Abstract
A single-nucleotide polymorphism (rs2274223: A5780G:His1927Arg) in the phospholipase C epsilon gene (PLCε) was recently identified as a susceptibility locus for esophageal cancer in Chinese subjects. To determine the underlying mechanisms of PLCε and this SNP in esophageal carcinogenesis, we analyzed PLCε genotypes, expression, and their correlation in esophageal cancer cell lines, non-transformed esophageal cells, 58 esophageal squamous cell carcinomas and 10,614 non-cancer subjects from China. We found that the G allele (AG or GG) was associated with increased PLCε mRNA and protein expression in esophageal cancer tissues and in esophageal cancer cell lines. G allele was also associated with higher enzyme activity, which might be associated with increased protein expression. Quantitative analysis of the C2 domain sequences revealed that A:G allelic imbalance was strongly linked to esophageal malignancy. Moreover, the analysis of 10,614 non-cancer subjects demonstrated that the G allele was strongly associated with moderate to severe esophagitis in the subjects from the high-incidence areas of China (OR 6.03, 95% CI 1.59–22.9 in high-incidence area vs. OR 0.74, 95% CI 0.33–1.64 in low-incidence area; P = 0.008). In conclusion, the PLCε gene, particularly the 5780G allele, might play a pivotal role in esophageal carcinogenesis via upregulating PLCε mRNA, protein, and enzyme activity, and augmenting inflammatory process in esophageal epithelium. Thus, 5780G allele may constitute a promising biomarker for esophageal squamous cell carcinoma risk stratification, early detection, and progression prediction.
Keywords: phospholipase C epsilon, esophageal cancer, esophagitis
INTRODUCTION
Phospholipase C epsilon (PLCε or PLCE 1) is a phosphoinositide-specific enzyme phospholipase C that converts phosphatidylinositol 4,5-biphosphate (PtdIns (4, 5) P2) [1, 2] to two intracellular second messengers, diacylglycerol and inositol 1,4,5-trisphosphate, which regulate protein kinase C activity and calcium mobilization, respectively [3, 4]. These responses are involved in the regulation of cell growth, differentiation, and oncogenesis [5]. To date, six members of the PLC family (PLCβ, γ, δ, ε, η, and ζ) have been identified in mammalian cells. These members share similar catalytic properties and are characterized by distinct regulatory mechanisms [6, 7], indicating that PLCs exert diverse functions in carcinogenesis. PLCε binds to and is activated by the Ras family GTPases [1, 8, 9]. It participates in murine skin cancer development through augmentation of inflammatory cytokine production and signaling [10–12]. A more recent report also showed that inhibition of intestinal tumorigenesis in ApcMin/+ mice by loss of PLCε occurs via reducing cytokine-mediated inflammation in local tissue [13]. However, the involvement of PLCε in human carcinogenesis, particularly in esophageal cancer, was not been recognized until a susceptible SNP was recently identified in GWAS studies of more than 20,000 Chinese subjects [14, 15]. This SNP, located within exon 26 of the PLCε gene at chromosome 10q23, leads to a nonsynonymous alteration from CAC (A allele, encoding histidine) to CGC (G allele, encoding arginine). These previous data showed that a G at position 5780 in the C2 domain of PLCε was associated with an increased risk of both squamous cell carcinoma (SCC) and esophagus-cardia gastric adenocarcinoma (ECA). In the current report, we demonstrate that the presence of the G allele at this locus leads to increased PLCε mRNA and protein expression and enzyme activity in esophageal cancer cells in vitro, and that A:G allelic imbalance is strongly associated with esophageal malignancy in primary human tissues. Interestingly, we show that the presence of the G allele is also strongly associated with moderate to severe esophagitis in non-cancer subjects from the areas of high-incidence of esophageal cancer in China.
MATERIALS AND METHODS
Human Subjects
Frozen tissues from 58 esophageal SCCs and white blood cell DNA from 10,614 non-cancer subjects were collected from an ongoing hospital-based SCC and EAC case–control study, involving multiple hospitals throughout high- and low-risk areas for esophageal SCC in China since 2007. The genotypes of PLCE in non-SCC patients were based on TaqMan genotyping methods, as described recently [14]. All subjects had undergone esophagogastroduodeno-scopy, and non-cancer subjects with esophagitis were determined by endoscopic examination and histopathology. The diagnosis of the degree of esophagitis was made by at least three pathologists. All procedures were conducted according to Declaration of Helsinki principles and had been approved by respective institutional review boards.
DNA Extraction and PCR-Based PCLε Exon 26 Sequencing
Genomic DNA was extracted from the following materials: SCC and adjacent normal esophageal epithelia, esophageal SCC cell lines (TE-1, TE-2, TE-7, TE-8, TE-12, G5, HCE4, HCE7, EC171, EC8712, EC109, EC9706, SHEEC) and non-transformed esophageal epithelial cell lines (HET1A, HEEpic) using a DNA extraction kit (QIAGEN, Valencia, CA). PCR for PLCε exon 26, in which the C2 domain is located, was performed using the following primers: forward (Ex26F): 5′-TGTTCTTGGGATTCCTTTGC-3′ and reverse (Ex26R): 5′-TGCTTCTTAATTCAACTTCTTTATAGG-3′. The PCR product was directly sequenced using an ABI sequencing system (Applied Biosystems, Inc., Foster City, CA). Allelic imbalance was analyzed using Mutation Surveyor software (Softgenetics, College Station, PA).
RNA Extraction, Quantitative Real-Time PCR, Immunoblotting, and Immunohistochemical Staining
Total RNA was extracted from human esophageal cancer cell lines and tissues, samples were then subjected to reverse transcription; cDNA was used for quantitative PCR analyses of PLCε expression at the mRNA level. The following primers that cover the C2 domain were used for quantitative PCR analysis: forward 5′-TGTGGAACGAGCAGTTTCTG-3′ and reverse 5′-ATCGAAGAGGC TGACATGGT-3′. Cell lysate was made from esophageal cancer cell lines, followed by immunoblotting and probing with anti-PLCε antibody (provide by Dr. J. Lomasney, North-western University, Chicago, IL). Immunoblotting intensities were quantified using Quanty One software (Bio-Rad, Hercules, CA). PLCε immunohistochemical staining was performed as described recently [14], and immunohistochemical staining was scored by at least three pathologists using the following criteria: 0, no staining or staining area was <10%; 1, positive area was between 10% and 50%; and 2, positive area was more than 50%, similar as we reported [16].
PLCε Enzyme Activity Assay
PLCε enzymatic activity was determined by measurement of total [3H]inositol phosphates accumulation in esophageal cancer cells containing AG and AA alleles (TE-2, -7, -8, and -12). 1-Oleoyl-l-α-lyso-phosphatidic acid (LPA) was purchased from Sigma– Aldrich (St. Louis, MO) and dissolved in water containing 1.0% fatty acid-free bovine serum albumin. Briefly, cells were seeded in a 24-well plate at a density of 200,000 cells per well. 18 h later, medium was replaced with inositol-and serum-free DMEM containing 1 µCi/well [3H]myo-inositol (American Radiolabeled Chemicals, St. Louis, MO). Phospholipase C activity was quantified 16 h after labeling by incubation in inositol-free DMEM containing 10 mM LiCl, either in the absence of LPA or in the presence of 10 µM LPA. The reaction was stopped after 30 min by aspiration of the medium and addition of an ice-cold buffer containing 0.6 M perchloric acid and 0.2 mM IP6. After neutralization with buffer containing 1 M K2CO3 and 40 mM EDTA, the accumulation of [3H] inositol phosphates was quantified by Dowex chromatography. 0.1% Triton X-100/0.1 M NaOH was added to cells in each well to determine total lipids. Each experiment was performed in triplicate, and these triplicate experiments were also repeated independently three times.
RESULTS AND DISCUSSION
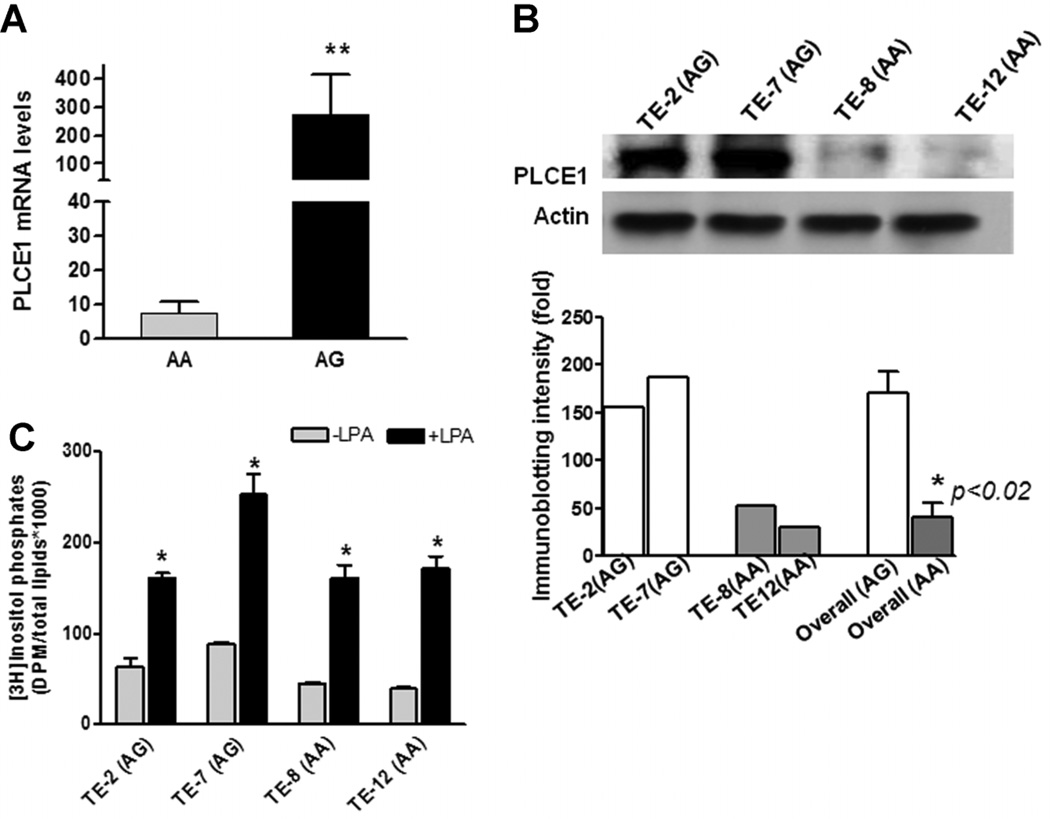
To investigate whether the G allele of the PLCε gene SNP at position 5780 alters PLCε expression, we determined PLCε genotypes and mRNA levels in 13 human esophageal squamous cancer cell lines (TE-1, TE-2, TE-7, TE-8, TE-12, G5, HCE4, HCE7, EC171, EC8712, EC109, EC9706, SHEEC). Six SCC cell lines (G5, TE-8, TE-12, HCE4, EC171, EC8712) were AA, while the remaining 7 (TE-1, TE-2, TE-7, HCE7, EC109, EC9706, SHEEC) were AG for PLCε at position 5780. Quantitative RT-PCR (qRT-PCR) revealed that PLCε mRNA levels in the seven SCC AG cell lines increased approximately 37-fold relative to the 6 AA SCC cell lines (Figure 1A; 271 ± 144 in AG cells vs. 7.3 ± 3.3 in AA cells, P < 0.01). Increased PLCε mRNA levels also correlated with higher PLCε protein levels measured by immuno-blotting (Figure 1B). The overall (average) intensity of PLCE1 protein in AG cell lines were higher than those in the AA cell lines (P < 0.02). Interestingly, two immortalized human esophageal epithelial cell lines derived from normal cells, HET1A (AG allele) and HEEPic (AA allele) also exhibited a similar association pattern: cells with the AG allele expressed higher levels of PLCε mRNA (23.6 in HET1A vs. 3.6 in HEEPic) and protein (data not shown).
Figure 1.
Correlation of A5780G genotypes with PLCε mRNA, total protein and enzyme activity levels in SCC cell lines. (A) Quantitative determination of PLCε mRNA levels in SCC cell lines (n = 13) by qRT-PCR. Bars represent the mean ± SD for each tumor type. **P < 0.01. (B) A representative of immunoblotting showed that PLCE1 protein levels were higher in AG cell lines (TE-2 and TE-7) and were lower in AA cell lines (TE-8 and TE-12), using specific anti-PLCε antibody. Histograms showed immunoblotting intensities quantified by Quanty One software (Bio-Rad, Hercules, CA). *P < 0.02, overall (average) intensity of PLCE1 protein in AG cell lines in comparison with those in AA cell lines. (C) PLCε enzymatic activity was determined by measurement of [3H] inositol triphosphate in esophageal cancer cells with AA and AG alleles. Endogenous PLCε baseline activity (gray bars) was nearly twice as high in the two AG cell lines (TE-2 and TE-7) than those in the two AA cell lines (TE-8 and TE-12) (76 ± 20 vs. 42 ± 7, P < 0.05). LPA was used as a ligand to stimulate PLCε activity, and PLCε activities were significantly induced (black bars). Bars represent the mean ± SD for three replicates of experiments performed. *P < 0.001, compared to individual cell line without LPA treatment.
We then examined whether elevated PLCε mRNA and protein levels correlated with PLCε enzyme activity. For these experiments, we chose the two AG cell lines (TE-7 and TE-2) that expressed the highest levels of PLCε and the two AA cell lines (TE-8 and TE-12) with the lowest levels of PLCε mRNA and protein. Consistent with findings for protein levels, endogenous PLCε baseline activity was nearly twice as high in the two AG cell lines (TE-2 and TE-7) than those in the two AA cell lines (TE-8 and TE-12) (76 ± 20 vs. 42 ± 7, P < 0.05). The increased enzyme activities might be linked to the increased protein expression. Previous studies have demonstrated that overexpression of activated small Rho family GTPases leads to marked elevation of intracellular inositol phosphate accumulation [17], and PLCε is a direct effector of activated Rho [18]. Similarly, the expression of G protein α-subunits Gα 12 and Gα 13 resulted in PLCε-dependent accumulation of inositol phosphates [19, 20]. Therefore, agonists of G Protein coupled receptors that couple to Gα 12 and Gα 13, such as LPA (1-Oleoyl-l-α-lysophosphatidic acid), activate PLCε in a Rho-dependent manner [21]. To determine the response of PLCε activity to LPA in cells containing different alleles (AA vs. AG), we treated the two groups of ESCC cell lines with LPA and surprisingly found that AG cell lines exhibited only 2.5-fold average induction of PLCε activity, whereas AA cell lines were induced average fourfold (Figure 1C; P < 0.05). This finding may have been due to the failure of adapted AG allele cells to fully activate PLCε after interacting with harmful environmental factors (e.g., bile acids, bacterial infection, carcinogens, or other stressors), which reduces subsequential induction of cytokine and chemokine and development of inflammation in esophageal epithelium as a defensive response to the environmental detrimental stimulation. As a consequence, the lack of fully activated PLCε enzyme could cause epithelial cells to produce more PLCε mRNA and protein as a compensatory response through a feedback mechanism. From analysis of homology modeling of the PLCε C2 domain structure, we observed that changing His1927 to Arg in the C2 domain may affect protein–protein interaction and/or lipid recognition, but is unlikely to have an impact on ion binding by this enzyme (Supplementary Figure 1). However, further investigations using mutant allele plasmids are needed to confirm this modeling result.
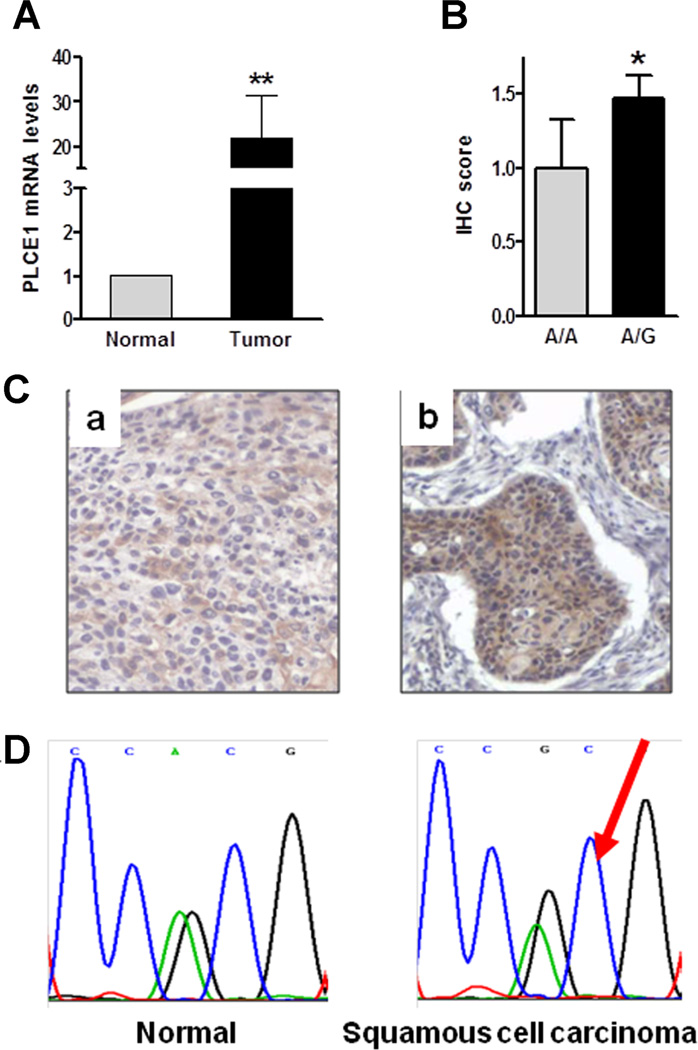
PLCε-mediated cell growth promotion has been reported in various cell types [5, 22, 23], and the mitogenic effect of PLCε may facilitate cancer progression. We measured PLCε mRNA levels in 26 primary human esophageal SCCs and adjacent normal esophageal epithelial tissues by quantitative RT-PCR. As shown in Figure 2A, PLCε mRNA levels were significantly higher in esophageal SCCs than in adjacent normal tissues (normal, 1.0 vs. SCC, 22.0; P < 0.01). Consistent with these mRNA expression levels, SCC tissues with heterozygous AG expressed 1.5-fold higher protein levels than did homozygous AA tissues (1.5 ± 0.1 vs. 1.0 ± 0.3, P < 0.05; Figure 2B), assayed by immunohistochemical staining using an anti-PLCε antibody (Figure 2C). Our results were different from recent report that showed decreased PLCε mRNA and no changes of protein in esophageal cancer tissues compared to the adjacent normal tissues, assayed by qRT-PCR and immunohistochemical staining, respectively [24]. This discrepancy could be resulted from the patients selection: our patients were from the North Henan area, the most high incidence area of esophageal cancer in the world, the patients used in the recent report were from Shanghai area [24], the low incidence area of esophageal cancer. The different pattern of PLCε in high- and low-incidence areas was observed in esophagitis (Figure 3). We showed the results in detail later. Interestingly, allelic imbalance analysis showed an increased G allele copy number in 44% (15/34) of AG SCC tissues when compared to matching normal esophageal control tissues (Figure 2D), using Mutation Surveyor software (Soft-genetics, College Station, PA). These findings suggest that G allele is associated with overexpression of PLCε in primary SCC tissues.
Figure 2.
Genetic and expression-level alterations of PLCε in human esophageal cancer tissues. (A) Increased PLCε mRNA levels were determined by RT-PCR in SCC tissues (n = 26) compared to adjacent normal control tissues. Bars represent the mean ± SD of tumor and adjacent normal tissues. **P < 0.02. (B) Immunohistochemical staining showed higher PLCε expression scores in SCC than in adjacent normal epithelia (1.5 ± 0.1 vs. 1.0 ± 0.3, *P < 0.05). (C) Representative PLCε1 immunohistochemical staining in a AA SCC (a) and an AG SCC (b). (D) Representative sequencing results showed A5780G allelic imbalance in SCC versus normal esophageal control epithelium using Mutation Surveyor software (Softgenetics, College Station, PA). Red arrow indicates a gain of G allele in squamous cell carcinoma.
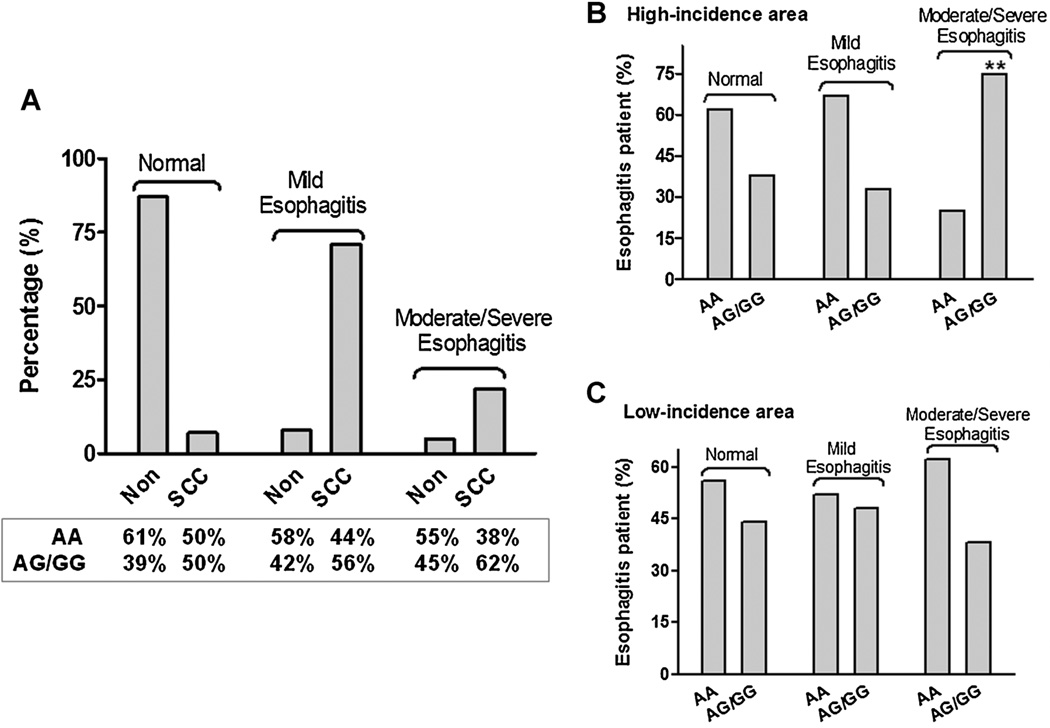
Figure 3.
Association between PLCε genotypes and esophagitis in SCC and Non-SCC individuals. (A) The severity of esophagitis (mild, moderate and severe) in SCC (n = 58) and Non-SCC subjects (n = 1,0614) was correlated with the three PLCε genotypes. (B,C) Association between PLCε genotypes and esophagitis in Non-SCC individuals from high- and low-risk areas. The distribution and numbers in each group are provided in Table 1. **P < 0.01.
Overexpression of PLCε protein and enhancement of PLCε enzyme activity have been reported to activate PKC and induce elevation of intracellular calcium levels [25], leading to cytokine- or chemo-kine-mediated inflammation in local tissues [12]. Furthermore, an association between esophagitis and the development of esophageal squamous cell cancer has been recognized and documented previously [26]. To investigate any possible association between A5780G and esophageal inflammation, we correlated SNP genotypes with presence or absence of esophagitis in individuals with cancer (SCC) and without cancer (non-SCC). 52 (89.7%) of the 58 SCC patients exhibited various degrees of esophagitis (mild, moderate, or severe), whereas only 1517 (14.3%) of the 10,614 non-SCC subjects had any esophagitis, determined by endoscopic examination and confirmed by histopathology (P < 0.0001). Importantly, the severity of esophagitis was associated with the AG/GG allele in these SCC patients. Eight (62%) of the 13 SCC patients with moderate or severe esophagitis were AG/GG, while only 5 (38%) of the 13 SCC patients with moderate or severe esophagitis had the AA genotype (Figure 3A). However, when non-SCC individuals with esophagitis were classified into high- or low-incidence areas for esophageal cancer, a significant association between severe esophagitis and the G allele of the PLCε gene was observed: 77% of the severe esophagitis individuals in high-incidence areas had AG/GG genotypes (Figure 3B), versus only 37% of these subjects in low-incidence areas (Figure 3C) (OR 6.03 with 95% CI 1.59–22.9 vs. OR 0.74 with 95% CI 0.33–1.64; P = 0.008; Table 1). These data support the hypothesis that the interaction of potential environmental factors with PLCε, particularly in the individuals with AG or GG allele, not only exists in high-incidence areas for esophageal cancer development in China, but also correlates with the severity of esophagitis. Currently, this group of individuals is under long-term follow up. Correlations between this risk allele, esophagitis, and cancer development will be clarified in future studies.
Table 1.
Association Between PLCE1 Genotypes and Esophagitis in High- and Low-Risk Areas of China
| High-incidence area % (n) |
Low-incidence area % (n) |
|||||
|---|---|---|---|---|---|---|
| Genotypes | AA | AG/GG | OR (95% CI) | AA | AG/GG | OR (95% CI) |
| Mild esophagitis | 86% (126) | 80% (76) | 1.00 | 92% (296) | 91% (230) | 1.00 |
| Moderate | 12% (18) | 9% (9) | 0.84 (0.36–1.97) | 3% (10) | 5% (13) | 1.67 (0.72–3.89) |
| Severe | 2% (3) | 11% (10) | 6.03 (1.59–22.9)* | 5% (17) | 4% (10) | 0.73 (0.33–1.64) |
OR, odds ratio; CI, confidence interval.
P = 0.008.
In conclusion, PLCε is likely to play a pivotal role in esophageal carcinogenesis: the presence of the 5780G allele may not only predict a high risk of future esophageal cancer development, but may also participate in esophageal cancer growth and progression by upregulating levels of PLCε mRNA, protein, and enzyme activity, ultimately leading to augmentation of the inflammatory process in esophageal epithelium. Thus, the 5780G allele in PLCε may constitute a promising biomarker for esophageal squamous carcinoma risk stratification, early detection, and progression prediction.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the members of Dr. Wang’s group at Xinxiang Medical University Cancer Research Center (Xinxiang, China) and to the collaborators in China for sample collection and data analysis. We would also like to thank Dr. Friedhelm Hildebrandt (University of Michigan, Ann Arbor, MI) for providing PLCε sequencing primers, thank Dr. Qingyi Wei (University of Texas MD Anderson Cancer Center, Houston, TX) for statistic analysis, Dr. Jon Lomasney (Northwestern University, Chicago, IL) for providing the PLCε antibody, and thank Dr. Alan Smrcka (University of Rochester, Rochester, NY) and Jun-Yan Hong (University of Medicine and Dentistry of New Jersey, Piscataway, NJ) for critical reading and discussing of this manuscript.
Contract grant sponsor: National Institutes of Health, USA; Contract grant number: CA112081 (to W. Yang); Contract grant sponsor:; Contract grant number: CA146799 and DK087454 (to S.J. Meltzer); Contract grant sponsor: Xinxiang Medical University Key Scientific Program 2009-5; Contract grant sponsor: China National Natural Science Foundation; Contract grant numbers: 30670956; 30971133 (to L.D. Wang); 91229115 (to W. Yang); Contract grant sponsor: Program of Liaoning Excellent Talents in University (LETU); Contract grant number: LJQ2011002 (to X. Bi); Contract grant sponsor: Intramural Research Program of the NIH/National Institute of Environmental Health Sciences (to S. Shears).
The sponsors of this study had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- PLCε
phospholipase C epsilon
- SCC
squamous cell carcinoma
- LPA
1-Oleoyl-L-α-lysophosphatidic acid
- RT-PCR
reverse transcriptional polymerase chain reaction.
Footnotes
Additional supporting information may be found in the online version of this article.
Conflict of interest: nothing to declare.
REFERENCES
- 1.Bunney TD, Katan M. Phospholipase C epsilon: Linking second messengers and small GTPases. Trends Cell Biol. 2006;16:640–648. doi: 10.1016/j.tcb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Bunney TD, Baxendale RW, Katan M. Regulatory links between PLC enzymes and Ras superfamily GTPases: Signalling via PLCepsilon. Adv Enzyme Regul. 2009;49:54–58. doi: 10.1016/j.advenzreg.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Bunney TD, Katan M. Phosphoinositide signalling in cancer: Beyond PI3K and PTEN. Nat Rev Cancer. 2010;10:342–352. doi: 10.1038/nrc2842. [DOI] [PubMed] [Google Scholar]
- 4.Singer WD, Brown HA, Sternweis PC. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- 5.Citro S, Malik S, Oestreich EA, et al. Phospholipase Cepsilon is a nexus for Rho and Rap-mediated G protein-coupled receptor-induced astrocyte proliferation. Proc Natl Acad Sci USA. 2007;104:15543–15548. doi: 10.1073/pnas.0702943104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley GG, Kaproth-Joslin KA, Reks SE, Smrcka AV, Wojcikiewicz RJ. G-protein-coupled receptor agonists activate endogenous phospholipase Cepsilon and phospholipase Cbeta3 in a temporally distinct manner. J Biol Chem. 2006;281:2639–2648. doi: 10.1074/jbc.M507681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley GG, Reks SE, Smrcka AV. Hormonal regulation of phospholipase Cepsilon through distinct and overlapping pathways involving G12 and Ras family G-proteins. Biochem J. 2004;378:129–139. doi: 10.1042/BJ20031370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C(epsilon): A novel Ras effector. EMBO J. 2001;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunney TD, Harris R, Gandarillas NL, et al. Structural and mechanistic insights into ras association domains of phospholipase C epsilon. Mol Cell. 2006;21:495–507. doi: 10.1016/j.molcel.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Bai Y, Edamatsu H, Maeda S, et al. Crucial role of phospholipase Cepsilon in chemical carcinogen-induced skin tumor development. Cancer Res. 2004;64:8808–8810. doi: 10.1158/0008-5472.CAN-04-3143. [DOI] [PubMed] [Google Scholar]
- 11.Ikuta S, Edamatsu H, Li M, Hu L, Kataoka T. Crucial role of phospholipase C epsilon in skin inflammation induced by tumor-promoting phorbol ester. Cancer Res. 2008;68:64–72. doi: 10.1158/0008-5472.CAN-07-3245. [DOI] [PubMed] [Google Scholar]
- 12.Hu L, Edamatsu H, Takenaka N, Ikuta S, Kataoka T. Crucial role of phospholipase Cepsilon in induction of local skin inflammatory reactions in the elicitation stage of allergic contact hypersensitivity. J Immunol. 2010;184:993–1002. doi: 10.4049/jimmunol.0901816. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Edamatsu H, Kitazawa R, Kitazawa S, Kataoka T. Phospholipase Cepsilon promotes intestinal tumorigenesis of Apc(Min/þ) mice through augmentation of inflammation and angiogenesis. Carcinogenesis. 2009;30:1424–1432. doi: 10.1093/carcin/bgp125. [DOI] [PubMed] [Google Scholar]
- 14.Wang LD, Zhou FY, Li XM, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42:759–763. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 15.Abnet CC, Freedman ND, Hu N, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010;42:764–767. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T, Yang W, Li M, et al. Expression of selenium-binding protein 1 characterizes intestinal cell maturation and predicts survival for patients with colorectal cancer. Mol Nutr Food Res. 2008;52:1289–1299. doi: 10.1002/mnfr.200700331. [DOI] [PubMed] [Google Scholar]
- 17.Wing MR, Snyder JT, Sondek J, Harden TK. Direct activation of phospholipase C-epsilon by Rho. J Biol Chem. 2003;278:41253–41258. doi: 10.1074/jbc.M306904200. [DOI] [PubMed] [Google Scholar]
- 18.Seifert JP, Wing MR, Snyder JT, Gershburg S, Sondek J, Harden TK. RhoA activates purified phospholipase C-epsilon by a guanine nucleotide-dependent mechanism. J Biol Chem. 2004;279:47992–47997. doi: 10.1074/jbc.M407111200. [DOI] [PubMed] [Google Scholar]
- 19.Wing MR, Houston D, Kelley GG, Der CJ, Siderovski DP, Harden TK. Activation of phospholipase C-epsilon by heterotrimeric G protein betagamma-subunits. J Biol Chem. 2001;276:48257–48261. doi: 10.1074/jbc.C100574200. [DOI] [PubMed] [Google Scholar]
- 20.Lopez I, Mak EC, Ding J, Hamm HE, Lomasney JW. A novel bifunctional phospholipase c that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. J Biol Chem. 2001;276:2758–2765. doi: 10.1074/jbc.M008119200. [DOI] [PubMed] [Google Scholar]
- 21.Hains MD, Wing MR, Maddileti S, Siderovski DP, Harden TK. Galpha12/13- and rho-dependent activation of phospholipase C-epsilon by lysophosphatidic acid and thrombin receptors. Mol Pharmacol. 2006;69:2068–2075. doi: 10.1124/mol.105.017921. [DOI] [PubMed] [Google Scholar]
- 22.Song C, Satoh T, Edamatsu H, et al. Differential roles of Ras and Rap1 in growth factor-dependent activation of phospholipase C epsilon. Oncogene. 2002;21:8105–8113. doi: 10.1038/sj.onc.1206003. [DOI] [PubMed] [Google Scholar]
- 23.Yun S, Hong WP, Choi JH, et al. Phospholipase C-epsilon augments epidermal growth factor-dependent cell growth by inhibiting epidermal growth factor receptor downregulation. J Biol Chem. 2008;283:341–349. doi: 10.1074/jbc.M704180200. [DOI] [PubMed] [Google Scholar]
- 24.Hu H, Yang J, Sun Y, et al. Putatively functional PLCE1 variants and susceptibility to esophageal squamous cell carcinoma (ESCC): A case-control study in Eastern Chinese populations. Ann Surg Oncol. 2012;19:2403–2410. doi: 10.1245/s10434-011-2160-y. [DOI] [PubMed] [Google Scholar]
- 25.Oestreich EA, Malik S, Goonasekera SA, et al. Epac and phospholipase Cepsilon regulate Ca2þ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz N, Crespi M, Grassi A, Qing WG, Qiong S, Cai LZ. Precursor lesions of oesophageal cancer in high-risk populations in Iran and China. Lancet. 1982;1:876–879. doi: 10.1016/s0140-6736(82)92151-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.