Abstract
Specific cleavage of the transmembrane molecule, CUB domain-containing protein-1 (CDCP1), by plasmin-like serine proteases induces outside–in signal transduction that facilitates early stages of spontaneous metastasis leading to tumor cell intravasation, namely cell escape from the primary tumor, stromal invasion and transendothelial migration. We identified active β1 integrin as a biochemical and functional partner of the membrane-retained 70-kDa CDCP1 fragment, newly generated from its full-length 135-kDa precursor though proteolytic cleavage by serine proteases. Both in cell cultures and in live animals, active β1 integrin complexed preferentially with functionally activated, phosphorylated 70-kDa CDCP1. Complexing of β1 integrin the 70-kDa with CDCP1 fragment induced intracellular phosphorylation signaling, involving focal adhesion kinase-1 (FAK) and PI3 kinase (PI3K)-dependent Akt activation. Thus, inhibition of FAK/PI3K activities by specific inhibitors as well as short-hairpin RNA downregulation of β1 integrin significantly reduced FAK/Akt phosphorylation under conditions where CDCP1 was processed by serine proteases, indicating that FAK/PI3K/Akt pathway operates downstream of cleaved CDCP1 complexed with β1 integrin. Furthermore, this complex-dependent signaling correlated positively with high levels of tumor cell intravasation and dissemination. Correspondingly, abrogation in vivo of CDCP1 cleavage either by unique cleavage-blocking monoclonal antibody 10-D7 or by inhibition of proteolytic activity of plasmin-like serine proteases with aprotinin prevented β1 integrin/CDCP1 complexing and downstream FAK/Akt signaling concomitant with significant reduction of stromal invasion and spontaneous metastasis. Therefore, β1 integrin appears to serve as a motility-regulating partner mediating cross-talk between proteolytically cleaved, membrane-retained CDCP1 and members of FAK/PI3K/Akt pathway. This CDCP1 cleavage-induced signaling cascade constitutes a unique mechanism, independent of extracellular matrix remodeling, whereby a proteolytically cleaved CDCP1 regulates in vivo locomotion and metastasis of tumor cells through β1 integrin partnering. Our findings indicate that CDCP1 cleavage, occurring at the apex of a β1 integrin/FAK/PI3K/Akt signaling cascade, may represent a therapeutic target for CDCP1-positive cancers.
Keywords: metastasis, intravasation, invasion, transendothelial migration, Src kinase, prostate cancer
INTRODUCTION
Enhanced expression of CUB domain-containing protein-1 (CDCP1) correlates with poor prognosis in patients with distinct cancer types, including carcinomas of gastro-intestinal tract,1–4 pancreas,5 lung,1,4,6 kidney,7,8 breast4 and prostate.9 Highlighting the functional importance of CDCP1 in cancer, a number of independent approaches10–14 identified CDCP1 as a metastasis-associated cell surface protein, following its initial discovery at the gene level.1 In addition, forced downregulation of CDCP1 expression in animal models resulted in inhibition of metastatic dissemination of human lung and gastric cancer cells.3,15
CDCP1 has been implicated in regulation of tumor cell signaling via specific molecules such as Src and PKCδ that complex with the C-terminus of CDCP1.4,5,8,14–20 We have recently demonstrated that to achieve maximal signaling capacity in vivo, the full-length 135-kDa molecule CDCP1 should be proteolytically cleaved by the serine protease, plasmin, generating membrane-retained 70-kDa CDCP1 fragment.20 We also showed that to become functionally active, cleaved CDCP1 should be phosphorylated by the active Src. The C-terminus of cleaved and phosphorylated 70-kDa CDCP1 then acts as an in vivo docking platform for PKCδ, which itself becomes activated in a CDCP1/Src-dependent manner. This outside–in signaling, induced by external CDCP1 cleavage in vivo, ultimately results in intracellular Akt activation and suppression of PARP1-mediated cell apoptosis and leads to enhanced survival of tumor cells at late stages of the metastatic cascade, namely, during vascular extravasation and tissue colonization.20,21 However, specific molecular partners capable of transducing signals from phosphorylated 70-kDa CDCP1 to the Akt molecule have not been identified, thereby leaving critical gaps in the CDCP1 → Akt signaling. Furthermore, although we demonstrated that treatment with anti-CDCP1 cleavage-blocking antibodies inhibited tissue colonization of prostate carcinoma (PC) cells, it remained unclear whether CDCP1 and its cleavage may have any functional role during early steps of spontaneous metastasis preceding tumor cell arrest, extravasation and tissue colonization.
Using several metastasis models employing human PC-hi/diss and human fibrosarcoma (HT-hi/diss) cells naturally expressing high levels of CDCP1, we have demonstrated herein that proteolytic cleavage of CDCP1 is specifically, and independently of protease-mediated pathway clearing, involved in such early metastasis events as cell escape from the primary tumor, stroma invasion and intravasation. As directional cell motility is required for accomplishing each and all of these distinct metastasis-related processes, we focused our research on elucidating putative cell motility-regulating element(s), which could be structurally and functionally linked to in vivo cleaved CDCP1.
In the current study, we have identified β1 integrin as a critical 70-kDa CDCP1 partner and demonstrated that complexing of activated β1 integrin with the cleaved and phosphorylated CDCP1 induces β1 integrin signaling involving focal adhesion kinase-1 (FAK) phosphorylation and PI3 kinase (PI3K)-dependent Akt activation. This signal transduction pathway represents a unique molecular mechanism, in which proteolytic cleavage of a transmembrane molecule, namely CDCP1, is a prerequisite for its functional induction and complexing with a specific motility-mediating partner, namely β1 integrin. This novel ‘cleaved CDCP1 → β1 integrin → phospho Akt’ signaling axis mechanistically underscores the functional role of CDCP1 cleavage in the enhanced motility of aggressive cancer cells that is required for their efficient escape from primary tumors, invasion of adjacent stroma and crossing over endothelial barriers. As all these CDCP1 cleavage-dependent processes ultimately culminate in spontaneous tumor cell intravasation and vascular metastasis, targeted abrogation of CDCP1 cleavage in vivo, for example, with unique cleavage-blocking anti-CDCP1 monoclonal antibodies (mAbs), may therefore represent a therapeutic approach to control enhanced motility of aggressive cancer cells independent of controlling path-making matrix proteolysis.
RESULTS
CDCP1 cleavage is involved in spontaneous dissemination of carcinoma cells in a mouse orthotopic model for prostate cancer
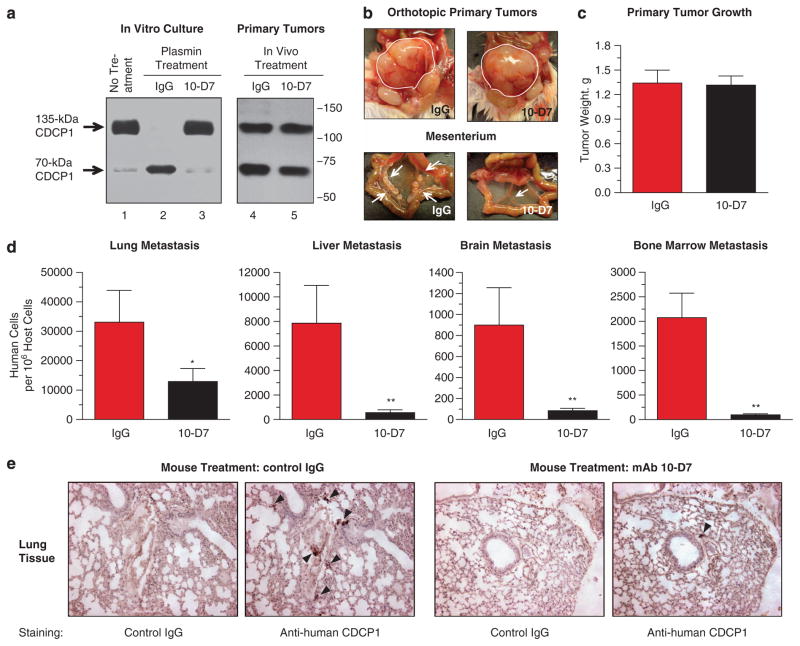
A distinct role for in vivo cleavage of CDCP1 during spontaneous metastasis was initially demonstrated in a mouse model of prostate cancer. PC-hi/diss cells were orthotopically implanted into the prostates of immunodeficient mice and allowed to establish primary tumors for 7 days, and then mice were treated with control IgG or CDCP1-specific mAb 10-D7 that is capable of completely blocking plasmin-induced cleavage of CDCP1 in vitro (Figure 1a, lanes 1–3). Treatment with mAb 10-D7 did not affect overall size and appearance of PC-hi/diss tumors (Figure 1b, top) or their weight (Figure 1c), but dramatically reduced the tumor colonization of mesenterium (Figure 1b, bottom). Furthermore, as quantified by Alu-quantitative PCR (qPCR), mAb 10-D7 significantly inhibited the rates of spontaneous metastasis of PC-hi/diss cells from primary prostate tumors into distinct internal organs such as lungs, liver, brain and bone marrow (Figure 1d). In addition, immunohistochemical analysis demonstrates small nodules of CDCP1-positive human cells in the lungs of control mice and almost complete absence of positively stained cells in the lungs from mice treated with mAb 10-D7 (Figure 1e), which is completely consistent with Alu-qPCR data. These data strongly indicate that cleavage of CDCP1 is involved in spontaneous dissemination of PC-hi/diss cells despite that the extent of CDCP1 processing in the bulk of primary tumors was not significantly diminished by mAb 10-D7 (Figure 1a, lanes 4–5). The latter finding could be attributed to the delay in tumor treatment with mAb 10-D7 (up to 6 –7 days post surgery) as well as to relatively low retention of inoculated antibodies in the mice (Supplementary Figure S1), which independently or in combination would allow for substantial cleavage of CDCP1 in the bulk tumor. However, taking into account the dramatic inhibition of metastasis at secondary sites (Figure 1d) and functional capacity of mAb 10-D7 to inhibit CDCP1 cleavage and CDCP1 signaling in vivo,20 we hypothesized that mAb 10-D7 blocked proteolytic processing of CDCP1 in a small subset of tumor cells, which in the absence of mAb 10-D7 would efficiently escape the primary site, invade the adjacent stroma and reach tumor-associated vasculature.
Figure 1.
CDCP1 cleavage facilitates spontaneous metastasis of PC cells in an orthotopic mouse model of human prostate cancer. (a) Analysis of CDCP1 expression and CDCP1 cleavage in PC-hi/diss cells in culture and in orthotopic primary tumors. Left panel, western blot analysis performed under non-reducing conditions with polyclonal anti-CDCP1 antibody on cell lysates from control EDTA-treated cultures (lane 1) or cultures pre-treated either with 20 μg/ml control IgG (lane 2) or CDCP1 mAb 10-D7 (lane 3), followed by 30-min exposure to 500 nM plasmin. Right panel, prostate tumors were initiated in immunodeficient mice by orthotopic implantation of PC-hi/diss cells. The mice with developing tumors were inoculated intraperitoneally, 2 times per week, with 100 μg control IgG (lane 4) or mAb 10-D7 (lane 5). Western blot analysis of CDCP1 was performed on lysates of primary tumors excised from mice euthanized at 4–5 weeks after surgery. The full-length 135-kDa and cleaved 70-kDa CDCP1 species are indicated in kDa on the left. The position of molecular weight markers is indicated on the right. (b) Effects of mAb 10-D7 treatment on tumor growth and intraperitoneal spread. Treatment with CDCP1 cleavage-blocking mAb 10-D7 does not affect overall appearance of prostate xenografts (top), but dramatically reduced the extent of mesenterium colonization (bottom). Prostate tumor xenografts are delineated with white lines, while tumor cell colonies on the mesenterium are indicated with arrows. (c) Anti-CDCP1 cleavage-blocking mAb does not affect tumorigenesis. Primary tumors from the IgG- and mAb 10-D7-treated mice were excised and weighed to determine the effect of mAb 10-D7 treatment on tumor growth. (d) Anti-CDCP1 cleavage-blocking mAb inhibits spontaneous metastasis of PC-hi/diss cells. Lungs, liver, brain and bone marrow were harvested and processed by Alu-qPCR to determine the number of metastasized human tumor cells within host tissues. Data are presented as means±s.e.m. determined from two independent experiments, involving 8–10 mice per treatment. * and **, P<0.05, one-tailed and two-tailed unpaired Student’s t-tests, respectively. (e) Immunohistochemical analysis of murine lung tissue for CDCP1-positive human cells. Consecutive paraffin sections of lung tissue harvested from the mice bearing orthotopically implanted PC-hi/diss tumors treated in vivo with control mouse IgG or mAb 10-D7, were immunostained with mAb 41-2 against human CDCP1 or control IgG. The small nodules of positively stained human cells (dark brown) are indicated by black arrowheads.
Serine protease-mediated cleavage of CDCP1 is required for dissemination of PC cells in the chick embryo model of spontaneous metastasis
To investigate our hypothesis that proteolytic cleavage of CDCP1 can determine the fate of spontaneously metastasizing tumor cells, we have employed a number of chick embryo models that are readily amenable for analyzing individual steps of the metastatic cascade, including those early stages that culminate in tumor cell intravasation. In a spontaneous metastasis model, where primary tumors can be initiated in the presence or absence of blocking reagents from the time of cell inoculation, PC-hi/diss cells were grafted onto the chorioallantoic membrane (CAM) of chick embryos along with mAb 10-D7 or control IgG, and received additional topical applications of corresponding antibodies. On day 7, samples of distal CAM and liver were analyzed by Alu-qPCR for the levels of human tumor cell intravasation and metastasis, respectively, while primary tumors were probed for cleavage status of CDCP1. Treatment with CDCP1-specific mAb 10-D7 resulted in a dramatic, 97–98%, decrease in the number of tumor cells that intravasated to the CAM (Figure 2a) and metastasized to the liver (Figure 2b). This diminishment in spontaneous dissemination was independent of primary tumor growth as indicated by similar tumor weights (Figure 2c). Although the majority of CDCP1 was not cleaved in PC-hi/diss cells before grafting on the CAM (Figure 2d, lane 1), by day 7 a significant portion of CDCP1 in IgG-treated embryos was represented by a 70-kDa species (Figure 2d, lane 2). However, this conversion of full-length 135-kDa CDCP1 to the 70-kDa CDCP1 fragment was completely abrogated in vivo by mAb 10-D7 (Figure 2d, lane 3). Therefore, decreased levels of spontaneous intravasation and metastasis from PC-hi/diss tumors appear to correlate directly with the lack of proteolytic cleavage of CDCP1 in the mAb 10-D7-treated hosts.
Figure 2.
Blocking of CDCP1 cleavage inhibits dissemination of PC cells in chick embryo CAM model for spontaneous metastasis. (a–c) Analysis of tumor growth and dissemination. PC-hi/diss cells were grafted onto the CAM of chick embryos (2 ×106 per embryo) in the presence of 50 μg control IgG or mAb 10-D7 or 0.1 TIU aprotinin. Developing tumors were treated additionally on days 2 and 4 with topical applications of corresponding agents. On day 7, the levels of tumor cell intravasation to the CAM (a) and metastasis to the liver (b) were quantified by Alu-qPCR. Primary tumors were excised and weighed to determine the effect of treatments on tumor growth (c). Presented are means±s.e.m. determined in pooled data from three independent experiments, each employing from 24–36 embryos per treatment variant. ***P<0.001. (d) Analysis of CDCP1 cleavage. Portions of primary tumors were lysed and analyzed for the status of CDCP cleavage by immunoprecipitation with anti-CDCP1 mAb 41–2 and western blotting under non-reducing conditions with poly-clonal anti-CDCP1 antibody. Lane 1, analysis of CDCP1 in the cells inoculated onto the CAM. Lanes 2–4, analysis of CDCP1 in primary CAM tumors treated with control IgG (lane 2), mAb 10-D7 (lane 3) or aprotinin (lane 4). The full-length 135-kDa and cleaved 70-kDa CDCP1 species are indicated on the right. The position of molecular weight markers is indicated in kDa on the left. Asterisk in lane 2 indicates the lower portion of the protein band containing 150-kDa IgG crossreacting with the secondary antibody.
Treatment of primary PC-hi/diss tumors with aprotinin, a potent inhibitor of plasmin-like serine proteases, substantially decreased the levels of tumor cell dissemination to the CAM and liver (by 94% and 99%, respectively), although not affecting tumor growth (Figure 2a–c). Consistent with inhibition of the proteolytic activity of plasmin in aprotinin-treated embryos, the diminishment in tumor cell dissemination by aprotinin was accompanied by a complete lack of cleaved CDCP1 identifiable in primary tumors (Figure 2d, lane 4). Such inhibition was confirmed independently by measuring direct activity of plasmin-like serine proteases in the plasma of embryos inoculated with PC-hi/diss cells alone or with aprotinin (Supplementary Figure S2).
Thus, spontaneous dissemination of prostate cancer cells in two distinct animal models appears to be critically dependent on tumor cell functions that are inhibited by the specific CDCP1 cleavage-blocking antibody before or during intravasation.
CDCP1 cleavage facilitates early steps of the metastatic cascade
To investigate the mechanisms underlying the role of cleaved CDCP1 in tumor cell intravasation, we performed kinetic analyses of PC-hi/diss dissemination in the CAM metastasis model (Figure 3a). This analysis indicates that in contrast to primary tumor growth, the levels of spontaneous dissemination of PC-hi/diss cells do not increase progressively with time, but rather cell dissemination manifests itself after several days of tumor formation in an apparent wave of tumor cell intravasation observed between day 6 and day 7. Consistent with the kinetic data, a single application of mAb 10-D7 conducted on day 6 after cell grafting, that is, at the time frame when the intravasation process is just initiated in already developed primary tumors, almost completely abrogated PC-hi/diss dissemination analyzed on day 7 (Figure 3b). Similar pronounced inhibitory effects on PC-hi/diss dissemination also were observed when 6-day-old tumors were treated with aprotinin (Figure 3b), affirming the notion that the proteolytic activity of CDCP1-cleaving, plasmin-like serine proteases is important for tumor cell intravasation and liver metastasis. To verify whether the treatments of day 6 primary tumors just delayed the onset of proliferation of intravasated cells, we performed an additional analysis of CAM tissue 2 days after a singular application of mAb 10-D7. This analysis indicated that PC-hi/diss dissemination to the distal CAM (that is, intravasation) was still fully inhibited on day 8 even though primary tumors continued to grow (Figure 3c).
Figure 3.
Kinetic analysis of PC cell dissemination in the chick embryo CAM model. (a) Time-course analysis of tumor cell dissemination. PC-hi/diss cells were grafted on the CAM of chick embryos. Primary tumors, distal portions of the CAM and the liver were harvested at the indicated time points to determine tumor weight and levels of CAM intravasation and liver metastasis by Alu-qPCR. (b–c) Effects of delays in CDCP1 cleavage-blocking treatments on PC cell dissemination. PC-hi/diss cells were grafted on the CAM of chick embryos and allowed to develop for 6 days, at which time point primary tumors were treated topically with 50 μg control IgG or mAb 10-D7 or 0.5 TIU aprotinin. The levels of CAM intravasation (left), liver metastasis (middle) and weight of primary tumors (right) were determined on day 7 (b), or on day 7 and day 8 (c). Presented are means±s.e.m. determined in pooled data from three independent experiments employing from 18 to 28 embryos per treatment variant. ***P<0.001. (d) Analysis of CDCP1 in primary CAM tumors. CDCP1 was immunoprecipitated with mAb 41–2 from primary CAM tumors treated with control IgG (lane 1), mAb 10-D7 (lane 2) or aprotinin (lane 3) as described in (b) and harvested the following day (day 7 after cell grafting). Western blot analysis was performed with polyclonal anti-CDCP1 antibody. The full-length 135-kDa and cleaved 70-kDa CDCP1 species are indicated on the right. The position of molecular weight markers is indicated in kDa on the left.
Western blot analysis of day 7 primary tumors performed 24 h after they were treated with mAb 10-D7 and aprotinin indicated a substantial build up of 135-kDa forms compared with control IgG-treated tumors, consistent with newly synthesized full-length CDCP1 being prevented from cleavage (Figure 3d). However, primary tumors had similar levels of 70-kDa CDCP1 cleavage species, suggesting that the membrane-retained CDCP1 fragments are relatively stable and their complete replacement by newly synthesized full-length CDCP1 can take more than 24 h. Our in vitro kinetic analyses of de novo synthesis of CDCP1 employing two distinct CDCP1-positive cell lines indicates that although newly synthesized, 135-kDa CDCP1 could be detected as early as 9 h following complete proteolytic cleavage of CDCP1, substantial quantities of cleaved, 70-kDa CDCP1 could be still present 48 h after treatment (Supplementary Figure S3). Taken together, these data indicate that inhibition of tumor cell dissemination in both the chick embryo CAM model and orthotopic mouse model of spontaneous metastasis could be achieved even though biochemical detection of cleavage inhibition within the bulk of the primary tumor is not apparent because of the stability and persistence of the 70-kDa cleaved CDCP1.
Our findings suggested that CDCP1 cleavage could be functionally important for a subset tumor cells that are actively escaping from the primary tumor and reaching and/or entering the vasculature although we do not have direct proof of this possibility. However, we examined an alternative possibility, namely that PC-hi/diss cells from mAb 10-D7-treated tumors were actually able to intravasate despite CDCP1 ligation by mAb 10-D7, but were then rapidly cleared from CAM tissue because of impaired vascular arrest and/or extravasation. Such clearance would have been interpreted as inhibition of vascular intravasation. To this end, PC-hi/diss cells were pre-treated with excess mAb 10-D7 to ligate CDCP1, washed and inoculated directly into the circulation. The levels of cell arrest (2 h), clearance (24 h) and colonization (5 days) in the CAM tissue were comparable or only slightly reduced in all mAb 10-D7-treated groups (Supplementary Figure S4) and thereby unable to account for the 97–98% inhibition of intravasation when primary tumors were treated with excess mAb 10-D7 (Figure 3).
Collectively, our findings support the notion that CDCP1 cleavage can be functionally important for a subpopulation of tumor cells during early metastatic events that lead to intravasation, namely during cell escape from the primary tumor, stromal invasion and/or transendothelial migration at the vascular entry step.
Specific roles of CDCP1 cleavage in tumor escape, stromal invasion and transendothelial migration
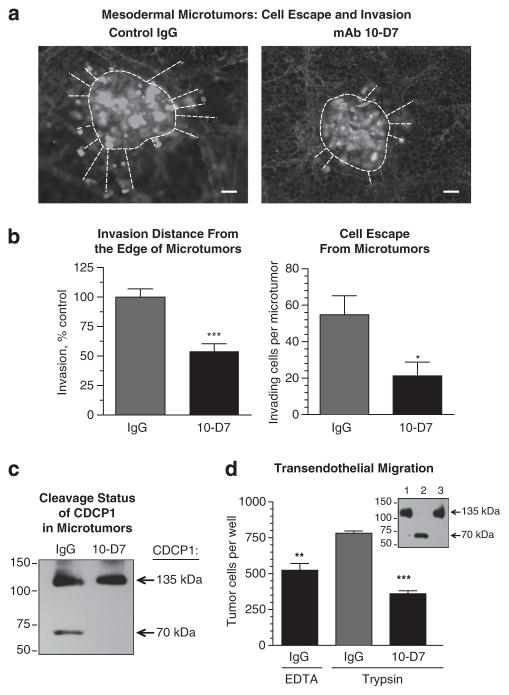
To verify whether in vivo cleavage of CDCP1 facilitates tumor cell escape and invasion, we have employed our intramesodermal model of stromal invasion.22 In this model, fluorescently tagged tumor cells are inoculated directly into the CAM mesoderm, where they form microtumors. Within 2–3 days, highly migratory cells initiate evacuation from the mesodermal microtumors and begin interstitial invasion that could be visualized by epifluorescent microscopy and quantified as migration distance covered by escaped cells. CellTracker-labeled PC-hi/diss cells were inoculated into the CAM and developing PC-hi/diss microtumors were treated locally with mAb 10-D7 or control IgG. As shown in Figure 4a, mAb 10-D7 significantly decreased stromal invasion of PC-hi/diss cells visualized on day 6 following intramesodermal inoculations. Quantitative analysis indicated 50–60% reduction both in productive distance traveled by escaped PC-hi/diss cells and in the number of cells that had escaped from microtumors treated with mAb 10-D7 (Figure 4b). Furthermore, immunoblot analysis of immunoprecipitated CDCP1 indicated the presence of cleaved 70-kDa CDCP1 in control IgG-treated microtumors, whereas only the 135-kDa species of CDCP1 was found in microtumors treated with mAb 10-D7 (Figure 4c). Similar results were obtained with another highly disseminating variant of a human tumor cell line, the HT-hi/diss variant of HT-1080 fibrosarcoma. Treatment of HT-hi/diss microtumors with mAb 10-D7 significantly reduced intrameso-dermal invasion of escaped cells and also abrogated 135-kDa → 70-kDa conversion of CDCP1 (Supplementary Figure S5).
Figure 4.
Inhibition of CDCP1 cleavage diminishes carcinoma cell escape from primary tumors, stromal invasion and transendothelial migration. (a) Cell escape and invasion in the intramesodermal microtumor model. Fluorescent-labeled PC-hi/diss cells (green) were inoculated directly into CAM mesoderm. Developing microtumors were treated twice, on days 2 and 4, with control IgG or mAb 10-D7. On day 6, the embryos were inoculated intravenously with rhodamine-tagged LCA to highlight the vasculature (red). Imaging of CAM microtumors and vasculature were performed at ×10 magnification. Dotted lines depict microtumor border and invasion distances covered by escaped tumor cells. Scale bar, 50 μm. (b) Quantification of tumor cell escape and invasion. Mean invasion distance (±s.e.m.) covered by escaped cells from the edge of the microtumor (left graph) and the mean number (±s.e.m.) of cells escaped from the microtumor (right graph) were determined in acquired images. * and ***, respectively, P<0.05 and <0.001. (c) Analysis of CDCP1 cleavage status in the CAM tissue. Following microscopy, mesodermal microtumors with adjacent stromal tissue were dissected from the CAM of embryos treated with control IgG or mAb 10-D7 as described in (a). CDCP1 was immunoprecipitated from tissue lysates with mAb 41–2 and analyzed by western blotting with polyclonal anti-CDCP1 antibody. The full-length 135-kDa and cleaved 70-kDa CDCP1 species are indicated on the right. The position of molecular weight markers is indicated in kDa on the left. (d) Transendothelial migration of PC cells. Fluorescent-tagged PC-hi/diss cells were pre-treated with 20 μg/ml IgG or mAb 10-D7, detached either with EDTA or trypsin, and placed into Transwell inserts with endothelial monolayers pre-grown on the Matrigel-covered undersurfaces. Tumor cells that crossed endothelial barriers were quantified after 2 days by flow cytometry. Data are means±s.e.m. from a representative experiment (out of three independent experiments) performed in triplicate. ** and ***, respectively, P<0.01 and <0.001, unpaired two-tailed Student’s t-tests. Inset, western blot analysis of CDCP1 immunoprecipitated from the transmigrated cells recovered from outer chambers 2 days after cells were pre-treated with IgG and EDTA (lane 1), IgG and trypsin (lane 2), or mAb 10-D7 and trypsin (lane 3), and placed into inserts. CDCP1 species are indicated on the right.
Tumor cell escape and invasion can lead aggressive tumor cells to angiogenic blood vessels, the preferred vascular conduits for intravasating tumor cells. To analyze whether CDCP1 cleavage may also facilitate crossing of vascular barriers, we performed a series of transendothelial migration experiments. To mimic in vivo conditions where tumor cells would face the abluminal surface of endothelial monolayers, human endothelial cells were grown on the Transwell undersurfaces pre-coated with Matrigel. Overall, serine protease-mediated proteolysis was reproduced by trypsin treatment of PC-hi/diss cells pre-incubated with control IgG. Alternatively, PC-hi/diss cells were treated with mAb 10-D7 to block cleavage of CDCP1, or control IgG-incubated cells were treated with ethylenediaminetetraacetic acid (EDTA) to preserve non-cleaved status of CDCP1. Following washing, PC-hi/diss cells were placed into flipped inserts and allowed to migrate, confronting first the Matrigel and extracellular matrix proteins deposited by endothelial cells and then the endothelial monolayers themselves.
Quantification of tumor cells in the lower chambers indicated that serine protease treatment caused approximately a 1.5-fold increase in the ability of PC-hi/diss cells to cross the endothelial monolayer. However, the rates of transendothelial migration of proteolytically treated cells were significantly inhibited by mAb 10-D7, on average by 62% (Figure 4d). As human endothelial cells express little or no CDCP1 (Supplementary Figure S6), the inhibitory effects of mAb 10-D7 on transendothelial migration could be attributed to inhibition of CDCP1 expressed in the PC-hi/diss cells. Further solidifying the link between CDCP1 cleavage and cleavage-mediated cell motility, immunoprecipitation analysis confirmed that 135-kDa CDCP1 was completely processed to the 70-kDa form in the protease/IgG-treated cells recovered from the outer chamber of Transwells, while demonstrating that CDCP1 was not cleaved in the transmigrated cells either pre-treated with EDTA or mAb 10-D7 (Figure 4d, inset).
Proteolysis-mediated cleavage of CDCP1 induces complex formation between phosphorylated 70-kDa CDCP1 and activated β1 integrin
The above-presented findings suggested that CDCP1 can mediate signal transduction via motility-regulating molecules specifically associated with the 70-kDa cleaved CDCP1. As β1 integrins are the critical molecules regulating cell adhesion, migration and invasion, we focused our analyses on a putative association of β1 integrin with the cleaved CDCP1. The PC-hi/diss cells were first pre-treated with either control IgG or mAb 10-D7, incubated with plasmin to induce CDCP1 processing, and then lysed, after which the CDCP1 protein was immunoprecipitated from the cell lysates (Figure 5a). Western blot analysis of precipitated CDCP1 confirmed its complete conversion to 70-kDa form in IgG-treated cells and preservation of 135-kDa form by mAb 10-D7. Indicating its functional activation, plasmin-cleaved CDCP1 was found strongly phosphorylated. Immunoprobing for integrins co-precipitated with CDCP1 demonstrated the presence of β1 integrin subunit, but exclusively with the plasmin-cleaved 70-kDa CDCP1. In contrast, when cleavage of CDCP1 was prevented by mAb 10-D7, no β1 integrin subunit was identified among proteins co-precipitated with the 135-kDa CDCP1. The apparent 130 kDa mol wt of β1 integrin co-precipitated with cleaved CDCP1 indicates that complex formation involves the mature β1 integrin. Furthermore, probing of eluted complexes with mAb P1E6 indicated presence of α2 integrin subunit, but again only under conditions allowing for CDCP1 cleavage (Figure 5a). Interestingly, probing CDCP1–IP complexes for β3 integrin failed to demonstrate complexing between β3 integrins either with cleaved or full-length CDCP1 (Supplementary Figure S7).
Figure 5.
Proteolytic cleavage of CDCP1 induces Src-dependent CDCP1 phosphorylation, β1 integrin complexing with the 70-kDa CDCP1, and intracellular phosphorylation signaling involving FAK and Akt activation. (a) In vitro plasmin-executed CDCP1 cleavage results in complexing of β1 integrin with the 70-kDa CDCP1. PC-hi/diss cells were pre-treated with 20 μg/ml control mouse IgG or mAb 10-D7, and then treated with 500 nM plasmin. CDCP1 was immunoprecipitated from cell lysates with mAb 41–2. Eluted proteins were analyzed by western blotting under reducing conditions with either polyclonal anti-CDCP1 antibody, polyclonal antibody recognizing phosphorylated CDCP1 or mAbs against human β1 or α2 integrin subunits. (b) Complexing of activated β1 integrin with the 70-kDa CDCP1 requires Src-dependent phosphorylation of cleaved CDCP1. PC-hi/diss cells were pre-treated with 20 μg/ml control mouse IgG or mAb 10-D7 or 0.5 μM SFK inhibitor dasatinib and then treated with EDTA (lane 1) or trypsin/EDTA (lanes 2–4) and lysed. Cleavage status of CDCP1 was confirmed by western blotting of cell lysates with polyclonal anti-CDCP1 antibody. Integrin complexes were precipitated from tissue lysates with the antibody HUTS-4 recognizing activated form of β1 integrin. Eluted proteins were then analyzed for the presence of co-precipitated CDCP1 and phospho CDCP1. Equal levels of precipitated activated β1 integrin are indicated by western blotting with the mAb 17783 recognizing β1 integrin after SDS–PAGE. Position of molecular weight markers in kDa is indicated on the left. (c) Signaling pathways induced by CDCP1 cleavage in vivo in the mouse lung retention model. PC-hi/diss cells were inoculated via tail vein into mice along with 100 μg control mouse IgG or mAb 10-D7. After 24 h, the lungs were excised from euthanized mice and lysed. Tissue lysates were subjected to western blot analysis for CDCP1 cleavage status or immunoprecipitation with anti-activated β1 integrin antibody HUTS-4. Eluted proteins were analyzed for β1 integrin levels with the mAb 17783 and for presence of CDCP1 and phosphorylated CDCP1. In addition, lung tissue lysates were analyzed for the levels of activated (phospho FAK) versus total FAK and activated (phospho Akt) versus total Akt.
Reciprocally, β1 integrins were precipitated from the lysates of PC-hi/diss cells with the HUTS-4 mAb recognizing the activated form of β1 integrin.23 Precipitated β1 integrin was associated only with the 70-kDa CDCP1 present in the IgG control cells treated with trypsin (Figure 5b, lane 2). Accordingly, CDCP1 was not co-precipitated with β1 integrin from the cells in which CDCP1 cleavage was prevented by treatment either with EDTA or mAb 10-D7 (Figure 5b, lanes 1 and 3). To verify whether the association of activated β1 integrin with cleaved CDCP1 depended on phosphorylation of cleaved CDCP1, PC-hi/diss cells were pre-treated with a potent inhibitor of Src kinases, dasatinib. We previously have demonstrated that dasatinib does not prevent cleavage of CDCP1, but completely abrogates Src-mediated phosphorylation of cleaved CDCP1.20 In agreement with these results, dasatinib did not prevent cleavage of CDCP1 in PC-hi/diss cells but prevented complex formation between activated β1 integrin and cleaved, but not phosphorylated, CDCP1 (Figure 5b, lane 4). Thus, it appears that complexing of activated β1 integrin with cleaved CDCP1 depends on Src activity and the phosphorylation status of CDCP1. As β1 integrin was immunoprecipitated by antibodies against activated integrin and equal amounts of precipitated integrin are shown across all four lanes by probing with ‘pan’ β1 integrin antibody, our data also indicate that dasatinib apparently did not affect activation status of β1 integrin. To further investigate whether complexing of cleaved CDCP1 with β1 integrin is facilitated by active status of integrin, we analyzed the presence of β1 integrin in CDCP1 complexes immunoprecipitated from cells treated with Mn2+, known to induce active conformation of β1 integrin recognizable by mAb HUTS-4.23 Flow cytometry analysis of PC-hi/diss cells demonstrated a threefold increase in the level of HUTS-4 epitope in the cells incubated in HEPES-NaCl-D-glucose-Ca2+/Mg2+ buffer additionally supplemented with 1 mM Mn2+ as compared with the cells incubated in the absence of Mn2+ (data not shown). Western blot analysis, depicted in Supplementary Figure S8, demonstrates that significantly more HUTS-4-reactive β1 integrin is co-precipitated with CDCP1 by mAb 10-D7 from Mn2+-treated PC-hi/diss cells. Together, these data strengthen the notion that Src activity and Src-mediated phosphorylation of cleaved CDCP1 are prerequisite for complexing of CDCP1 with activated β1 integrin.
To confirm that formation of β1 integrin/70-kDa CDCP1 complexes and complex-dependent signal transduction occurs in vivo, we employed our recently introduced lung retention assay in mice, which allows for investigation of plasmin-induced proteolysis of CDCP1 and proteolysis-associated signaling in live animals.20 To this end, PC-hi/diss cells were intravenously inoculated into mice along with control IgG or mAb 10-D7, and lung tissue with retained tumor cells was analyzed 24 h later (Figure 5c). Western blotting of total lung lysates confirmed that CDCP1 was completely cleaved in control IgG-treated cells, while CDCP1 cleavage was abrogated by mAb 10-D7. Furthermore, when β1 integrin was precipitated from the lung tissue, its complexing with CDCP1 was demonstrated only in control IgG-treated animals, that is, where CDCP1 was completely cleaved and, importantly, phosphorylated. In contrast, prevention of CDCP1 cleavage and thus the lack of CDCP1 phosphorylation completely abrogated complex formation between β1 integrin and full-length CDCP1 (Figure 5c).
To analyze the signaling that might be operating downstream of complex formation between CDCP1 and β1 integrin, the lung tissue was probed for the levels of phosphorylated FAK. Less activated FAK was demonstrated in the lung tissue of mAb 10-D7-treated animals as compared with IgG control. Similar analysis for Akt indicated significantly lower levels of activated Akt in the lung tissue where CDCP1 cleavage was prevented by mAb 10-D7 (Figure 5c). The involvement of FAK and Akt pathways was also confirmed for CAM tissue by treatment of chick embryos that had been inoculated with PC-hi/diss cells with FAK inhibitor 14 (FAKI-14) or PI3K/Akt inhibitor (wortmannin), respectively. Both inhibitors significantly diminished levels of CAM colonization, concomitant with almost complete abrogation of phosphorylated FAK and Akt in the CAM tissue (Supplementary Figure S9). Demonstration in two independent models (the mouse lung retention model and in chick embryo CAM model) that the inhibition of CDCP1/β1 integrin complexing correlates with diminishment of FAK and Akt activation is a significant observation as these signal activation processes have now been documented in vivo, namely in tumor cells that encountered the proteolytic activity of de novo generated plasmin (ref. 20 and Supplementary Figure S2).
To more directly demonstrate that outside–in signal transduction downstream of CDCP1 cleavage indeed involves signaling from β1 integrin, we stably knocked down β1 integrin expression in PC-hi/diss cells by a lentiviral FG12-tdTomato construct, generating the PC-β1KD cell line. Control cells, PC-Ctrl, were generated by infection with viral vector encoding control short-hairpin RNA sequence (see Supplementary Information). Downregulation of β1 integrin in PC-β1KD cells was confirmed by flow cytometry (data not shown) and western blot analyses (Supplementary Figure S10). Control and β1KD cells were treated in vitro with trypsin to induce cell surface cleavage of CDCP1 and then the cells were plated onto type I collagen-coated dishes for a 30-min adhesion to induce ‘outside-in’ β1 integrin signaling. Alternatively, the cells were treated with EDTA to preserve the original, uncleaved status of CDCP1 (Supplementary Figure S10, lanes 1–2 versus 3–4). The cell lysates from both cell types were analyzed for CDCP1 cleavage, β1 integrin expression, and levels of FAK and Akt activation in response to cell adhesion. These analyses have indicated that despite the cleaved status of CDCP1, downregulation of β1 integrin in trypsin-treated PC-β1KD cells resulted in significantly reduced levels of phospho FAK and also phospho Akt compared with the levels observed in control cells (Supplementary Figure S10, lane 3 versus lane 4). These data indicate that outside–in signal transduction downstream of CDCP1 cleavage involves FAK and Akt pathways that appear to require expression of β1 integrin.
Together our in vitro and in vivo findings strongly indicate that proteolytic cleavage of CDCP1 results in complex formation between 70-kDa phosphorylated CDCP1 and β1 integrin, concomitant with the induction of downstream signaling through phosphorylated FAK and Akt, two downstream cascade molecules involved in β1 integrin-mediated tumor cell locomotion.
Complex formation between plasmin-cleaved CDCP1 and β1 integrin regulates tumor invasion and transendothelial migration via Akt signaling pathway
To link mechanistically β1 integrin and cleaved CDCP1 with tumor cell dissemination, Akt signaling was abrogated under conditions facilitating plasmin cleavage of CDCP1 and cleavage-dependent complexing of β1 integrin with 70-kDa CDCP1. Akt signaling downstream of β1 integrin/70-kDa CDCP1 complex formation was inhibited in vitro and in vivo by wortmannin, a potent inhibitor of PI3K, activity of which is required for Akt phosphorylation.
PC-hi/diss cells were treated with plasmin in the presence of either aprotinin to inhibit plasmin-mediated CDCP1 cleavage, or vehicle to allow for plasmin-induced CDCP1 cleavage and cleavage-associated signaling; under the latter condition, a portion of cells was also treated with wortmannin to inhibit PI3K-mediated Akt activation. As expected, aprotinin prevented plasmin-induced cleavage of CDCP1, concomitant with the simultaneous lack of CDCP1 phosphorylation, CDCP1-associated β1 integrin and very low levels of activated Akt (Figure 6a, lane 1). In contrast, plasmin-induced cleavage of CDCP1 was associated with phosphorylation of the 70-kDa CDCP1, its complexing with β1 integrin and induction of Akt activation (Figure 6a, lane 2). Consistent with the position of PI3K downstream of β1 integrin, wortmannin did not change the cleavage status of CDCP1 or prevent β1 integrin association with the 70-kDa CDCP1, but completely inhibited Akt phosphorylation (Figure 6a, lane 3).
Figure 6.
Serine protease-induced Akt signaling regulates stromal invasion and transendothelial migration of tumor cells through complex formation between β1 integrin with cleaved 70-kDa CDCP1. (a) Akt signaling pathway is induced by plasmin-mediated cleavage of CDCP1 in a PI3K-dependent manner. PC-hi/diss cells were treated with 500 nM plasmin in the presence of serine protease inhibitor aprotinin (0.1 TIU/ml), vehicle (PBS-1% dimethyl sulfoxide (DMSO)) or PI3K inhibitor wortmannin (1 μM). CDCP1 was immunoprecipitated from cells lysates and analyzed for CDCP1 cleavage status, CDCP1 phosphorylation and presence of co-precipitated β1 integrin. In addition, whole cell lysates were analyzed for the levels of activated and total Akt. (b) Akt signaling regulates stromal invasion of PC cells. Fluorescent-tagged PC-hi/diss cells were inoculated directly into CAM mesoderm. Developing microtumors were treated twice on days 2 and 4 with 0.1 ml of aprotinin (0.1 TIU/ml), vehicle (PBS-2% DMSO) or wortmannin (2 μM). Invasion distances were determined in digitally acquired images as described in Figure 4. The data are mean±s.e.m. of invasion expressed as percentage of aprotinin-treated conditions (100%), that is, in the absence of serine protease activity. One of three independent experiments is presented. ***P<0.001. (c) Akt signaling pathway, induced in vitro under conditions allowing for activity of plasmin-like serine proteases, regulates transendothelial migration of PC cells. Fluorescent-tagged PC-hi/diss cells were incubated with aprotinin (0.1 TIU/ml), vehicle (PBS-1% DMSO) or wortmannin (1 μM). The cells were then treated with trypsin in the presence of the corresponding agents, washed and placed into Transwell inserts, the undersurface of which was covered by a monolayer of human endothelial cells pre-grown on a layer of Matrigel. Tumor cells were allowed to migrate for 2 days to the outer chamber filled with Dulbecco’s modified Eagle’s medium–5% fetal calf serum and corresponding pre-treatment agents. The number of green fluorescent cells that crossed the endothelial barrier was determined in individual outer chambers by flow cytometry. The data are mean±s.e.m. of migration expressed as percentage of aprotinin-treated conditions (100%), that is, in the absence of serine protease activity. One of four independent experiments is presented. **P<0.01.
We next investigated whether wortmannin-mediated inhibition of Akt signaling would affect the ability of PC-hi/diss cells to escape from primary tumors and invade CAM stroma in the in vivo setting where CDCP1 is cleaved in a plasmin-dependent manner. Thus, PC-hi/diss cells were injected into the CAM mesoderm, and developing microtumors were treated by local applications of vehicle, aprotinin or wortmannin. Analysis of cell invasion on day 6 indicated that PC-hi/diss invasion was significantly increased in the vehicle-treated animals compared with aprotinin-treated animals, but this CDCP1 cleavage-associated induction of invasion was completely abrogated by wortmannin treatment (Figure 6b).
In a similar fashion, we carried out a series of transendothelial migration assays where PC-hi/diss cells were exposed to plasmin treatment in the presence of aprotinin, vehicle or wortmannin, and then confronted with endothelial cell monolayers (Figure 6c). Consistent with the above-described findings on in vivo invasion, the levels of transendothelial migration induced by plasmin in control conditions were inhibited by wortmannin to levels below those observed in the presence of aprotinin. While lack of cell toxicity to a wide range of aprotinin concentration is known and has been verified in our previous studies,20,24 we specifically demonstrated herein that wortmannin was also well tolerated by PC-hi/diss cells and did not affect their proliferation (Supplementary Figure S11). Therefore, it appears that putative inhibition of cell proliferation or viability can be excluded from inhibitory effects of wortmannin on tumor migration and invasion. Thus, our data solidify the link between plasmin-mediated cleavage of CDCP1 and CDCP1-associated β1 integrin transducing signals regulating tumor cell migration and invasion via a PI3K/Akt activation pathway.
Functional role of CDCP1 cleavage and cleavage-associated β1 integrin signaling in tumor escape, stromal invasion and spontaneous metastasis involves FAK and PI3K/Akt pathways
To determine whether activation of Akt and FAK was required for spontaneous metastasis under physiological conditions sustaining CDCP1 cleavage, PC-hi/diss cells were grafted on the CAM of chick embryos and developing tumors were treated with wortmannin or FAKI-14. While neither wortmannin nor FAKI-14 affect tumor growth, both inhibitors dramatically diminished PC-hi/diss cell intravasation and metastasis by 80–99% compared with vehicle control (Figure 7a). Complementary to pronounced inhibition of PC-hi/diss dissemination by wortmannin, biochemical analyses of wortmannin-treated tumors indicated significantly reduced Akt activation despite evident CDCP1 cleavage, phosphorylation of cleaved CDCP1 and complexing of cleaved CDCP1 with α2β1 integrin (Figure 7b). Similar biochemical analyses of tumors treated with FAKI-14 indicated significantly reduced levels of activated FAK associated specifically with β1 integrin as well as activated FAK in whole tumor lysates (Figure 7c). Lower levels of Akt activation were also confirmed in FAKI-14-treated tumors despite CDCP1 cleavage and complexing of β1 integrin with cleaved CDCP1 (Figure 7c).
Figure 7.
Spontaneous dissemination and stromal invasion of PC cells involves FAK and PI3K/Akt pathways induced in vivo downstream of β1 integrin complexing with the cleaved phosphorylated CDCP1. (a) Spontaneous tumor cell dissemination, but not primary tumor growth, is inhibited by abrogating FAK and Akt activation. PC-hi/diss cells were grafted on the CAM of chick embryos and developing tumors were treated twice with 0.1 ml vehicle (PBS-1% DMSO), PI3K inhibitor wortmannin (1 μM) or FAK inhibitor 14 (10 μM). On day 7, primary tumors were harvested and weighed. Portions of distal CAM and liver were excised, processed and analyzed by Alu-qPCR to determine the number of tumor cells that intravasated into CAM vasculature and metastasized to the liver. Data (means±s.e.m.) are from two independent experiments for each inhibitor employing 13 and 11 embryos treated with wortmannin and FAK inhibitor, respectively, and a total of 31 control embryos. *, **, and *** indicate P<0.05, <0.01, <0.001, respectively. (b) Analysis of β1 integrin complexing with cleaved phosphorylated CDCP1 and activation of Akt signaling. CDCP1 was immunoprecipitated from primary CAM tumors harvested on day 7 from the embryos treated with vehicle or wortmannin as described above in (a). Presence of cleaved and phosphorylated CDCP1 and co-precipitated β1 and α2 integrin subunits was confirmed in eluted complexes by western blotting performed with corresponding specific mAbs. Total CAM tissue lysates were analyzed for the levels of phosphorylated Akt versus total Akt. (c) Analysis of FAK and Akt phosphorylation downstream of complexing between β1 integrin with cleaved CDCP1. β1 integrin was immunoprecipitated from primary CAM tumors harvested on day 7 from the embryos treated with vehicle or FAK inhibitor 14 as described above in (a). Eluted complexes were analyzed for the presence of β1 integrin subunit, cleavage status of CDDP1 and levels of phospho FAK versus total FAK specifically associated with β1 integrin. Total tissue lysates were also analyzed for the levels of phospho FAK and Akt versus corresponding total proteins. (d) Stromal invasion of PC-hi/diss cells depends on the activity of FAK and β1 integrin. Intramesodermal PC-hi/diss microtumors were treated once on day 3 with FAK inhibitor 14 (25 μl of 20 μM solution per bolus) or anti-β1 integrin function-blocking mAb P5D2 (25 μl of 50 μg/ml solution per bolus). Control microtumors were treated with 25 μl vehicle (2% DMSO in PBS). Invasion distances were measured on day 6 after cell grafting. ***P<0.001. Primary microtumors and invasion distances are indicated by dotted lines in representative images on the right. Scale bar, 50 μm.
Finally, we verified that the early steps of spontaneous dissemination, namely, tumor cell escape and invasion, also depended on FAK- and β1 integrin-mediated functions. To this end, intramesodermal PC-hi/diss microtumors were treated with FAKI-14 or anti-β1 integrin function-blocking mAb P5D2. As shown in Figure 7d, while neither treatment caused inhibition of microtumor development, both agents significantly reduced tumor cell invasion, indicating the involvement of β1 integrin and FAK pathways in the cell motility mechanisms involved in tumor cell dissemination in our model systems.
Together, our findings strongly indicate that spontaneous dissemination of PC-hi/diss cells requires execution of FAK and Akt pathways downstream of plasmin-mediated cleavage of CDCP1 and complexing of β1 integrin with cleaved CDCP1.
DISCUSSION
In this study, we have demonstrated for the first time that limited processing of cell surface CDCP1 by distinct serine proteases has an important role in spontaneous dissemination of human tumor cells, including PC and fibrosarcoma cells. Reaching this conclusion has been facilitated by the use of unique CDCP1-specific mAbs 10-D7 and 41-2 highly capable of blocking CDCP1 proteolysis, both in cell cultures and in live animals.20 Our in vivo and in vitro studies indicate that the anti-CDCP1 function-blocking antibodies, mAb 10-D7 and mAb 41-2, both of which were selected for the ability to supress cancer cell metastasis,10 inhibit overall CDCP1 function by preventing cleavage of CDCP1 and its functional activation by active Src. We have demonstrated that specific blocking of CDCP1 cleavage leads to inhibition of Akt activation and therefore, it could be anticipated that these cleavage-blocking CDCP1 antibodies would affect various Akt-dependent cell functions, such as tumor cell survival20 and tumor cell motility involved in tumor cell escape, invasion, and intravasation, the main highlights of the present study. These anti-CDCP1 mAbs prevent in vivo processing of CDCP1 by plasmin, the major host serine protease shown to be responsible for generation of the membrane-retained CDCP1 fragment from its full-length precursor during lung colonization in the experimental metastasis model in mice.20 CDCP1 can be cleaved also by trypsin, yielding the same membrane-retained 70-kDa fragment as plasmin. There is indication that CDCP1 can also be cleaved in vitro by the recombinant catalytic domain of matriptase,20,25 but cell surface-expressed matriptase was shown to be non-essential for proteolytic processing of CDCP1 in prostate cancer cells, including PC-3,18 the parental cells of our PC-hi/diss variant. The lack of CDCP1 cleavage in plasminogen-knockout mice or in wild-type mice inoculated with mAb 10-D7, as well as rescue of CDCP1 cleavage in plasminogen-knockout mice by supplementation with plasmin or plasminogen,20,26 further indicate that there are no major proteases other than plasmin that cleave CDCP1 in vivo, at least during the first 24 h when tumor cells are accessible to de novo generated enzyme.
In the orthotopic model for spontaneous dissemination of human prostate cancer used herein, mAb 10-D7 substantially diminished levels of PC-hi/diss metastasis into distinct murine organs, without affecting tumor growth or cleavage status of CDCP1 in the bulk of primary tumors. While these observations indicate that inhibition of cell proliferation was not contributing significantly to lowering metastasis levels, the findings also suggest that in vivo processing of CDCP1 was blocked by mAb 10-D7 in a small subset of primary tumor cells prevented from reaching and entering tumor-associated vasculature. This notion was elaborated and confirmed in the much more rapid and facile chick embryo model for spontaneous metastasis, where mAb 10-D7 treatment or direct inhibition of plasmin activity with aprotinin not only completely blocked CDCP1 cleavage in primary tumors but also dramatically decreased levels of tumor cells intravasation and dissemination to the liver.
The overall contribution of CDCP1 to tumor invasion in vivo has been previously indicated when CDCP1 expression was diminished by RNA interference in orthotopically implanted scirrhous carcinoma cells,3 but the precise functional role of CDCP1 cleavage was not evaluated. The present study uniquely shows that CDCP1 cleavage determines the efficiency of those steps of metastasis that precede tumor cell intravasation, namely tumor escape, stromal invasion and transendothelial migration. By specifically blocking CDCP1 processing with anti-CDCP1 cleavage-blocking mAb 10-D7, the escape from microtumors and stromal invasion was significantly reduced for PC and fibrosarcoma cells in our in vivo intramesodermal model. In the same model, inhibition of plasmin generation with anti-uPA mAb-112 or direct inhibition of plasmin activity with aprotinin (and therefore inhibition of plasmin-mediated processing of CDCP1) results in a >50% reduction of PC-hi/diss cell invasion,22 which now can at least partially be attributed to prevention of CDCP1 processing. This study also indicates a critical role of CDCP1 cleavage for tumor cell intravasation as prevention of CDCP1 processing by mAb 10-D7 diminished crossing over endothelial barriers by PC-hi/diss cells.
As enhanced cell motility in vivo underlies all early tumor dissemination events, our findings further indicate that cleaved CDCP1 initiates outside–in signaling regulating tumor cell motility in vivo. As the C-terminal domain of CDCP1 possesses several tyrosine residues and serves as a SFK substrate,10 CDCP1 functions were a priori linked to phosphorylation signaling. Several in vitro studies have implicated CDCP1 phosphorylation in cell adhesion14,15,25,27 and motility;3,5,19,27 however, the role of CDCP1 phosphorylation in distinct cell functions remains controversial and the reported CDCP1 phosphorylation was not generally linked to the cleavage status of CDCP1. Recently, we have specifically linked CDCP1 phosphorylation and outside–in signaling to plasmin-mediated CDCP1 cleavage that was critically important for PC cell survival during late steps of metastasis, namely tissue colonization.20 Further support for the link between CDCP1 cleavage and Akt phosphorylation signaling is provided by diminished levels of activated Akt in HEK cells transfected with a cleavage-resistant CDCP1 mutant in contrast to wild-type CDCP1 control.20 The current signaling investigation has been aimed to establish whether phosphorylation signaling induced by in vivo cleaved CDCP1 also regulates early dissemination of PC cells, specifically during those steps that critically depend on cell motility and precede vascular arrest and extravasation at secondary sites.
The present study demonstrates for the first time that cleavage of CDCP1 and generation of the 70-kDa CDCP1 fragment in vivo is a prerequisite for effective CDCP1 signal transduction during spontaneous dissemination of PC cells. Furthermore, we show that acquisition of signaling capacity by 70-kDa CDCP1 requires its phosphorylation, which occurs only when active Src binds to the cleaved molecule. In addition, our data show that although both active and non-active Src can dock the 70-kDa CDCP1, proteolytic cleavage is a necessary step for efficient induction of Src binding and phosphorylation of CDCP1 by active Src. This specific SFK-dependent cascade, whereby proteolytic processing of CDCP1 leads to its functional activation and phosphorylation-dependent signaling, is an important mechanism that operates in vivo, both during early stages of spontaneous metastasis, as shown herein, and during late phases of dissemination, as we demonstrated previously,20 and also in culture conditions in vitro.18,28 CDCP1 cleavage-dependent signaling involves Src-dependent phosphorylation of PKCδ, which specifically docks onto the functionally activated CDCP1 fragment.18,20 Although in vitro data showed SFK-dependent phosphorylation of PKCδ after its binding to CDCP1–SFK complexes,15,16,19,28 our studies emphasize that phosphorylation of both cleaved CDCP1 and CDCP1-bound PKCδ in vivo strictly requires the Src activity. The Akt kinase is another key signaling member of CDCP1 cleavage-induced cascade, the functional role of which was demonstrated previously for tumor cell survival in experimental metastasis.20 In this study, we illuminated a novel function of Akt, acting in a CDCP1 cleavage-dependent manner during such early stages of spontaneous metastasis as tumor cell escape, stromal invasion and transendothelial migration.
In search for those member(s) of the CDCP1 → Akt signaling cascade that would be capable of transducing signals for enhanced motility from the protease-generated, cell membrane-retained CDCP1 to the cytoplasmic Akt, we identified activated β1 integrin as a specific molecular partner of cleaved 70-kDa CDCP1. Reciprocally, the cleaved and phosphorylated CDCP1 fragment was found within the in vivo formed β1 integrin complexes, thereby solidifying the link between functionally active CDCP1 and β1 integrin. The precipitated full-length 135-kDa CDCP1, protected from proteolytic cleavage by mAb 10-D7, and the cleaved 70-kDa CDCP1, protected from phosphorylation by the Src inhibitor dasatinib, were essentially free of β1 integrin, thereby indicating that complexing with β1 integrin requires both proteolytic processing and functional activation of CDCP1. It also appears that β1 integrin has to be activated in order to complex with cleaved and phosphorylated CDCP1. Furthermore, our biochemical analyses indicate that membrane-retained, cleaved CDCP1 complexes specifically with mature 130-kDa β1 integrin and that this complexing occurs within the plasma membrane of intact cells in sync with the proteolytic cleavage of CDCP1 and not within cell lysates, which in addition to mature β1 integrin molecules would also contain a pool of immature intracellular β1 integrin represented by 100-kDa precursor molecules. We also have demonstrated that α2β1 integrin is among those integrins within the β1 integrin family that can complex with the cleaved CDCP1, although other α subunits are likely to be found in stable CDCP1–β1 integrin complexes, depending on the cell type or particular conditions that might induce specific α integrin expression or activation. In addition, our biochemical analyses did not demonstrate β3 integrin subunit in the CDCP1-immuno-precipitated complexes regardless of CDCP1 cleavage status, pointing to a possibility that β1 integrin(s) could represent specific partner(s) of cleaved CDCP1.
Complex formation between β1 integrin and functionally activated, cleaved CDCP1 may provide an additional means for β1 integrin heteromolecular clustering, an important mechanism implicated in the initiation of intracellular signal transduction by several transmembrane molecules.29,30 In agreement, our study demonstrates that β1 integrin/70-kDa CDCP1 complex formation in vivo is accompanied by enhancement of FAK phosphorylation and dramatic induction of Akt activation, thereby further extending our newly established CDCP1–Src-β1 integrin signaling cascade. In several studies employing cell culture systems, CDCP1 phosphorylation and FAK phosphorylation were shown to mutually dampen each other, possibly because of substrate competition for SFK.19,27,28,31 Under specific anchorage-independent culture conditions, CDCP1 signaling has also been shown to interfere with β1 integrin-mediated cell attachment.27 It appears that in our model systems, where CDCP1 is naturally expressed in bona fide tumor cells and is cleaved by the natural serine protease plasmin, the enhanced phosphorylation of FAK is consistent with its docking to the CDCP1-complexed β1 integrin. Studies in breast cancer point to FAK as a core component of integrin signaling that promotes Src-mediated phosphorylation functions and PI3K-dependent tumor migration, invasion and metastasis.32 In support of reported important roles of FAK activation in vascular dissemination of tumor cells,33,34 inhibition of FAK activity significantly reduced the levels of PC intravasation and liver metastasis in this study. Therefore, our data indicate that in prostate cancer, the synchronized cooperation between phosphorylated cleaved CDCP1 and β1 integrin-associated FAK also results in synergistic signaling that facilitates tumor cell motility and dissemination through PI3K-dependent Akt activation. The involvement of FAK and Akt downstream of CDCP1 cleavage and β1 integrin complexing was confirmed by the use of a specific FAK inhibitor (FAKI-14) and a potent and specific inhibitor of the direct Akt activator, PI3K (wortmannin). These two inhibitors respectively inhibited FAK and Akt activation concomitant with diminishment of CDCP1-dependent stromal invasion, transendothelial migration and vascular dissemination. Moreover, CDCP1-mediated induction of these important tumor cell functions was abrogated by FAKI-14 and wortmannin, despite complex formation between 70-kDa phosphorylated CDCP1 and β1 integrin, confirming that activated FAK and Akt function downstream of CDCP1 cleavage-induced signaling cascade. Finally, by employing an anti-β1 integrin function-blocking mAb, we demonstrated that CDCP1 cleavage-mediated tumor cell escape and invasion indeed depends on β1 integrin activity.
Our full appreciation of the role of CDCP1 signaling in metastasis went from the discovery of CDCP1 at the protein level and demonstration of overall importance of CDCP1 expression in tumor cells,10 to the indication of when and where CDCP1 functions during metastatic dissemination,21 to the demonstration of the functional importance of CDCP1 cleavage in tumor cell functions in vitro18 and tumor colonization in vivo,20 and now in the present study, to the discovery of the functional importance of complexing between cleaved CDCP1 and β1 integrin and complex-dependent signaling via the FAK/PI3K/Akt pathway. Our ongoing structure–function analysis will allow us to identify those specific domains in the β1 integrin and CDCP1 molecules that are responsible for partner complexing and downstream FAK/PI3K/Akt signaling, further deepening our understanding of the role of CDCP1 in cancer. Taken together, our findings have illuminated a novel molecular mechanism, whereby the enhanced motility of aggressive CDCP1-expressing tumor cells during early stages of their metastatic dissemination is supported by tightly linked and coordinated biochemical events, cascading from plasmin-induced cleavage of cell surface 135-kDa CDCP1 to Src-mediated phosphorylation of newly generated 70-kDa CDCP1 fragment, to complex formation between phosphorylated cleaved CDCP1 and activated β1 integrin, to β1 integrin-mediated FAK activation, ultimately culminating in PI3K-mediated Akt activation (Figure 8). The proteolytic cleavage and functional activation of the transmembrane molecule, CDCP1, accompanied by complexing of the membrane-retained, functionally activated fragment with the major motility-regulating element, β1 integrin, constitutes a novel proteolysis-dependent mechanism, acting independent of the path-making, matrix proteolysis events that also occur during early steps of cancer spreading. Abrogation of CDCP1 cleavage specifically at the apex of this complex signaling cascade may represent an efficient therapeutic approach to prevent vascular dissemination of dysregulated CDCP1-overexpressing cancer cells.
Figure 8.
Proteolytic cleavage of CDCP1 contributes to enhanced tumor cell survival and motility through the FAK and Akt signaling pathways downstream of complex formation between cleaved CDCP1 and β1 integrin. Schematic depicts a sequence of events that follow proteolytic cleavage of the cell surface CDCP1 molecule, consisting of three extracellular CUB domains, a transmembrane domain (TM) and a C-terminal domain (CT) containing several tyrosine residues (Tyr). Specifically, the serine protease plasmin executes singular cleavage of the full-length 135-kDa CDCP1, clipping off a 65-kDa fragment and generating the 70-kDa membrane-retained fragment. This 70-kDa fragment serves as a docking platform for Src family kinases, including Src. If the activity of Src kinase is inhibited by the Src inhibitor dasatinib, the cleaved CDCP1 does not become phosphorylated although inactive Src still docks onto the C-terminus of cleaved 70-kDa CDCP1. If cytoplasmic Src is active (phosphorylated, pSrc), CDCP1-bound pSrc phosphorylates the C-terminus of CDCP1 (70-kDa pCDCP1). Phosphorylation of 70-kDa CDCP1 at Tyr residues facilitates docking of PKCδ, which becomes activated (pPKCδ) by CDCP1-bound active Src. The phosphorylated 70-kDa CDCP1 forms stable complexes with β1 integrins (e.g., α2β1), which in turn enhances phosphorylation of integrin-associated FAK (pFAK) and leads to PI3K-dependent activation of Akt. Inhibition of FAK activity (e.g, by specific FAK inhibitor 14) prevents FAK phosphorylation, leading to diminished phosphorylation of Akt (pAkt). Akt activation downstream of FAK can be abrogated completely through inhibition of the direct activator of Akt, PI3K (e.g, by wortmannin). Ultimately, the inhibition of FAK/PI3K/Akt pathway reduces signaling for cell survival and motility. While CDCP1 cleavage signaling is crucial for tumor cell survival during late stages of metastasis, namely tissue colonization at the secondary sites, cleaved CDCP1 signals for enhanced motility during early stages of spontaneous dissemination, namely cell escape from the primary tumor, stromal invasion and intravasation. Synergistically, these motility-involving processes contribute to enhanced metastatic dissemination of aggressive CDCP1-expressing tumor cells.
MATERIALS AND METHODS
Reagents
The CDCP1-specific antibodies mAb 41–2 and 10-D7 were generated in our laboratory10 and described in Deryugina et al.21 Additional information about antibodies and reagents can be found in the Supplementary information.
Cell lines and culture conditions
Highly disseminating variants of the human PC-3 cell line, PC3-hi/diss, and fibrosarcoma HT-1080, HT-hi/diss, were isolated in vivo and described.35,36 The generation of PC-control and PC-β1KD cells, stably expressing control short-hairpin RNA or short-hairpin RNA knocking down β1 integrin, is described in the Supplementary information. Unless otherwise specified, the cells were passaged using enzyme-free cell dissociation solution (Millipore, Billerica, MA, USA) and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (D-10). Human dermal microvascular endothelial cells were from Lonza (Anaheim, CA, USA).
Intramesodermal model for tumor cell escape and invasion in vivo
Following labeling with 5 μM CellTracker Green, PC-hi/diss and HT-hi/diss cells were resuspended at 2 × 106/ml and 1 × 106/ml, respectively. Five to seven small boluses of tumor cells (3–5 μl) were injected directly into the CAM mesoderm of chick embryos as described.36 Individual microtumors were treated twice on days 2 and 4 (PC-hi/diss) or once on day 2 (HT-hi/diss) with mAb 10-D7 or control IgG (20 μg/ml), aprotinin (0.1 TIU/ml) or wortmannin (1 μM) in 20 μl phosphate-buffered saline (PBS)-1% dimethyl sulfoxide. On day 5 (HT-hi/diss) or day 6 (PC-hi/diss), embryos were inoculated with rhodamine-conjugated agglutinin (LCA; Vector Labs, Burlingame, CA, USA) to highlight the vasculature. Portions of the CAM with microtumors were excised and imaged using a Carl Zeiss Axio Imager (Carl Zeiss Microscopy GmbH, Germany). Quantification of tumor cell escape and invasion was performed using ImageJ software (NIH, Bethesda, MD, USA). The mean number of the cells escaped and the mean of invasion distances from the microtumor–CAM border was determined for each microtumor. A total of 11 to 13 individual microtumors from six to eight embryos were analyzed for each variable in two independent experiments.
Lung retention assay
The assay was performed essentially as recently described.20 Six- to eight-week-old mice were inoculated via lateral tail vein with 1 × 106 cells along with 50 μg mAb 10-D7 or control IgG. After 24 h, lung tissue lysates were analyzed by immunoprecipitation and/or western blotting.
Orthotopic prostate tumor model, chick embryo model for spontaneous metastasis, quantification of human cells by Alu-qPCR, transendothelial migration, flow cytometry, western blotting and immunoprecipitation
These well-established or already-published procedures are described in the Supplementary information.
Statistical analysis and data presentation
Data processing and statistical analyses were done using GraphPad Prism software (GraphPad, San Diego, CA, USA). Levels of tumor cell dissemination are expressed as number of human cells determined by Alu-qPCR within 106 host cells and presented as means±s.e.m. calculated from numerical data from a representative or pooled independent experiments. Unless otherwise indicated, unpaired Student’s t-test was used to determine P-values for the differences between the experimental data sets; P<0.05 was considered as statistically significant.
Supplementary Material
Acknowledgments
This study was supported by NIH grants R01 CA 129484 and R01 CA 105412 (to JPQ), NIH/NCRR/STSI Grant RR 025774 (Pilot Award to EID), Postdoctoral Fellowship from the Science and Innovation Ministry of Spain (to BC), Research Fellowships from Nachwuchsförderungskredit/Stiefel-Zangger Foundation, University of Zurich (to IR), and NIH grants HL56595 and HL57900 (to SJS).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- 1.Scherl-Mostageer M, Sommergruber W, Abseher R, Hauptmann R, Ambros P, Schweifer N. Identification of a novel gene, CDCP1, overexpressed in human colorectal cancer. Oncogene. 2001;20:4402–4408. doi: 10.1038/sj.onc.1204566. [DOI] [PubMed] [Google Scholar]
- 2.Perry SE, Robinson P, Melcher A, Quirke P, Buhring HJ, Cook GP, et al. Expression of the CUB domain containing protein 1 (CDCP1) gene in colorectal tumour cells. FEBS Lett. 2007;581:1137–1142. doi: 10.1016/j.febslet.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Uekita T, Tanaka M, Takigahira M, Miyazawa Y, Nakanishi Y, Kanai Y, et al. CUB-domain-containing protein 1 regulates peritoneal dissemination of gastric scirrhous carcinoma. Am J Pathol. 2008;172:1729–1739. doi: 10.2353/ajpath.2008.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CH, Baehner FL, Spassov DS, Ahuja D, Wang D, Hann B, et al. Phosphorylation of the SRC epithelial substrate trask is tightly regulated in normal epithelia but widespread in many human epithelial cancers. Clin Cancer Res. 2009;15:2311–2322. doi: 10.1158/1078-0432.CCR-08-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazawa Y, Uekita T, Hiraoka N, Fujii S, Kosuge T, Kanai Y, et al. CUB domain-containing protein 1, a prognostic factor for human pancreatic cancers, promotes cell migration and extracellular matrix degradation. Cancer Res. 2010;70:5136–5146. doi: 10.1158/0008-5472.CAN-10-0220. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda J, Oda T, Inoue M, Uekita T, Sakai R, Okumura M, et al. Expression of CUB domain containing protein (CDCP1) is correlated with prognosis and survival of patients with adenocarcinoma of lung. Cancer Sci. 2009;100:429–433. doi: 10.1111/j.1349-7006.2008.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awakura Y, Nakamura E, Takahashi T, Kotani H, Mikami Y, Kadowaki T, et al. Microarray-based identification of CUB-domain containing protein 1 as a potential prognostic marker in conventional renal cell carcinoma. J Cancer Res Clin Oncol. 2008;134:1363–1369. doi: 10.1007/s00432-008-0412-4. [DOI] [PubMed] [Google Scholar]
- 8.Razorenova OV, Finger EC, Colavitti R, Chernikova SB, Boiko AD, Chan CK, et al. VHL loss in renal cell carcinoma leads to up-regulation of CUB domain-containing protein 1 to stimulate PKCdelta-driven migration. Proc Natl Acad Sci USA. 2011;108:1931–1936. doi: 10.1073/pnas.1011777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siva AC, Wild MA, Kirkland RE, Nolan MJ, Lin B, Maruyama T, et al. Targeting CUB domain-containing protein 1 with a monoclonal antibody inhibits metastasis in a prostate cancer model. Cancer Res. 2008;68:3759–3766. doi: 10.1158/0008-5472.CAN-07-1657. [DOI] [PubMed] [Google Scholar]
- 10.Hooper JD, Zijlstra A, Aimes RT, Liang H, Claassen GF, Tarin D, et al. Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen. Oncogene. 2003;22:1783–1794. doi: 10.1038/sj.onc.1206220. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Nyalwidhe JO, Guo S, Drake RR, Semmes OJ. Targeted identification of metastasis-associated cell-surface sialoglycoproteins in prostate cancer. Mol Cell Proteomics. 2011;10:M110.007294. doi: 10.1074/mcp.M110.007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Leroy C, Fialin C, Sirvent A, Simon V, Urbach S, Poncet J, et al. Quantitative phosphoproteomics reveals a cluster of tyrosine kinases that mediates SRC invasive activity in advanced colon carcinoma cells. Cancer Res. 2009;69:2279–2286. doi: 10.1158/0008-5472.CAN-08-2354. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Ong SE, Badu-Nkansah K, Schindler J, White FM, Hynes RO. CUB-domain-containing protein 1 (CDCP1) activates Src to promote melanoma metastasis. Proc Natl Acad Sci USA. 2011;108:1379–1384. doi: 10.1073/pnas.1017228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121:271–280. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Uekita T, Jia L, Narisawa-Saito M, Yokota J, Kiyono T, Sakai R. CUB domain-containing protein 1 is a novel regulator of anoikis resistance in lung adenocarcinoma. Mol Cell Biol. 2007;27:7649–7660. doi: 10.1128/MCB.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvares SM, Dunn CA, Brown TA, Wayner EE, Carter WG. The role of membrane microdomains in transmembrane signaling through the epithelial glycoprotein Gp140/CDCP1. Biochim Biophys Acta. 2008;1780:486–496. doi: 10.1016/j.bbagen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Wortmann A, Burke LJ, Reid JC, Adams MN, Abdul-Jabbar I, et al. Proteolysis-induced N-terminal ectodomain shedding of the integral membrane glycoprotein CUB domain-containing protein 1 (CDCP1) is accompanied by tyrosine phosphorylation of its C-terminal domain and recruitment of Src and PKCdelta. J Biol Chem. 2010;285:26162–26173. doi: 10.1074/jbc.M109.096453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benes CH, Poulogiannis G, Cantley LC, Soltoff SP. The SRC-associated protein CUB domain-containing protein-1 regulates adhesion and motility. Oncogene. 2012;31:653–663. doi: 10.1038/onc.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casar B, He Y, Iconomou M, Hooper JD, Quigley JP, Deryugina EI. Blocking of CDCP1 cleavage in vivo prevents Akt-dependent survival and inhibits metastatic colonization through PARP1-mediated apoptosis of cancer cells. Oncogene. 2012;31:3924–3938. doi: 10.1038/onc.2011.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deryugina EI, Conn EM, Wortmann A, Partridge JJ, Kupriyanova TA, Ardi VC, et al. Functional role of cell surface CUB domain-containing protein 1 in tumor cell dissemination. Mol Cancer Res. 2009;7:1197–1211. doi: 10.1158/1541-7786.MCR-09-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekes EM, Deryugina EI, Kupriyanova TA, Zajac E, Botkjaer KA, Andreasen P, et al. Activation of pro-uPA is critical for initial escape from the primary tumor and hematogenous dieesmination of human carcinoma cells. Neoplasia. 2011;13:806–821. doi: 10.1593/neo.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 24.Bekes EM, Deryugina EI, Kupriyanova TA, Zajac E, Botkjaer KA, Andreasen PA, et al. Activation of pro-uPA is critical for initial escape from the primary tumor and hematogenous dissemination of human carcinoma cells. Neoplasia. 2011;13:806–821. doi: 10.1593/neo.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt AS, Erdjument-Bromage H, Tempst P, Craik CS, Moasser MM. Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene. 2005;24:5333–5343. doi: 10.1038/sj.onc.1208582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deryugina EI, Quigley JP. Cell surface remodeling by plasmin: a new function for an old enzyme. J Biomed Biotechnol. 2012;2012:564529. doi: 10.1155/2012/564259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spassov DS, Wong CH, Sergina N, Ahuja D, Fried M, Sheppard D, et al. Phosphorylation of Trask by Src kinases inhibits integrin clustering and functions in exclusion with focal adhesion signaling. Mol Cell Biol. 2011;31:766–782. doi: 10.1128/MCB.00841-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wortmann A, He Y, Christensen ME, Linn M, Lumley JW, Pollock PM, et al. Cellular settings mediating Src substrate switching between focal adhesion kinase tyrosine 861 and CUB-domain-containing protein 1 (CDCP1) tyrosine 734. J Biol Chem. 2011;286:42303–42315. doi: 10.1074/jbc.M111.227462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown TA, Yang TM, Zaitsevskaia T, Xia Y, Dunn CA, Sigle RO, et al. Adhesion or plasmin regulates tyrosine phosphorylation of a novel membrane glycoprotein p80/gp140/CUB domain-containing protein 1 in epithelia. J Biol Chem. 2004;279:14772–14783. doi: 10.1074/jbc.M309678200. [DOI] [PubMed] [Google Scholar]
- 32.Pylayeva Y, Gillen KM, Gerald W, Beggs HE, Reichardt LF, Giancotti FG. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009;119:252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63:610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deryugina EI, Zijlstra A, Partridge JJ, Kupriyanova TA, Madsen MA, Papagiannakopoulos T, et al. Unexpected effect of matrix metalloproteinase down-regulation on vascular intravasation and metastasis of human fibrosarcoma cells selected in vivo for high rates of dissemination. Cancer Res. 2005;65:10959–10969. doi: 10.1158/0008-5472.CAN-05-2228. [DOI] [PubMed] [Google Scholar]
- 36.Conn EM, Botkjaer KA, Kupriyanova TA, Andreasen PA, Deryugina EI, Quigley JP. Comparative analysis of metastasis variants derived from human prostate carcinoma cells. Roles in intravasation of VEGF-mediated angiogenesis and uPA-mediated invasion. Am J Pathol. 2009;175:1638–1652. doi: 10.2353/ajpath.2009.090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.