PubMed] [Google Scholar]
- 95.Schlumberger M. Management of refractory thyroid cancers. Ann Endocrinol (Paris) 2011;72:149–157. doi: 10.1016/j.ando.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 96.Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 97.Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 98.Miyauchi A, Kudo T, Miya A, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. 2011;21:707–716. doi: 10.1089/thy.2010.0355. [DOI] [PubMed] [Google Scholar]
- 99.Deandreis D, Al GA, Leboulleux S, et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr Relat Cancer. 2011;18:159–169. doi: 10.1677/ERC-10-0233. [DOI] [PubMed] [Google Scholar]
- 100.Carr LL, Mankoff DA, Goulart BH, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010;16:5260–5268. doi: 10.1158/1078-0432.CCR-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Oncol (R Coll Radiol) 2010;22:464–468. doi: 10.1016/j.clon.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 102.Fagin JA. How thyroid tumors start and why it matters: kinase mutants as targets for solid cancer pharmacotherapy. J Endocrinol. 2004;183:249–256. doi: 10.1677/joe.1.05895. [DOI] [PubMed] [Google Scholar]
- 103.Ringel MD, Hayre N, Saito J, et al. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001;61:6105–6111. [PubMed] [Google Scholar]
- 104.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid. 2010;20:697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol. 1999;155:1967–1976. doi: 10.1016/S0002-9440(10)65515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897–905. doi: 10.1016/S1470-2045(12)70335-2. [DOI] [PubMed] [Google Scholar]
- 107.Ahmed M, Barbachano Y, Riddell A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol. 2011;165:315–322. doi: 10.1530/EJE-11-0129. [DOI] [PubMed] [Google Scholar]
- 108.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoftijzer H, Heemstra KA, Morreau H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161:923–931. doi: 10.1530/EJE-09-0702. [DOI] [PubMed] [Google Scholar]
- 111.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 112.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bible KC, Suman VJ, Molina JR, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sherman SI, Jarzab B, Cabanillas ME, et al. A phase II trial of the multi-targeted kinase inhibitor, lenvatinib (E7080), in advanced radioiodine -refractory differentiated thyroid cancer (DTC) J Clin Oncol. 2011;29(suppl):5503. abstr. [Google Scholar]
- 115.Cabanillas ME, Brose MS, Ramies DA, et al. Antitumor activity of cabozantinib (XL184) in a cohort of patients with differentiated thyroid cancer (DTC) J Clin Oncol. 2012;30(suppl):5547. abstr. [Google Scholar]
- 116.Bayer HealthCare Pharmaceuticals, Onyx Pharmaceuticals. [(accessed Jan 3, 2013)];Phase 3 DECISION trial of Nexavar (sorafenib) meets primary endpoint of improving progression-free survival in patients with radioactive iodine refractory differentiated thyroid cancer. 2013 http://finance.yahoo.com/news/phase-3-decision-trial-nexavar-073000142.html.
- 117.Hou P, Bojdani E, Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J Clin Endocrinol Metab. 2010;95:820–828. doi: 10.1210/jc.2009-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsimberidou AM, Vaklavas C, Wen S, et al. Phase I clinical trials in 56 patients with thyroid cancer: the MD Anderson Cancer Center experience. J Clin Endocrinol Metab. 2009;94:4423–4432. doi: 10.1210/jc.2009-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Massicotte M, Brassard M, Claude-Desroches M, et al. Off -label tyrosine kinas inhibitor treatments in patients with metastatic thyroid carcinomas: a study of the TUTHYREF network. 82nd annual meeting of the American Thyroid Association; Québec City; Sept. 2012:19–23. Abstract O21. [Google Scholar]
- 121.Sherman EJ, Ho AL, Fury MG, et al. A phase II study of temsirolimus/sorafenib in patients with radioactive iodine (RAI)-refractory thyroid carcinoma. J Clin Oncol. 2012;30(suppl):5514. abstr. [Google Scholar]
- 122.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu D, Hou P, Liu Z, et al. Genetic alterations in the phosphoinositide 3-kinase/Akt signaling pathway confer sensitivity of thyroid cancer cells to therapeutic targeting of Akt and mammalian target of rapamycin. Cancer Res. 2009;69:7311–7319. doi: 10.1158/0008-5472.CAN-09-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu R, Liu D, Trink E, et al. The Akt-specific inhibitor MK2206 selectively inhibits thyroid cancer cells harboring mutations that can activate the PI3K/Akt pathway. J Clin Endocrinol Metab. 2011;96:E577–E585. doi: 10.1210/jc.2010-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bass MB, Sherman SI, Schlumberger MJ, et al. Biomarkers as predictors of response to treatment with motesanib in patients with progressive advanced thyroid cancer. J Clin Endocrinol Metab. 2010;95:5018–5027. doi: 10.1210/jc.2010-0947. [DOI] [PubMed] [Google Scholar]
- 126.Ball DW, Sherman SI, Jarzab B, et al. Lenvatinib treatment of RAI-refractory differentiated thyroid cancer (DTC): cytokine and angiogenic factor (CAF) profiling in combination with tumor genetic analysis to identify markers associated with response. ASCO Annual Meeting; June 1–5, 2012; Chicago, IL. Abstract 5518. [Google Scholar]
- 127.Spano JP, Vano Y, Vignot S, et al. GEMOX regimen in the treatment of metastatic differentiated refractory thyroid carcinoma. Med Oncol. 2012;29:1421–1428. doi: 10.1007/s12032-011-0070-2. [DOI] [PubMed] [Google Scholar]
- 128.Crouzeix G, Michels JJ, Sevin E, et al. Unusual short-term complete response to two regimens of cytotoxic chemotherapy in a patient with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2012;97:3046–3050. doi: 10.1210/jc.2012-1630. [DOI] [PubMed] [Google Scholar]