Abstract
OBJECTIVE
To compare illicit drug and smoking use in pregnancies with and without stillbirth.
METHODS
The Stillbirth Collaborative Research Network conducted a case-control study from March 2006 to September 2008, covering more than 90% of deliveries to residents of five a priori defined geographically diverse regions. The study attempted to include all stillbirths and representative liveborn controls. Umbilical cord samples from cases and controls were collected and frozen for subsequent batch analysis. Maternal serum was collected at delivery and batch analyzed for cotinine.
RESULTS
For 663 stillbirth deliveries, 418 (63%) had cord homogenate and 579 (87%) had maternal cotinine assays performed. For 1,932 live birth deliveries, 1,050 (54%) had cord homogenate toxicology and 1,545 (80%) had maternal cotinine assays performed. A positive cord homogenate test for any illicit drug was associated with stillbirth (OR 1.94; 95% CI 1.16, 3.27). The most common individual drug was cannabis (OR 2.34; 95% CI 1.13, 4.81), although the effect was partially confounded by smoking. Both maternal self-reported smoking history and maternal serum cotinine levels were associated in a dose-response relationship with stillbirth. Positive serum cotinine < 3 ng/ml and no reported history of smoking (proxy for passive smoke exposure) also was associated with stillbirth (OR 2.06; 95% CI 1.24, 3.41).
CONCLUSION
Cannabis, smoking, illicit drug use, and apparent exposure to second-hand smoke, separately or in combination, during pregnancy were associated with an increased risk of stillbirth. As cannabis use may be increasing with increased legalization, the relevance of these findings may increase as well.
INTRODUCTION
The second half of the twentieth century witnessed a substantial decrease in the perinatal mortality rate in the United States (US). Although the US stillbirth rate also gradually decreased during this epoch, from 18 per 1000 births in 1950 to 6.05 per 1000 births in 2006,1 this decrease has been substantially less in comparison to infant mortality and the stillbirth rate remains higher than that of many other developed countries. In fact, the US stillbirth rate is similar to the infant death rate (6.51 per 1000 births) and affects almost 26,000 babies per year.1
Smoking and drug abuse during pregnancy are potential modifiable risk factors for stillbirth.2–12 However, the association between smoking and illicit drugs and stillbirth is primarily based on studies relying on self-reporting of smoking and drug abuse. Our objective was to determine the association of smoking and illicit drug use to stillbirth by measurement of metabolites in maternal serum and umbilical cord homogenate in deliveries complicated by stillbirth compared to live births.
METHODS
The Stillbirth Collaborative Research Network (SCRN) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) conducted a population-based case-control study of stillbirth (fetal death ≥ 20 weeks of gestation) in five a priori defined geographically diverse regions, with screening and enrollment at the time of delivery between March 2006 and September 2008. Details of methods and study design13 and sample size considerations14 have previously been published. Attempts were made to enroll all eligible women whose delivery resulted in one or more stillborn fetuses, and a representative sample of eligible women whose delivery resulted in only liveborn infants, supplemented by oversampling of women with live birth delivering at less than 32 weeks of gestation and those of African descent delivering at 32 weeks of gestation or greater.13 Approval was obtained from the Institutional Review Board of each clinical site and the data-coordinating center. An advisory board reviewed the progress and safety of the study. All participants gave written informed consent.
A stillborn fetus was defined by Apgar scores of 0 at 1 and 5 minutes, and no signs of life by direct observation. Deliveries resulting from the intentional termination of a live fetus were excluded. Gestational age was determined by the best clinical estimate using multiple sources including assisted reproduction (if applicable), first day of the last menstrual period and obstetrical sonograms as previously described.15 Stillbirths and live births were classified as small for gestational age (SGA) if the birth weight was less than the 10th percentile for gestational age based on population norms.16
Study components included a comprehensive standardized fetal postmortem examination and uniform placental pathology evaluation performed by a perinatal pathologist.17,18 A standardized maternal interview during the delivery hospitalization and detailed chart abstraction of prenatal office visits, antepartum hospitalizations, and the delivery hospitalization were conducted. Biospecimens collected included maternal blood for serum and DNA, fetal blood from the umbilical cord (when available), placental tissue, and in cases, fetal tissue. The consent process provided participants the option to decline consent to one or more components of the study: interview, chart abstraction, blood draw, placental examination, autopsy, genetic studies, storage and future use of biospecimens, and future contact for additional research. The consent form discussed planned testing of the afterbirth for legal and illegal drugs, the de-identification of results and the protections afforded by the Certificate of Confidentiality that had been obtained for the study. No special consent was obtained for cotinine or toxicology testing.
Umbilical cord segments from cases and controls were collected in sterile containers and frozen at −80°C until assay. Cords were homogenized prior to batch ELISA analyses for amphetamine, methamphetamine, cocaine (benzoylecgonine), pethidine, meperidine, hydrocodone, and tetrahydrocannabinolic acid (THCA) (United States Drug Testing Laboratories, Des Plaines, IL). All samples were initially tested by ELISA and presumptive positives were confirmed using appropriate mass spectrometric assays using established and validated procedures.19
Maternal blood for serum samples was collected at delivery and centrifuged for 15 minutes at 1300g at room temperature at all participating clinical sites. Serum samples were then frozen at −80°C until assay. After completion of the study enrollment, serum aliquots were shipped to the University of Utah Center for Human Toxicology and batch-analyzed for cotinine using solid phase extraction and liquid chromatography. The personnel performing the assays were blinded to clinical outcomes.
Medical records from all deliveries with positive cord homogenate narcotic results were reviewed for evidence of prescribed narcotic administration for any reason prior to delivery. Only those with positive cord homogenate testing and medical records with no evidence of narcotic administration prior to delivery were considered positive for illicit narcotic use.
Nicotine and cotinine metabolism is accelerated in pregnancy20 and the maternal serum cotinine per cigarette ratio is typically less in pregnant compared to non-pregnant women.21 Thus, the threshold for defining exposure may be different in pregnant and non-pregnant women. We addressed this issue by using quartiles, established in our controls, in addition to a 3 ng/ml threshold to assess cotinine exposure.22 A positive serum cotinine < 3 ng/ml in women who denied smoking was used as a proxy for passive exposure among non-smokers.22
The delivery, defined as a case if there were any stillbirths delivered and as a control if all live births were delivered, was the unit of analysis. The analyses were weighted for the oversampling of live births and other aspects of the study design, as well as for differential consent among the women with stillbirth and among the women with live birth using SUDAAN software, Version 11.0.23 The construction of the weights has been previously described.13 The weighted samples of live births and stillbirths are intended to approximate random selections of live births and stillbirths in the catchment areas over the enrollment period. Crude and adjusted odds ratios (OR and aOR) and 95% confidence intervals were calculated from univariate and multivariable logistic regression models, respectively. Predictor variables in the models were treated as categorical. However, for ordered categories on smoking history in the trimester the baby was born and cotinine levels, tests for linear and quadratic trends in the log odds of stillbirth were also conducted using orthogonal contrasts. All tests were performed at a nominal significance level of α=0.05. All single degree of freedom tests were 2-sided without correction for multiple comparisons.
Adjusted odds ratios were computed to account for stillbirth risk factors known at pregnancy confirmation (baseline) using a modification to a risk factor score for stillbirth that was developed on the logit scale using the coefficients from a logistic regression model. Variables contributing to the baseline risk factor score were those described previously,14 specifically, the following maternal characteristics: age, race/ethnicity, marital status, education, pregnancy history, body mass index, smoking status, alcohol use, illicit drug use, hypertension, diabetes, seizure disorder, blood type, Rh factor, and multiple gestation in current pregnancy, as well as paternal age, family income, insurance/method of payment and clinical site. All variables included in the score were categorical and an “average” of the regression coefficients associated with the categories was used when a variable was missing for an observation. The average was based on the sample-weighted proportion of live births by category. The modification to the risk factor score for this analysis was to exclude coefficients associated with smoking status and illicit drug use.
The relationships between cotinine levels (negative, ≤ 50th percentile, > 50th percentile), THCA and SGA fetus on pregnancy outcome were studied by comparing the stillbirth odds ratios for one of the factors with and without accounting for another in logistic regression models. A commonly used threshold of 10% reduction (or increase) in the odds ratio was taken as a measure of confounding. In addition, the interactions of high levels of cotinine (> 50th percentile) with SGA fetus and with preeclampsia were studied using logistic regression models with an interaction term and computing stillbirth odds ratios for high cotinine levels stratified by whether the fetus was SGA and by whether preeclampsia was a condition noted in the chart at delivery.
RESULTS
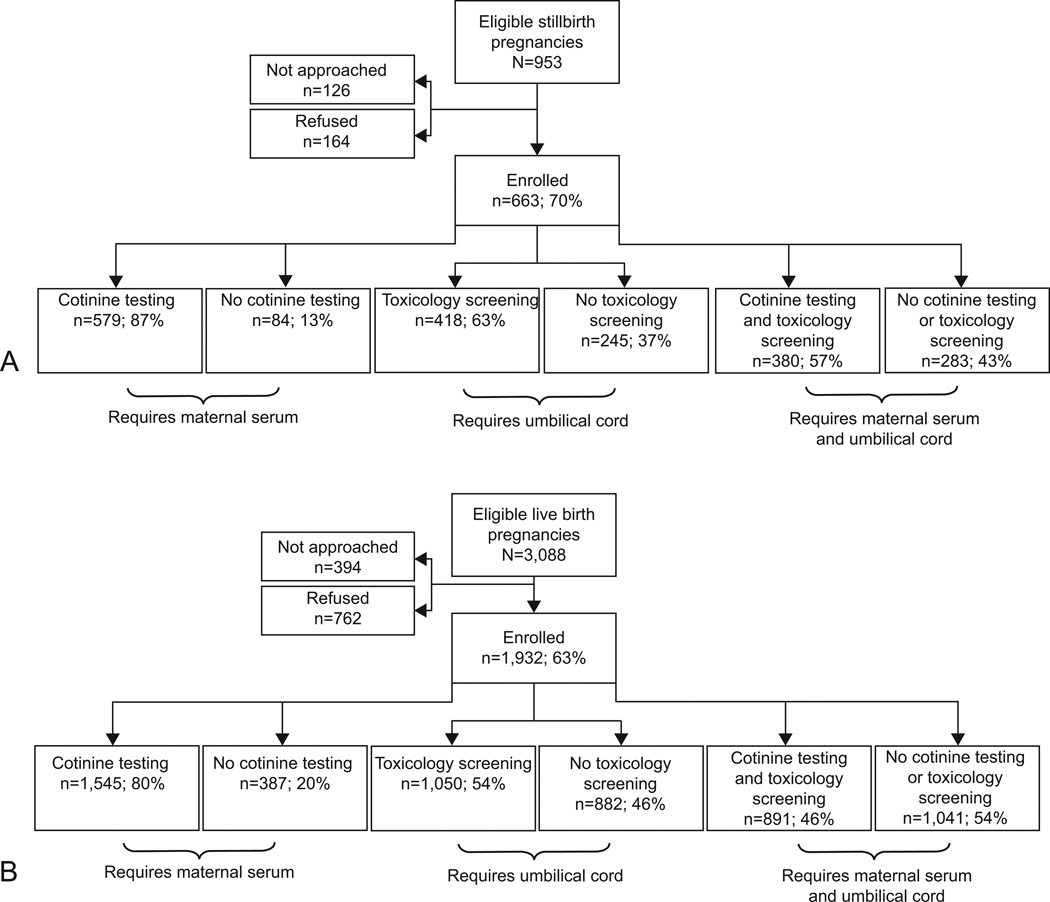
Enrollment to the SCRN study and inclusion in the serum cotinine and toxicology analyses are shown in Figure 1. For 663 stillbirth deliveries (cases), 418 (63%) had a cord segment collected for subsequent toxicology studies and 579 (87%) had maternal serum analyzed for cotinine. More than half (380 [57%]) had both maternal serum and cord segments for analysis. For 1,932 live birth deliveries (controls), 1,050 (54%) had cord segments collected for subsequent toxicology studies and 1,545 (80%) had maternal serum analyzed for cotinine. About half (891 [46%]) had both maternal serum and cord segments for analysis. Cotinine and toxicology testing was done on virtually all women with adequate blood or cord collected. Absence or insufficient sample was due to the participant declining sample collection, inconvenient timing, administrative error, and in the vast majority of cases for umbilical cord, discarding of the placenta before it could be retrieved for examination.
Figure 1.
Cotinine and toxicology analyses comparing results from stillbirth and live birth pregnancies. The Stillbirth Collaborative Research Network stillbirth case status (SCRN case status) is defined as follows. A pregnancy is categorized as a stillbirth pregnancy if there are any stillbirths delivered and as a live birth pregnancy if all live births are delivered. A fetal death is defined by Apgar scores of 0 at 1–5 minutes and no signs of life by direct observation. Fetal deaths are classified as stillbirths if the best clinical estimate of gestational age at death is 20 or more weeks. Fetal deaths at 18–19 weeks without good dating are also included as stillbirths.
Table 1 shows characteristics of cases and controls that did, and did not, undergo cotinine testing and toxicology screening. For both groups, those with cotinine testing and/or toxicology screening were more likely to be non-Hispanic white and less likely to be non-Hispanic black than those without testing. Cases and controls with both cotinine testing and toxicology screening were more likely to have commercial insurance and deliver at later gestational ages. Also, a disproportionate number of controls with testing were between 20–39 years of age compared to those without testing.
Table 1.
Sociodemographic and Pregnancy Characteristics by Cotinine Testing and Toxicology Screening Status
| Characteristic - Weighted %* | Cotinine Testing | Toxicology Screening | Cotinine Testing Toxicology Screening |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | P | No | Yes | P | No | Yes | P | |
| Stillbirth pregnancies | |||||||||
| Unweighted sample size, n | 84 | 579 | 245 | 418 | 283 | 380 | |||
| Weighted sample size, nw | 87 | 576 | 258 | 405 | 296 | 367 | |||
| Maternal age at delivery (years) | |||||||||
| <20 | 13.9 | 13.1 | 0.666 | 15.3 | 11.8 | 0.357 | 15.9 | 11.0 | 0.256 |
| 20–34 | 67.1 | 70.1 | 70.3 | 69.3 | 69.2 | 70.1 | |||
| 35–39 | 15.9 | 11.9 | 10.0 | 13.9 | 10.8 | 13.7 | |||
| 40+ | 3.1 | 5.0 | 4.4 | 4.9 | 4.1 | 5.2 | |||
| Maternal race/ethnicity | |||||||||
| White, non-Hispanic | 18.4 | 35.7 | <0.001 | 19.3 | 42.5 | <.001 | 20.2 | 44.1 | <.001 |
| Black, non-Hispanic | 41.5 | 20.6 | 31.8 | 17.9 | 32.8 | 15.7 | |||
| Hispanic | 31.3 | 37.1 | 40.9 | 33.3 | 39.5 | 33.7 | |||
| Other | 8.8 | 6.7 | 8.0 | 6.3 | 7.5 | 6.5 | |||
| Insurance/method of payment | |||||||||
| No insurance | 9.7 | 5.4 | 0.290 | 6.9 | 5.3 | 0.020 | 8.0 | 4.3 | 0.005 |
| Any public/private assistance | 50.8 | 54.0 | 59.5 | 49.8 | 57.9 | 50.1 | |||
| VA/commercial health ins/HMO | 39.6 | 40.7 | 33.6 | 44.9 | 34.1 | 45.7 | |||
| Gestational age (weeks) | |||||||||
| 18–19 | 3.6 | 2.3 | 0.123 | 4.7 | 1.1 | 0.028 | 4.1 | 1.2 | 0.014 |
| 20–23 | 46.3 | 32.0 | 38.2 | 31.1 | 39.1 | 29.6 | |||
| 24–27 | 16.5 | 15.7 | 15.8 | 15.9 | 15.8 | 15.9 | |||
| 28–31 | 10.9 | 13.0 | 9.7 | 14.6 | 9.7 | 15.2 | |||
| 32–36 | 11.2 | 19.9 | 17.2 | 19.8 | 16.7 | 20.5 | |||
| 37+ | 11.4 | 17.0 | 14.4 | 17.5 | 14.6 | 17.6 | |||
| Live birth pregnancies | |||||||||
| Unweighted sample size, n | 387 | 1,545 | 882 | 1,050 | 1,041 | 891 | |||
| Weighted sample size, nw | 256 | 1,183 | 565 | 874 | 697 | 742 | |||
| Maternal age at delivery (years) | |||||||||
| <20 | 13.9 | 9.5 | 0.029 | 11.7 | 9.4 | 0.465 | 12.4 | 8.4 | 0.019 |
| 20–34 | 71.4 | 76.6 | 75.0 | 76.1 | 73.9 | 77.3 | |||
| 35–39 | 10.6 | 12.2 | 11.1 | 12.5 | 10.9 | 12.9 | |||
| 40+ | 4.1 | 1.7 | 2.2 | 2.0 | 2.8 | 1.5 | |||
| Maternal race/ethnicity | |||||||||
| White, non-Hispanic | 38.9 | 47.3 | <.001 | 36.1 | 52.1 | <.001 | 37.9 | 53.2 | <.001 |
| Black, non-Hispanic | 22.5 | 9.4 | 18.8 | 7.2 | 17.4 | 6.4 | |||
| Hispanic | 29.2 | 36.1 | 37.8 | 32.9 | 36.7 | 33.1 | |||
| Other | 9.4 | 7.2 | 7.4 | 7.8 | 8.0 | 7.2 | |||
| Insurance/method of payment | |||||||||
| No insurance | 3.1 | 3.6 | 0.476 | 3.9 | 3.4 | 0.022 | 3.6 | 3.5 | 0.007 |
| Any public/private assistance | 52.1 | 47.9 | 53.2 | 45.7 | 53.1 | 44.5 | |||
| VA/commercial health ins/HMO | 44.8 | 48.5 | 43.0 | 50.9 | 43.4 | 52.0 | |||
| Gestational age (weeks) | |||||||||
| 20–23 | 0.3 | 0.4 | 0.291 | 0.5 | 0.3 | <.001 | 0.4 | 0.3 | <.001 |
| 24–27 | 1.0 | 0.7 | 1.4 | 0.3 | 1.2 | 0.3 | |||
| 28–31 | 1.2 | 1.0 | 2.0 | 0.4 | 1.7 | 0.4 | |||
| 32–36 | 10.6 | 8.2 | 13.0 | 5.8 | 11.6 | 5.8 | |||
| 37+ | 86.9 | 89.8 | 83.1 | 93.2 | 85.1 | 93.2 | |||
HMO, health maintenance organization.
Weighted percentages and p-values are shown by analysis inclusion status, i.e., whether specific testing (cotinine, toxicology, cotinine and toxicology) was done. The weights take into account the study design and differential consent based on characteristics recorded on all eligible pregnancies that were screened for the study. Unweighted and weighted samples sizes are also provided. The weighted sample sizes are not integers, but are shown rounded to the nearest integer. Sample sizes vary slightly by characteristic included in the table. Nw is a count of the observations according to their relative weight in the analysis.
Women who self-reported smoking were more likely than those who did not to be non-Hispanic white, 20–34 years of age, of low education, unmarried, and low income. Those who self-reported drug use were more likely than women who did not to be non-Hispanic white and unmarried (data not shown).
Self-reported smoking and drug use, cotinine levels and cord homogenate findings in all stillbirth and live birth deliveries are depicted in Table 2. There was an increase in the stillbirth odds ratio with increasing amounts of self-reported smoking in the trimester the baby was born (linear trend P = 0.0033). Compared to women who never smoked, women who reported smoking 1 – 9 cigarettes per day had a 1.77 OR for stillbirth (95% CI 1.13, 2.80); and those smoking ≥ 10 cigarettes per day had an OR for stillbirth of 2.17 (95% CI 1.25, 3.78). Similar results were noted with serum cotinine levels. Compared to women testing negative, those with positive cotinine concentrations ≤ 50th percentile had an OR of 2.04 (95% CI 1.39, 3.01); and those with cotinine levels > 50th percentile had an OR of 2.39 (95% CI 1.62, 3.52) (linear trend P <0.0001). Similar results were noted if cotinine concentrations between 0.25 – 2.99 and 3.00+ were used (linear trend P <0.0001). Women who denied smoking but had elevated cotinine levels had increased odds for stillbirth using either the 3 ng/mL cutpoint or percentiles (e.g., positive cotinine < 3 ng/ml OR 2.06; 95% CI 1.24, 3.41; positive cotinine > 3 ng/ml OR 2.61; 95% CI 1.39, 4.88).
Table 2.
Maternal Report and Testing Results for Smoking and Drug Use by Stillbirth Collaborative Research Network Case Status
| Characteristic - Weighted %* | Stillbirth | Live Birth | Odds Ratio (95% CI) | P |
|---|---|---|---|---|
| Maternal report of smoking | ||||
| Unweighted sample size – n = | 613 | 1,832 | ||
| Weighted sample size – nw = | 614 | 1,366 | ||
| Smoked trimester the baby was born (%) | ||||
| No, never smoked | 81.1 | 87.1 | Reference | 0.002 |
| No, smoked previously† | 8.8 | 7.2 | 1.31 (0.92, 1.86) | |
| Yes, 1–9 cigarettes/day on average | 5.9 | 3.6 | 1.77 (1.13, 2.80) | |
| Yes, 10+ cigarettes/day on average | 4.2 | 2.1 | 2.17 (1.25, 3.78) | |
| Test: linear trend | 0.003 | |||
| Cotinine testing | ||||
| Unweighted sample size – n | 579 | 1,545 | ||
| Weighted sample size – nw | 576 | 1,183 | ||
| Positive for Cotinine (%) | 18.5 | 9.3 | 2.22 (1.67, 2.95) | <.001 |
| Cotinine concentration (ng/ml) (%) | ||||
| Negative (<0.25)‡ | 81.5 | 90.7 | Reference | <.001 |
| Positive, <3 | 6.4 | 3.3 | 2.16 (1.39, 3.37) | |
| Positive, 3+ | 12.1 | 6.0 | 2.25 (1.59, 3.19) | |
| Test: linear trend | <.001 | |||
| Cotinine concentration (ng/ml), by quartile for positives (%) | ||||
| Negative (<0.25)‡ | 81.5 | 90.7 | Reference | <.001 |
| Positive, ≤1.49 | 4.8 | 2.3 | 2.36 (1.40, 3.97) | |
| Positive, 1.49 – 9.68 | 3.5 | 2.3 | 1.73 (0.99, 3.03) | |
| Positive, 9.68 – 23.62 | 4.1 | 2.4 | 1.96 (1.10, 3.47) | |
| Positive, >23.62 | 6.0 | 2.4 | 2.81 (1.69, 4.67) | |
| Test: linear trend | 0.004 | |||
| Cotinine concentration (ng/ml), by median for positives (%) | ||||
| Negative (<0.25)‡ | 81.5 | 90.7 | Reference | <.001 |
| Positive, ≤ 50th %tile (≤9.68) | 8.4 | 4.5 | 2.04 (1.39, 3.01) | |
| Positive, > 50th %tile (>9.68) | 10.1 | 4.7 | 2.39 (1.62, 3.52) | |
| Test: linear trend | <.001 | |||
| Maternal report of smoking and cotinine testing | ||||
| Unweighted sample size – n | 548 | 1,489 | ||
| Weighted sample size – nw | 546 | 1,144 | ||
| Cotinine and smoking during the trimester the baby was born (%) |

|
|||
| Negative cotinine (<0.25)‡ & never smoked | 75.8 | 84.5 | Reference | |
| Negative cotinine (<0.25)‡ & smoked previously† | 5.3 | 5.6 | 1.04 (0.66, 1.65) | |
| Negative cotinine (<0.25)‡ & smoked | 0.5 | 0.8 | 0.73 (0.20, 2.72) | |
| Positive cotinine, <3 ng/ml, & did not smoke | 5.0 | 2.7 | 2.06 (1.24, 3.41) | |
| Positive cotinine, 3+ ng/ml, & did not smoke | 3.6 | 1.5 | 2.61 (1.39, 4.88) | |
| Positive cotinine, any concentration, & smoked | 9.8 | 4.8 | 2.30 (1.54, 3.43) | |
| Cotinine and smoking during the trimester the baby was born (%) | ||||
| Negative cotinine (<0.25)‡ & never smoked | 75.8 | 84.5 | Reference | <.001 |
| Negative cotinine (<0.25)‡ & smoked previously† | 5.3 | 5.6 | 1.04 (0.66, 1.65) | |
| Negative cotinine (<0.25)‡ & smoked | 0.5 | 0.8 | 0.73 (0.20, 2.72) | |
| Positive cotinine, ≤ 50th %tile (≤9.68 ng/ml), & did not smoke | 5.9 | 3.5 | 1.89 (1.19, 3.00) | |
| Positive cotinine, > 50th %tile (>9.68 ng/ml), & did not smoke | 2.7 | 0.8 | 3.84 (1.74, 8.46) | |
| Maternal report of lifetime drug use | ||||
| Unweighted sample size – n | 611 | 1,823 | ||
| Weighted sample size – nw | 610 | 1,349 | ||
| Lifetime drug use(%) | ||||
| Reported never used drugs | 67.2 | 69.3 | Reference | 0.007 |
| Reported drug use | ||||
| Without addiction | 28.1 | 28.6 | 1.01 (0.81, 1.26) | |
| With addiction | 4.7 | 2.1 | 2.30 (1.37, 3.86) | |
| Toxicology screening§ | ||||
| Unweighted sample size – n | 418 | 1,050 | ||
| Weighted sample size – nw | 405 | 874 | ||
| Positive for any drug (%) | 7.0 | 3.7 | 1.94 (1.16, 3.27) | 0.012 |
| Positive for specific drugs (%) | ||||
| Morphine | 1.3 | 0.4 | 3.46 (0.86, 13.90) | 0.080 |
| Hydromorphone | 0.0 | 0.0 | — | — |
| Codeine | 0.6 | 0.2 | 2.80 (0.39, 20.27) | 0.307 |
| Hydrocodone | 0.4 | 0.0 | 152.57 (13.73, 1695.65) | <.001 |
| Pethidine / Meperidine | 0.0 | 1.0 | — | — |
| THCA (Tetrahydrocannabinolic acid) | 3.9 | 1.7 | 2.34 (1.13, 4.81) | 0.021 |
| Cocaine (Benzoylecgonine) | 0.9 | 0.6 | 1.59 (0.41, 6.14) | 0.501 |
| Amphetamine or Methamphetamine | 0.7 | 0.1 | 8.17 (0.84, 79.68) | 0.071 |
| None, single or multiple drugs detected (%) | ||||
| Negative for all drugs | 93.0 | 96.3 | Reference | 0.033 |
| Positive for 1 drug | 6.2 | 3.5 | 1.83 (1.06, 3.15) | |
| Positive for 2 drugs | 0.8 | 0.2 | 3.69 (0.64, 21.31) | |
| Maternal report of lifetime drug use and toxicology screening | ||||
| Unweighted sample size – n | 384 | 980 | ||
| Weighted sample size – nw | 373 | 813 | ||
| Umbilical cord toxicology and lifetime drug use (%) | ||||
| Negative for all drugs & reported never used drugs | 64.8 | 68.4 | Reference | 0.023 |
| Negative for all drugs & reported drug use | 28.3 | 28.2 | 1.06 (0.80, 1.41) | |
| Positive for any drug & reported never used drugs | 2.1 | 1.9 | 1.19 (0.50, 2.84) | |
| Positive for any drug & reported drug use | 4.8 | 1.5 | 3.30 (1.54, 7.03) | |
| Cotinine testing and toxicology screening | ||||
| Unweighted sample size – n | 380 | 891 | ||
| Weighted sample size – nw | 367 | 742 | ||
| Cotinine and drug use (%) | ||||
| Negative cotinine (<0.25)‡ & negative for all drugs | 80.6 | 88.6 | Reference | 0.001 |
| Positive cotinine & negative for all drugs | 12.4 | 8.0 | 1.70 (1.13, 2.56) | |
| Negative cotinine (<0.25)‡ & positive for any drug | 3.3 | 2.4 | 1.53 (0.71, 3.27) | |
| Positive cotinine & positive for any drug | 3.8 | 1.1 | 3.86 (1.61, 9.24) | |
CI, confidence interval.
Weighted percentages, odds ratios and p-values are shown. The weights take into account the study design and differential consent based on characteristics recorded on all eligible pregnancies that were screened for the study. Unweighted and weighted samples sizes are also provided. The weighted sample sizes are not integers, but are shown rounded to the nearest integer. For ordered categories on smoking history in the trimester the baby was born and cotinine levels, tests for linear and quadratic trends in the log odds of stillbirth were conducted using orthogonal contrasts. None of the quadratic trends was significant and their p-values are not reported. Nw is a count of the observations according to their relative weight in the analysis.
‘Previously’ indicates that the mother reported smoking 3 months prior to pregnancy or during pregnancy, but not during the trimester the baby was born.
Lower limit of detectability for the cotinine assay.
The toxicology screening panel can detect amphetamines (amphetamine, 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxy-N-ethylamphetamine (MDEA), N,N-Dimethyldopamine (DMDA), and methamphetamine), cannabinoids (carboxy-THC), cocaine (benzoylecgonine), opiates (codeine, hydrocodone, hydromorphine, morphine, 6-Monoacetylmorphine (6MAM), and meconin), phencyclidine (phencyclidine), 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), methadone, and barbiturates (amobarbital, butalbital, pentobarbital, Phenobarbital, and secobarbital).
Women with stillbirth were twice as likely as those with live birth to report having been addicted to an illicit drug (OR 2.30; 95% CI 1.37, 3.86). A positive test for any drug in the cord homogenate was associated with an OR for stillbirth of 1.94 (95% CI 1.16, 3.27). The OR was higher in women having a positive toxicology screen who also reported ever using illicit drugs (OR 3.30; 95% CI 1.54, 7.03). The most common individual drug, tetrahydrocannabinolic acid (THCA), was positive in 3.9% of cases and 1.7% of controls (OR for stillbirth 2.34; 95% CI 1.13, 4.81). Among women with testing for cotinine and illicit drugs, women who were positive for cotinine and not illicit drugs had an OR of 1.70 (95% CI 1.13, 2.56) compared to those who were negative for both; and women who were positive for both had an OR of 3.86 (95% CI 1.61, 9.24). However, the odds ratios for positive for both versus positive for cotinine only were not significantly different and there was evidence of confounding of the relationship between illicit drugs and stillbirth by cotinine.
Because they were already at higher risk for complications, we anticipated that smoking and illicit drugs would have less influence on pregnancies complicated by multiple gestation, obstetric complications or fetal aneuploidy. We therefore repeated these analyses in non-anomalous, singleton pregnancies excluding intrapartum stillbirths, as shown in Table 3. The OR for stillbirth in women with positive cotinine levels ≤ 50th percentile was 1.88 (95% CI 1.19, 2.97) and for those with levels > 50th percentile was 2.67 (95% CI 1.75, 4.07). Women with any positive toxicology screen had an increased odds of stillbirth of 2.23 (95% CI 1.29, 3.88). Positive cord homogenate THCA was associated with an increased odds of stillbirth of 2.83 (95% CI 1.34, 5.99).
Table 3.
Maternal Report and Testing Results for Smoking and Drug Use by Stillbirth Collaborative Research Network Case Status for Nonanomalous, Singleton Pregnancies Excluding Intrapartum Stillbirths
| Characteristic - Weighted %* | Stillbirth | Live Birth | Odds Ratio (95% CI) | P |
|---|---|---|---|---|
| Maternal report of smoking | ||||
| Unweighted sample size – n | 412 | 1,723 | ||
| Weighted sample size – nw | 405 | 1,304 | ||
| Smoked trimester the baby was born | ||||
| No, never smoked | 80.4 | 86.9 | Reference | 0.001 |
| No, smoked previously† | 9.5 | 7.4 | 1.40 (0.94, 2.07) | |
| Yes, 1–9 cigarettes/day on average | 6.0 | 3.7 | 1.78 (1.07, 2.97) | |
| Yes, 10+ cigarettes/day on average | 4.1 | 2.1 | 2.09 (1.10, 3.94) | |
| Test: linear trend | 0.017 | |||
| Cotinine Testing | ||||
| Unweighted sample size – n | 396 | 1,455 | ||
| Weighted sample size – nw | 387 | 1,131 | ||
| Positive for cotinine | 18.9 | 9.2 | 2.29 (1.66, 3.16) | <.001 |
| Cotinine concentration (ng/ml) | ||||
| Negative (<0.25)‡ | 81.1 | 90.8 | Reference | <.001 |
| Positive, <3 | 6.0 | 3.2 | 2.12 (1.27, 3.53) | |
| Positive, 3+ | 12.9 | 6.0 | 2.39 (1.62, 3.52) | |
| Test: linear trend | <.001 | |||
| Cotinine concentration (ng/ml), by quartile for positives | ||||
| Negative (<0.25)‡ | 81.1 | 90.8 | Reference | <.001 |
| Positive, ≤1.49 | 4.0 | 2.1 | 2.12 (1.14, 3.94) | |
| Positive, 1.49 – 9.68 | 3.4 | 2.3 | 1.66 (0.87, 3.17) | |
| Positive, 9.68 – 23.62 | 4.8 | 2.5 | 2.18 (1.18, 4.04) | |
| Positive, >23.62 | 6.7 | 2.4 | 3.18 (1.83, 5.53) | |
| Test: linear trend | 0.001 | |||
| Cotinine concentration (ng/ml), by median for positives | ||||
| Negative (<0.25)‡ | 81.1 | 90.8 | Reference | <.001 |
| Positive, ≤ 50th percentile (≤9.68) | 7.4 | 4.4 | 1.88 (1.19, 2.97) | |
| Positive, > 50th percentile (>9.68) | 11.5 | 4.8 | 2.67 (1.75, 4.07) | |
| Test: linear trend | <.001 | |||
| Maternal report of smoking and cotinine testing | ||||
| Unweighted sample size – n | 371 | 1,404 | ||
| Weighted sample size – nw | 363 | 1,095 | ||
| Cotinine and smoking during the trimester the baby was born |
<.001 |
|||
| Negative cotinine (<0.25)‡ & never smoked | 75.0 | 84.6 | Reference | |
| Negative cotinine (<0.25)‡ & smoked previously† | 6.0 | 5.6 | 1.20 (0.72, 1.98) | |
| Negative cotinine (<0.25)‡ & smoked | 0.5 | 0.8 | 0.71 (0.15, 3.29) | |
| Positive cotinine, <3 ng/ml, & did not smoke | 4.8 | 2.6 | 2.10 (1.18, 3.73) | |
| Positive cotinine, 3+ ng/ml, & did not smoke | 4.1 | 1.5 | 3.03 (1.52, 6.06) | |
| Positive cotinine, any concentration, & smoked | 9.6 | 4.8 | 2.24 (1.42, 3.52) | |
| Cotinine and smoking during the trimester the baby was born | ||||
| Negative cotinine (<0.25)† & never smoked | 75.0 | 84.6 | Reference | <.001 |
| Negative cotinine (<0.25)† & smoked previously† | 6.0 | 5.6 | 1.20 (0.72, 1.98) | |
| Negative cotinine (<0.25)‡ & smoked | 0.5 | 0.8 | 0.71 (0.15, 3.29) | |
| Positive cotinine, ≤ 50th %tile (≤9.68 ng/ml), & did not smoke | 5.6 | 3.3 | 1.88 (1.11, 3.19) | |
| Positive cotinine, > 50th %tile (>9.68 ng/ml), & did not smoke | 3.3 | 0.8 | 4.95 (2.10, 11.65) | |
| Maternal report of lifetime drug use | ||||
| Unweighted sample size – n | 410 | 1,714 | ||
| Weighted sample size – nw | 402 | 1,288 | ||
| Lifetime drug use | ||||
| Reported never used drugs | 66.6 | 69.7 | Reference | 0.017 |
| Reported drug use | ||||
| Without addiction | 28.5 | 28.1 | 1.06 (0.82, 1.37) | |
| With addiction | 4.9 | 2.2 | 2.33 (1.31, 4.17) | |
| Toxicology screening§ | ||||
| Unweighted sample size – n | 297 | 993 | ||
| Weighted sample size – nw | 284 | 842 | ||
| Positive for any drug | 8.2 | 3.8 | 2.23 (1.29, 3.88) | 0.004 |
| Positive for specific drugs | ||||
| Morphine | 1.5 | 0.4 | 4.19 (0.93, 18.98) | 0.063 |
| Hydromorphone | 0.0 | 0.0 | — | — |
| Codeine | 0.4 | 0.2 | 1.81 (0.16, 20.31) | 0.629 |
| Hydrocodone | 0.3 | 0.0 | 95.29 (5.93, 1531.34) | 0.001 |
| Pethidine / Meperidine | 0.0 | 1.0 | — | — |
| THCA (tetrahydrocannabinolic acid) | 4.9 | 1.8 | 2.83 (1.34, 5.99) | 0.007 |
| Cocaine (Benzoylecgonine) | 1.3 | 0.6 | 2.19 (0.57, 8.48) | 0.256 |
| Amphetamine or Methamphetamine | 0.7 | 0.1 | 7.61 (0.67, 85.98) | 0.101 |
| None, single or multiple drugs detected | ||||
| Negative for all drugs | 91.8 | 96.2 | Reference | 0.015 |
| Positive for 1 drug | 7.3 | 3.6 | 2.14 (1.20, 3.79) | |
| Positive for 2 drugs | 0.8 | 0.2 | 3.71 (0.54, 25.42) | |
| Maternal report of lifetime drug use and toxicology screening | ||||
| Unweighted sample size – n | 270 | 928 | ||
| Weighted sample size – nw | 259 | 784 | ||
| Umbilical cord toxicology and lifetime drug use | ||||
| Negative for all drugs & reported never used drugs | 61.4 | 68.7 | Reference | 0.008 |
| Negative for all drugs & reported drug use | 30.6 | 27.7 | 1.24 (0.90, 1.70) | |
| Positive for any drug & reported never used drugs | 2.7 | 2.0 | 1.56 (0.62, 3.87) | |
| Positive for any drug & reported drug use | 5.3 | 1.6 | 3.79 (1.69, 8.53) | |
| Cotinine testing and toxicology screening | ||||
| Unweighted sample size – n | 274 | 845 | ||
| Weighted sample size – nw | 262 | 715 | ||
| Cotinine and drug use | ||||
| Negative cotinine (<0.25)‡ & negative for all drugs | 78.1 | 88.8 | Reference | <0.001 |
| Positive cotinine & negative for all drugs | 13.5 | 7.6 | 2.02 (1.29, 3.16) | |
| Negative cotinine (<0.25)‡ & positive for any drug | 4.1 | 2.4 | 1.94 (0.88, 4.26) | |
| Positive cotinine & positive for any drug | 4.3 | 1.1 | 4.35 (1.74, 10.84) | |
Weighted percentages, odds ratios and p-values are shown. The weights take into account the study design and differential consent based on characteristics recorded on all eligible pregnancies that were screened for the study. Unweighted and weighted samples sizes are also provided. The weighted sample sizes are not integers, but are shown rounded to the nearest integer. For ordered categories on smoking history in the trimester the baby was born and cotinine levels, tests for linear and quadratic trends in the log odds of stillbirth were conducted using orthogonal contrasts. None of the quadratic trends was significant and their p-values are not reported. Nw is a count of the observations according to their relative weight in the analysis.
’Previously’ indicates that the mother reported smoking 3 months prior to pregnancy or during pregnancy, but not during the trimester the baby was born.
Lower limit of detectability for the cotinine assay.
The toxicology screening panel can detect amphetamines (amphetamine, 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxy-N-ethylamphetamine (MDEA), N,N-dimethyldopamine (DMDA), and methamphetamine), cannabinoids (carboxy-THC), cocaine (benzoylecgonine), opiates (codeine, hydrocodone, hydromorphine, morphine, 6-Monoacetylmorphine (6MAM), and meconin), phencyclidine (phencyclidine), 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP, methadone, and barbiturates (amobarbital, butalbital, pentobarbital, Phenobarbital, and secobarbital).
Selected odds ratios adjusted for pre-pregnancy risk factors for stillbirth are shown in Table 4. Self-reported smoking and elevated levels of cotinine were associated with stillbirth even after adjustment for other known risk factors. The aOR for stillbirth with positive cotinine levels ≤ 50th percentile was 2.05 (95% CI 1.33, 3.17) and for cotinine levels > 50th percentile was 2.56 (95% CI 1.66, 3.93). A positive test for drug use also was associated with stillbirth after adjustment. The adjusted results were also significant in the subgroup of non-anomalous, singleton pregnancies excluding intrapartum stillbirths. There were too few cases of positive results to assess adjusted odds ratios for each individual illicit drug.
Table 4.
Selected Adjusted Stillbirth Odds Ratios for Smoking and Drug Use
| Characteristic* | All Pregnancies | Nonanomalous,
Singleton Pregnancies, Excluding Intrapartum Stillbirths |
||
|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI) |
P | Adjusted Odds Ratio (95% CI) |
P | |
| Cotinine concentration (ng/ml), by median for positives | ||||
| Negative (<0.25)† | reference | <.001 | reference | <.001 |
| Positive, ≤ 50th %tile (≤9.68) | 2.05 (1.33, 3.17) | 1.84 (1.11, 3.05) | ||
| Positive, > 50th %tile (>9.68) | 2.56 (1.66, 3.93) | 2.70 (1.72, 4.25) | ||
| Test: linear trend | <.001 | <.001 | ||
| Cotinine and drug use‡ | ||||
| Negative cotinine (<0.25)† & negative for all drugs | reference | <0.001 | reference | <0.001 |
| Positive cotinine & negative for all drugs | 2.08 (1.31, 3.30) | 2.46 (1.49, 4.04) | ||
| Negative cotinine (<0.25)† & positive for any drug | 1.39 (0.59, 3.28) | 1.89 (0.82, 4.40) | ||
| Positive cotinine & positive for any drug | 4.53 (1.71, 12.05) | 4.00 (1.45, 10.97) | ||
CI, confidence interval.
Weighted stillbirth odds ratios and p-values are shown for smoking and drug use characteristics after adjustment for stillbirth risk factors known at pregnancy confirmation. The weights take into account the study design and differential consent based on characteristics recorded on all eligible pregnancies that were screened for the study. The adjustment for stillbirth risk factors is through a modified risk factor score for stillbirth developed on the logit scale using coefficients from a logistic regression model. The modification was to exclude coefficients associated with smoking status and illicit drug use. Weighted (unweighted) samples sizes for observations included in these adjusted analyses for cotinine are 548 (551) stillbirths and 1143 (1497) live births for all pregnancies and 367 (375) and 1094 (1410), respectively, for non-anomalous, singleton pregnancies, excluding intrapartum stillbirths. For cotinine and drug use, the sample sizes are 348 (359) and 708 (855), respectively, for all pregnancies and 246 (257) and 684 (811), respectively, for the subgroup. For ordered categories on cotinine levels, tests for linear and quadratic trends in the log odds of stillbirth were conducted using orthogonal contrasts. Neither of the quadratic trends was significant and their p-values are not reported.
Lower limit of detectability for the cotinine assay.
The toxicology screening panel can detect amphetamines (amphetamine, e,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxy-N-ethylamphetamine (MDEA), N,N-dimethyldopamine (DMDA,) and methamphetamine), cannabinoids (carboxy-THC), cocaine (benzoylecgonine), opiates (codeine, hydrocodone, hydromorphine, morphine, 6-Monoacetylmorphine (6MAM), and meconin), phencyclidine (phencyclidine (PCP)), 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), methadone), and barbiturates (amobarbital, butalbital, pentobarbital, Phenobarbital, and secobarbital).
Adjusting for whether the fetus was SGA reduced the stillbirth odds ratio for cotinine (≤ 50th percentile versus negative, and > 50th percentile versus negative) by greater than 10%. Thus, at least part of the association between smoking and stillbirth is mediated through fetal growth restriction. Furthermore, the interaction between high cotinine levels and fetal SGA was significant (p<0.02) and the stillbirth odds ratios for high cotinine levels among SGA and non-SGA fetuses were 2.43 (95% CI 1.53, 3.86) and 0.81 (95% CI 0.36, 1.82), respectively. In contrast, there was no significant interaction between high cotinine levels and preeclampsia in association with a stillbirth outcome of pregnancy.
Adjusting for cotinine level reduced the stillbirth odds ratio for THCA by greater than 10%, but adjusting for THCA did not reduce the stillbirth odds ratios for cotinine level. Thus, we cannot exclude the possibility that the association between cannabis and stillbirth is partially due to confounding by tobacco smoke. There was no evidence of confounding of the relationship between THCA and stillbirth by SGA fetus.
Among the 1,271 deliveries with both serum cotinine and drug testing, one woman was HIV-positive, seven were positive for hepatitis B and four were positive for hepatitis C. Only two of these women (both positive for hepatitis C) had either a positive cotinine or drug test. These small numbers preclude further analyses of the relationship between substance abuse and viral infection.
DISCUSSION
In this population-based study of stillbirth we noted a two-fold increase in stillbirth in women with positive umbilical cord homogenate screening. The most common drug detected was THCA, which was significantly associated with stillbirth (OR 2.34; 95% CI 1.13, 4.81). The effect was at least partially confounded with the effects of cotinine. Cannabis remains the most commonly used illicit drug in the United States. In 2009, 16.7 million persons reported using marijuana within the previous 30 days, a 2.3 million/month increase from 2007.24 Previous studies of cannabis use in pregnancy have been based on self report and either showed no association with adverse pregnancy outcomes or were associated with decreased fetal growth.25–28
Although numbers were small, hydrocodone and morphine trended towards an association with an increased odds of stillbirth, which is important given the epidemic of prescription opioid drug abuse.29 Approximately 1 in 20 of the United States population aged 12 or older has used opioid pain relievers non-medically24 and the potential exists that this could involve substantial numbers of pregnant women.
We also demonstrated a strong association between maternal smoking and stillbirth. Both self-reported smoking and maternal serum cotinine levels were associated with an increased stillbirth risk. Moreover, there was a general dose-response effect, strengthening the biological plausibility of the association. These data are similar to other reports associating self-reported maternal smoking with stillbirth.9–11 Prior studies also have noted a dose-dependent relationship between smoking and stillbirth and have demonstrated odds ratios in the range of 2.0.10,11,30 In this study, we used cotinine levels to objectively verify and quantitate smoking.
We also identified an increased risk of stillbirth among women exposed to second-hand smoke. We acknowledge that some of these women may have actually smoked but that number is likely small.31–33 Although recent studies have reported a relationship between second hand smoke and stillbirth,34,35,36 none used cotinine levels to verify and quantify the degree of exposure.
Our study had several limitations. First, participants who did not have cotinine and toxicology testing differed in race/ethnicity and gestational age from those whom samples were available for testing which may bias our findings. Second, drug use during pregnancy declines at term, which may have been another source of bias. Third, it is unclear whether exposure occurred prior to or after the stillbirth. Finally, despite the large number of women with stillbirth, we had a relatively small number of women testing positive for individual drugs. Thus, we lacked sample size to make definitive conclusions regarding the relationship between some individual drugs and stillbirth and between cannabis, smoking and stillbirth.
There were also several strengths of our study. The study was population based and racially and ethnically diverse. In addition, all participants were evaluated with a thorough standardized protocol that minimized variability in data and sample collection. Our study also included a maternal interview and medical record abstraction to allow for in-depth questions about smoking and drug use. Finally, in addition to self-reported substance abuse, exposure to tobacco and illicit drugs was confirmed by analyses that were blinded to the clinical outcome.
In summary, positive toxicology screen for illicit drugs was associated with a 2–3 fold increase in stillbirth risk. Documentation of THCA indicating cannabis use increased the odds of stillbirth two-fold. Cannabis users often smoke as well, and more research is needed to investigate the interaction of THCA and cigarette smoking. In addition, positive cotinine levels and smoking were associated with a two- to 2–2.5 fold increase in the risk of stillbirth. Furthermore even apparent passive smoking exposure was associated with stillbirth. Between 10 – 30% of pregnant women in developed countries continue to smoke during pregnancy.37 Women who quit smoking from their first to second pregnancy have been shown to reduce their risk of stillbirth to the same level as nonsmokers in the second pregnancy.38 In addition, cannabis use remains common during pregnancy with 2% of the women in this study with a positive cord homogenate (among live birth controls). Smoking and illicit drugs continue to be common and important modifiable risk factors for stillbirth. As cannabis use may be increasing with increased legalization, the relevance of our study’s findings may increase as well. Clinicians should be alert to these risks and should educate women regarding dangers associated with marijuana use and active and passive smoke exposure during pregnancy.
Supplementary Material
Acknowledgments
Funding:
Supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health: U10-HD045953 (Brown University, Rhode Island); U10-HD045925 (Emory University, Georgia); U10-HD045952 (University of Texas Medical Branch at Galveston); U10-HDO45955 (University of Texas Health Sciences Center at San Antonio); U10-HD045944 (University of Utah Health Sciences Center); and U01-HD045954 (RTI International, North Carolina).
The authors thank the following members of the National Institute of Child Health and Human Development Scientific Advisory and Safety Monitoring Board for their review of the study protocol, materials, and progress: Reverend Phillip Cato, PhD; James W. Collins Jr, MD, MPH; Terry Dwyer, MD, MPH; William P. Fifer, PhD; John Ilekis, PhD; Marc Incerpi, MD; George Macones, MD, MSCE; Richard M. Pauli, MD, PhD; Raymond W. Redline, MD; Elizabeth Thom, PhD (chair), as well as all of the other physicians, study coordinators, research nurses, and patients who participated in the Stillbirth Collaborative Research Network.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented in part at the 2011 Society for Maternal-Fetal Medicine, San Francisco, CA, February 10–12, 2011.
REFERENCES
- 1.MacDorman MF, Kirmeyer S, Wilson EC. National vital statistics reports. no 8. vol 60. Hyattsville, MD: National Center for Health Statistics; 2012. Fetal and perinatal mortality, United States, 2006. [PubMed] [Google Scholar]
- 2.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923–1935. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 3.Ananth CV, Liu S, Kinzler WL, Kramer SM. Stillbirths in the United States, 1981 – 2000: An age, period, and cohort analysis. Am J Public Health. 2005;95:2213–2217. doi: 10.2105/AJPH.2004.043885. [PMID:1449509]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludlow JP, Evans SF, Hulse G. Obstetric and perinatal outcomes in pregnancies associated with illicit substance abuse. Aust N Z J Obstet Gynaecol. 2004;44:302–306. doi: 10.1111/j.1479-828X.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 5.Plessinger MA. Prenatal exposure to amphetamines. Obstet Gynecol Clin North Am. 1998;25:119–138. doi: 10.1016/s0889-8545(05)70361-2. [DOI] [PubMed] [Google Scholar]
- 6.Addis A, Moretti ME, Syed FA, et al. Fetal effects of cocaine: an updated meta-analysis. Reprod Toxicol. 2001;15:341–369. doi: 10.1016/s0890-6238(01)00136-8. [DOI] [PubMed] [Google Scholar]
- 7.Bauer CR, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, et al. Maternal Lifestyles Study (MLS): Effects of substance exposure during pregnancy on acute maternal outcomes. Pediatr Res. 1996;39:257A. [Google Scholar]
- 8.Fretts R. Stillbirth epidemiology, risk factors, and opportunities for stillbirth prevention. Clin Obstet Gynecol. 2010;53:588–596. doi: 10.1097/GRF.0b013e3181eb63fc. [DOI] [PubMed] [Google Scholar]
- 9.Cnattingius S, Stephansson O. The epidemiology of stillbirth. Semin Perinatol. 2002;26:25–30. doi: 10.1053/sper.2002.29841. [DOI] [PubMed] [Google Scholar]
- 10.Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83:713–720. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. Exposure to tobacco smoke in utero and the risk of stillbirth and death in the first year of life. Am J Epidemiol. 2001;154:322–327. doi: 10.1093/aje/154.4.322. [DOI] [PubMed] [Google Scholar]
- 12.Kennare R, Heard A, Chan A. Substance use during pregnancy: risk factors and obstetric and perinatal outcomes in South Australia. Aust N Z J Obstet Gynaecol. 2005;45:220–225. doi: 10.1111/j.1479-828X.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 13.Parker CB, Hogue CJR, Koch MA, Willinger M, Reddy U, Thorsten VR, Dudley DJ, Silver RM, Coustan D, Saade GR, Conway D, Varner MW, Stoll B, Pinar H, Bukowski R, Carpenter M, Goldenberg R for the Stillbirth Collaborative Research Network. Stillbirth Collaborative Research Network: Design, methods and recruitment experience. Pediatr Perinatal Epidemiol. 2011;25:425–435. doi: 10.1111/j.1365-3016.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Stillbirth Collaborative Research Network Writing Group. Association between stillbirth and risk factors known at pregnancy confirmation. JAMA. 2011;306:2469–2479. doi: 10.1001/jama.2011.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey JC, Klebanoff MA, Hauth JC. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 16.Alexander GR, Himes JH, Kaufman RG, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 17.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Abramowsky CR, Thorsten VR, et al. The Stillbirth Collaborative Research Network Postmortem Examination Protocol. Am J Perinatol. 2012;29(3):187–202. doi: 10.1055/s-0031-1284228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Shehata B, Thorsten VR, et al. The Stillbirth Collaborative Research Network (SCRN) Placental and Umbilical Cord Examination Protocol. Am J Perinatol. 2011;28(10):781–792. doi: 10.1055/s-0031-1281509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol. 2006;26:11–14. doi: 10.1038/sj.jp.7211416. [DOI] [PubMed] [Google Scholar]
- 20.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 21.Rebagliato M, Bolumar F, Florey Cdu V, Jarvis MJ, Perez-Hoyos S, Hernandez-Aguado I, et al. Variations in cotinine levels in smokers during and after pregnancy. Am J Obstet Gynecol. 1998;178(3):568–571. doi: 10.1016/s0002-9378(98)70440-5. [DOI] [PubMed] [Google Scholar]
- 22.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 23.Research Triangle Institute. SUDAAN Language Manual, Volumes 1 and 2, Release 11. Research Triangle Park, NC: Research Triangle Institute; 2012. [Google Scholar]
- 24.Substance Abuse and Mental health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10–4586Findings) Rockville, MD: 2010. [Google Scholar]
- 25.Linn S, Schoenbaum SC, Monson RR, Rosner R, Stubblefield PC, Ryan KJ. The association of marijuana use with outcome of pregnancy. AJPH. 1983;73(10):1161–1164. doi: 10.2105/ajph.73.10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatch EE, Bracken MB. Effect of marijuana use in pregnancy on fetal growth. Am J Epidemiol. 1986;124(6):986–993. doi: 10.1093/oxfordjournals.aje.a114488. [DOI] [PubMed] [Google Scholar]
- 27.Fergusson DM, Horwood LJ, Northstone K. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109(1):21–27. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 28.El Marroun H, Tiemeier H, Steegers EA, Jaddoe VW, Hofman A, Verhulst FC, et al. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R Study. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1173–1181. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- 29.Paulozzi LJ, Jones CM, Mack KA, Rudd RA. Overdoses of prescription opioid pain relievers – United States, 1999–2008. MMWR. 2011;60:1487–1492. [PubMed] [Google Scholar]
- 30.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal weight, pregnancy weight gain, and the risk of antepartum stillbirth. Am J Obstet Gynecol. 2001;184:463–469. doi: 10.1067/mob.2001.109591. [DOI] [PubMed] [Google Scholar]
- 31.Klebanoff MA, Levine RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentrations and self-reported smoking during pregnancy. Am J Epidemiol. 1998;148(3):259–262. doi: 10.1093/oxfordjournals.aje.a009633. [DOI] [PubMed] [Google Scholar]
- 32.DeLorenza GN, Kharrazi M, Kaufman FL, Eskenazi B, Bernert JT. Exposure to environmental tobacco smoke in pregnant women: the association between self-report and serum cotinine. Environ Res. 2002;90(1):21–32. doi: 10.1006/enrs.2001.4380. [DOI] [PubMed] [Google Scholar]
- 33.Yeager DS, Krosnick JA. The validity of self-reported nicotine product use in the 2001–2008 National Health and Nutrition Examination survey. Med Care. 2010;48(12):1128–1132. doi: 10.1097/MLR.0b013e3181ef9948. [DOI] [PubMed] [Google Scholar]
- 34.Crane JM, Keough M, Murphy P, Burrage L, Hutchens D. Effects of environmental tobacco smoke on perinatal outcomes: a retrospective cohort study. BJOG. 2011;118(7):865–871. doi: 10.1111/j.1471-0528.2011.02941.x. [DOI] [PubMed] [Google Scholar]
- 35.Subramoney S, d’Espaignet ET, Gupta PC. Higher risk of stillbirth among lower and middle income women who do not use tobacco, but live with smokers. Acta Obstet Gynecol Scand. 2010;89(4):572–577. doi: 10.3109/00016341003801656. [DOI] [PubMed] [Google Scholar]
- 36.Leonardi-Bee J, Britton J, Venn A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: a meta-analysis. Pediatrics. 2011;127(4):734–741. doi: 10.1542/peds.2010-3041. [DOI] [PubMed] [Google Scholar]
- 37.Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. Exposure to tobacco smoke in utero and the risk of stillbirth and death in the first year of life. Am J Epidemiol. 2001;154(4):322–327. doi: 10.1093/aje/154.4.322. [DOI] [PubMed] [Google Scholar]
- 38.Hogbert L, Cnattingius S. The influence of maternal smoking habits on the risk of subsequent stillbirth: is there a causal relation? BJOG. 2007;114:699. doi: 10.1111/j.1471-0528.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.