Abstract
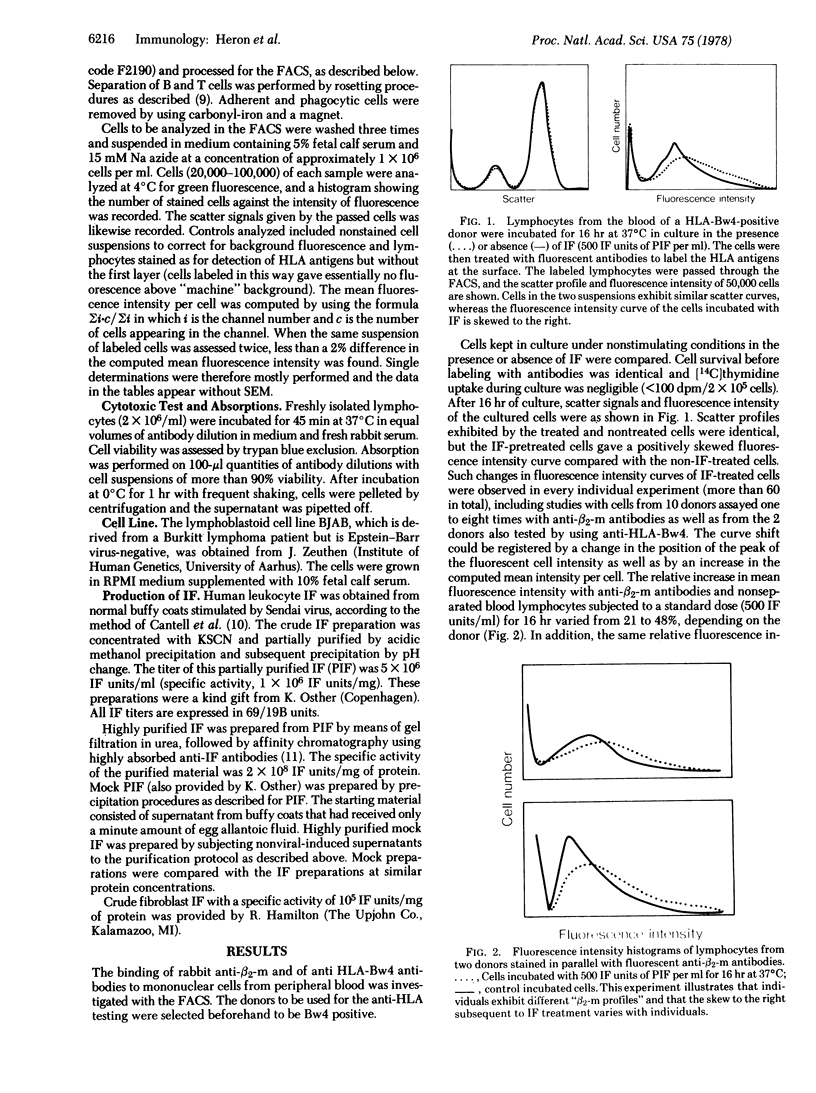
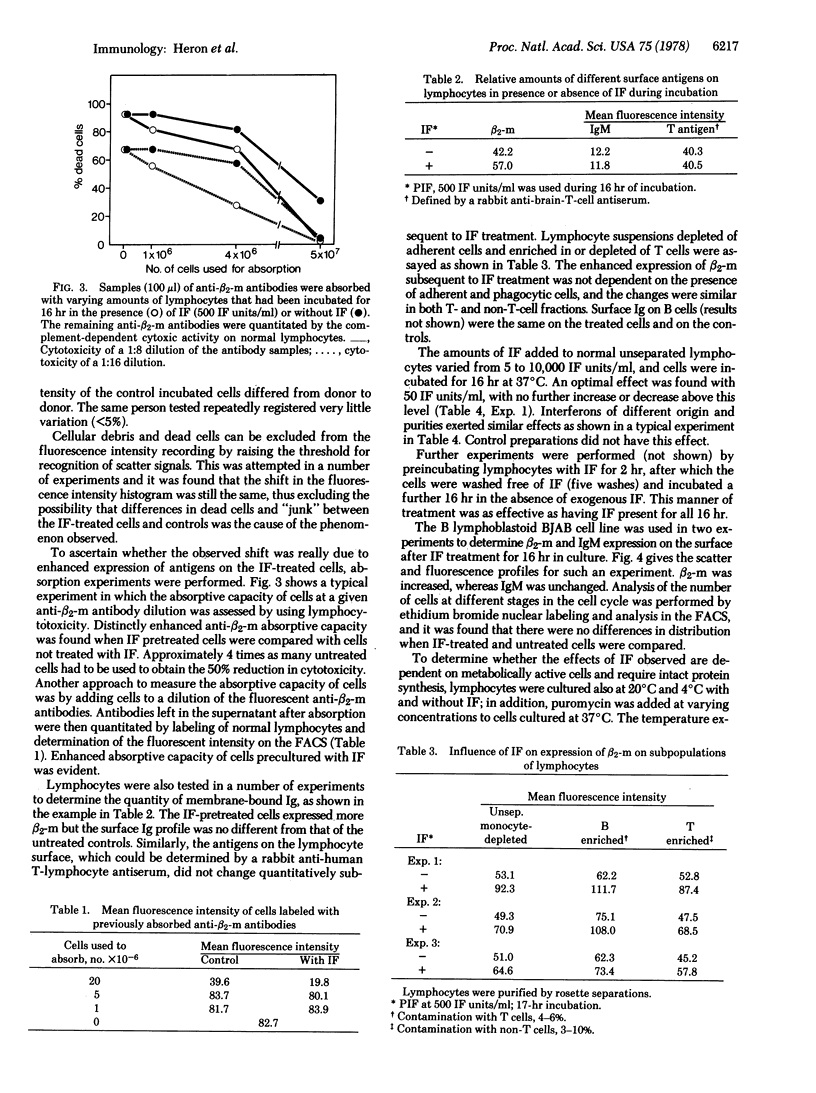
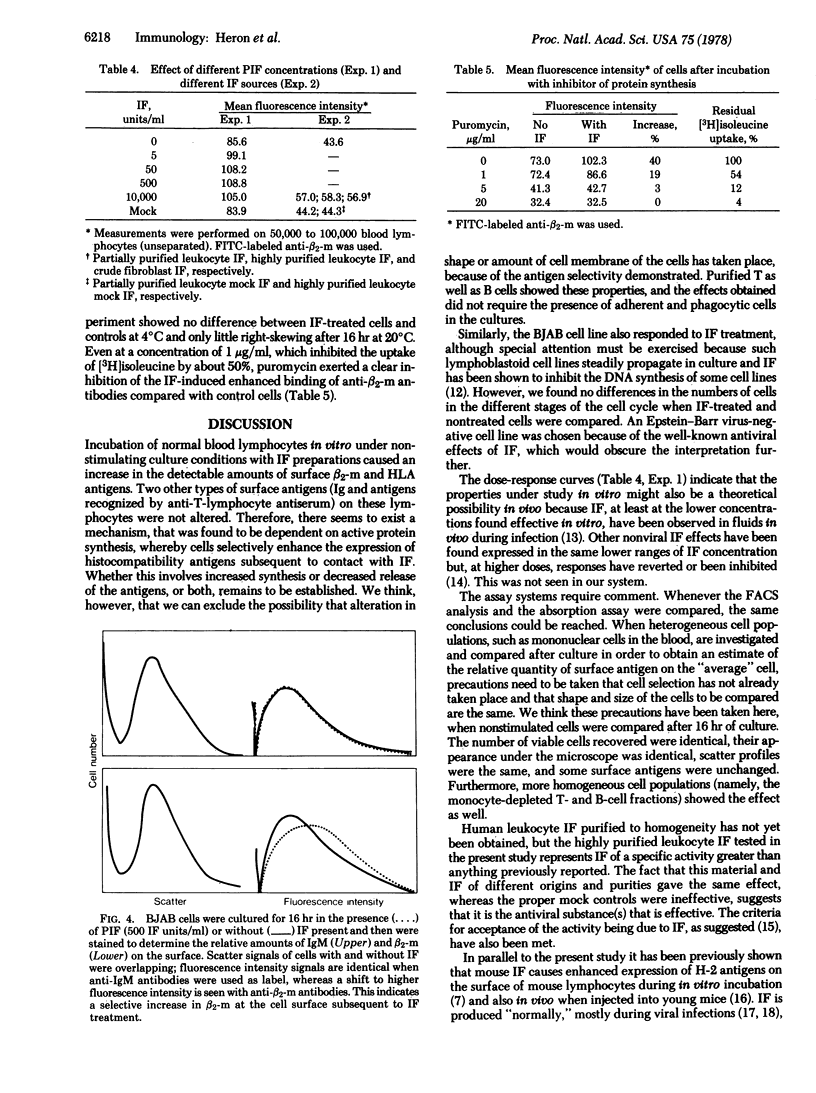
Mononuclear cells from the blood of healthy normal humans were kept in cultures under nonstimulating conditions for 16 hr in the presence or absence of human interferon. The relative quantities of HLA antigens and β2-microglobulin on the cultured cells were determined by quantitative immunofluorescence (fluorescence-activated cell sorter) and by the capacity of cells to absorb out cytotoxic antibodies against the relevant antigens. Interferons of different origin and purities enhanced the expression of HLA antigens and β2-microglobulins, whereas membrane immunoglobulins and antigens recognized by antiserum raised against human brain and T cells were the same on interferon-treated and control cells. Similar interferon effects were observed on an Epstein-Barrvirus-negative Burkitt lymphoma cell line. The enhanced expression of histocompatibility antigen subsequent to intereferon treatment was observed on B- and T-enriched lymphocyte populations and was found to be dose dependent with the optimum with “physiological” concentrations of interferon. Pretreatment of lymphocytes with interferon for 2 hr was found to be as effective as having interferon present during the total culture period. The interferon-induced enhancement of antigen expression on cells was dependent on active protein synthesis.
Keywords: histocompatibility antigens, membrane antigen expression
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARON S., DUBUY H. G., BUCKLER C. E., JOHNSON M. L. RELATIONSHIP OF INTERFERON PRODUCTION TO VIRUS GROWTH IN VIVO. Proc Soc Exp Biol Med. 1964 Nov;117:338–341. doi: 10.3181/00379727-117-29575. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T., Lindahl P., Gresser I. Inhibitory effect of interferon preparations and inducers on the multiplication of transplanted allogeneic spleen cells and syngeneic bone marrow cells. Nat New Biol. 1973 Apr 4;242(118):152–153. doi: 10.1038/newbio242152a0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- HEINEBERG H., GOLD E., ROBBINS F. C. DIFFERENCES IN INTERFERON CONTENT IN TISSUES OF MICE OF VARIOUS AGES INFECTED WITH COXSACKIE B1 VIRUS. Proc Soc Exp Biol Med. 1964 Apr;115:947–953. doi: 10.3181/00379727-115-29086. [DOI] [PubMed] [Google Scholar]
- Heron I., Berg K., Cantell K. Regulatory effect of interferon on T cells in vitro. J Immunol. 1976 Oct;117(4):1370–1373. [PubMed] [Google Scholar]
- Heron I., Berg K. The actions of interferon are potentiated at elevated temperature. Nature. 1978 Aug 3;274(5670):508–510. doi: 10.1038/274508a0. [DOI] [PubMed] [Google Scholar]
- Hilfenhaus J., Damm H., Karges H. E., Manthey K. F. Growth inhibition of human lymphoblastoid Daudi cells in vitro by interferon preparations. Arch Virol. 1976;51(1-2):87–97. doi: 10.1007/BF01317837. [DOI] [PubMed] [Google Scholar]
- Hokland M., Hokland P., Heron I. Two small lymphocyte subpopulations in human peripheral blood. I. Purification and surface marker profiles. Int Arch Allergy Appl Immunol. 1978;57(5):435–443. doi: 10.1159/000232135. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Baron S. Interferon: effects on the immune response and the mechanism of activation of the cellular response. CRC Crit Rev Biochem. 1976 Nov;4(2):203–227. doi: 10.3109/10409237609105459. [DOI] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Gresser I., Leary P., Tovey M. Interferon treatment of mice: enhanced expression of histocompatibility antigens on lymphoid cells. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1284–1287. doi: 10.1073/pnas.73.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the specific cytotoxicity of sensitized lymphocytes. Proc Natl Acad Sci U S A. 1972 Mar;69(3):721–725. doi: 10.1073/pnas.69.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Nimelstein S. H., Jones P. P., Lieberman M., McDevitt H. O. Increased synthesis and expression of H-2 antigens on thymocytes as a result of radiation leukemia virus infection: a possible mechanism for H-2 linked control of virus-induced neoplasia. J Exp Med. 1978 Feb 1;147(2):470–487. doi: 10.1084/jem.147.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miörner H., Landström L. E., Larner E., Larsson I., Lundgren E., Strannegård O. Regulation of mitogen-induced lymphocyte DNA synthesis by human interferon of different origins. Cell Immunol. 1978 Jan;35(1):15–24. doi: 10.1016/0008-8749(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]


