Abstract
Most medical curricula rely on human bodies for teaching macroscopic anatomy. Over the past 20 years, plastination has become an important means of preservation of organs, for well dissected specimens or for body slices. Here, several critical points regarding body donation with legal and ethical considerations for long-term preservation, the use of cadavers in teaching and the preparation of plastinates as an additional teaching tool will be discussed. Silicone S10 is the gold standard in the preparation of plastinates. An important point to respect is the preparation of specimens, since only very well dissected body parts or excellent tissue sections should be plastinated to show the extraordinary aspects of the human anatomy. The preparation of thin and transparent sections and preservation with P40 polyester provides an additional technique to prepare resistant body slices. A selection of samples prepared by S10 and P40 are shown and compared. In addition, Prussian or Berlin blue staining of brain slices is shown to discriminate better between gray and white matter and demonstrate neuroanatomical structures. These plastinates have been used for many years in teaching first-and second-year medical students and have not lost their appeal. Students and staff appreciate the use of such plastinates. One of the advantages is that their use is not restricted to the dissection hall; slices and body parts can be used in any lecture room or in small group teaching. Therefore, ethical and legal questions need to be addressed regarding their specific use. Plastinates do not replace the traditional dissection courses, since students learn best the anatomical features of a given region by hands-on dissection and by exploratory anatomy. Furthermore, plastinates are more rigid and do not allow demonstration of hidden structures; they also become more cumbersome for endoscopy or are too rigid for demonstrating mechanical features of joints. However, although not a replacement for traditional dissections, plastination provides an additional tool for long-term preservation and for teaching human anatomy.
Keywords: anatomy, body donation, ethics, long-term preservation, plastination, teaching
Introduction
Plastination of body parts is playing a more and more important role in the long-term preservation of tissue and anatomical teaching. The aim of this position paper is to address the importance of plastination and to discuss some problems inherent to plastination as an excellent method for preservation of tissue for a very long period of time. There are several plastination techniques with their advantages and disadvantages. Plastination, also called forced polymer impregnation, is an ideal method for long-term preservation of tissues, whole bodies or body parts. Given this, it is important to obtain written and informed consent from potential donors (SAMS, 2008, 2010). Anatomy departments depend on donations, therefore transparency and accurate information given to donors is essential. Plastinates need to be properly stored in a secure place when not in use. Since the use is not restricted to the dissection hall, any utilization outside the lab and by third parties needs to be defined to avoid unethical or disrespectful treatment of human remains.
Preparation of body parts may take considerable time and given that wet specimens can only be used for a few years, it has become more and more important to prepare specimens with care prior to plastination and their future use in teaching over several decades. Plastinated specimens have a long shelf life. However, now and then students try to test the strength of specimens, not aware being of how much work is needed and, ‘alas’, specimens may be lost forever.
Here, we will provide several examples of plastinates and how to use them in a complementary way. We will also compare the S10 and P40 techniques and their differences in revealing tissue parts. There are differences in the visualization of tissues. The S10 technique gives good and solid specimens. The P40 method provides thin tissue slices (4–6 mm) which are very resistant and more transparent, but may lead to differences in the discrimination of structures in the soft tissue.
Various aspects of the locomotor system, brain tissue or female pelvis will be highlighted, with their advantages and disadvantages. Overall, plastination can provide a supplementary method to demonstrate anatomical differences and it is an ideal method for long-term preservation of the most valuable preparations. In addition, plastinates are essential to complement the traditional dissection courses and contribute to a better preparation of postgraduates and clinicians. All specimens that are presented here have been used for several years in lectures and practical courses and so far have not lost their anatomical appeal.
Materials and methods
Bodies were obtained from the local donation program at the Department of Cell Biology and Morphology, University of Lausanne, Switzerland; each person has given prior written consent.
S10, the gold standard in plastination
Plastination of specimens was done by the standard S10 method described by von Hagens (von Hagens, 1985, 1986; DeJong & Henry, 2007). In short, the steps taken and the time schedule were as follows: dehydration was carried out at room temperature, with incubations in increasing concentrations of alcohol from 50 to 100%, changing every 3rd day, and finally three incubations in 100% ethanol. Specimens were placed for 24 h in acetone at 4 °C, and then at 3-day intervals in acetone baths at −20 °C. Specimens were placed for 24 h at −20 °C in S10 silicone containing a 1% S3 catalyst and chain extender, but without applying a vacuum. The next day, impregnation was started by reducing the air pressure gradually over a period of 4–5 days. After a week, a minimal air pressure of 5 mmHg was obtained, and vacuum-forced impregnation was stopped. Excess silicone was drained first at −20 °C and then at room temperature, and also wiped off with gauze and paper towels before and during the early phase of curing with S6 cross-linker.
P40, polymer impregnation of thin slices (3–6 mm) with UVA curing
The P40 polyester technique has been described previously for the production of thin body slices. Thin slices of 4–5 mm thickness are dehydrated and degreased with alcohol and acetone according the same principles as used for the S10 silicone method (Latorre & Henry, 2007). Slices are immersed in P40 without additives and placed in the vacuum chamber. Over the first 24 h, the vacuum pressure is only slightly lowered to vaporize acetone. The next day, vacuum pressure is lowered to 1 mmHg by the end of the day. Slices should be covered by resin all the time. For sheet plastination, slices are placed into casting chamber, between two glass plates, surrounded by a silicone gasket and fixed with fold-back clamps. P40 polyester was used with 1% A4 activator. As air interferes with light curing, the curing mixture should be degassed before pouring it into the curing chamber, bubbles removed, the mixture closed with a gasket seal and cured by exposure to UVA light for 4 h, while cooling with a fan.
Prussian blue impregnation of brain tissue
Prussian Blue (also called Berlin blue) coloration of brain tissue is a valuable staining procedure for macroscopic brain slices and neuroanatomical studies (Barnett et al. 1980). The selective gray matter staining with ferric ferrocyanide was described by Thompsett (1955). In short: fixed brain slices are first soaked in water, incubated for 5 min in a solution A (100 g crystalline phenol, 10 g copper sulfate, 2.5 mL conc. HCl in 2 L of distilled water) at 60–65 °C. Note that when the solution is too hot, white matter is also stained. Subsequently, sections were placed in ice-cold tap water for 20–30 s, followed by an incubation in 1% (w/v) ferric chloride (2 s up to 1 min at room temperature). The slices are placed for 2 min under running tap water, then for 10 s in 0.5% (w/v) potassium ferrocyanide. The slices are then placed for 24–48 h under running tap water and stored in 10% formol/0.2% citric acid or prepared for silicone S10 plastination. Note that plastinated slices break easily.
Results
Several specimens are presented that have been plastinated in the last 15 years and have been introduced in the teaching of human anatomy. These plastinates have not lost their appeal and beauty despite being heavily used in practical teaching. A comparison between the two methods, S10 and P40, clearly shows that the S10 method is ideal for dissected specimens, independent of their size, whereas P40 slices are more difficult to handle, as they are larger, but they become transparent and even show microscopic structures.
In Fig. 1, the medial aspect of a plastinated left elbow is shown (Fig. 1A), with the upper and lower surfaces, respectively (Fig. 1B,C). This slice provides an ideal training for recognizing the trajectory of blood vessels and muscles around the elbow joint (e.g. try to identify the bony structures of humerus, radius and ulna; where are the radial, median and ulnar nerves located?; observe their relationship to different blood vessels and muscles).
Figure 1.
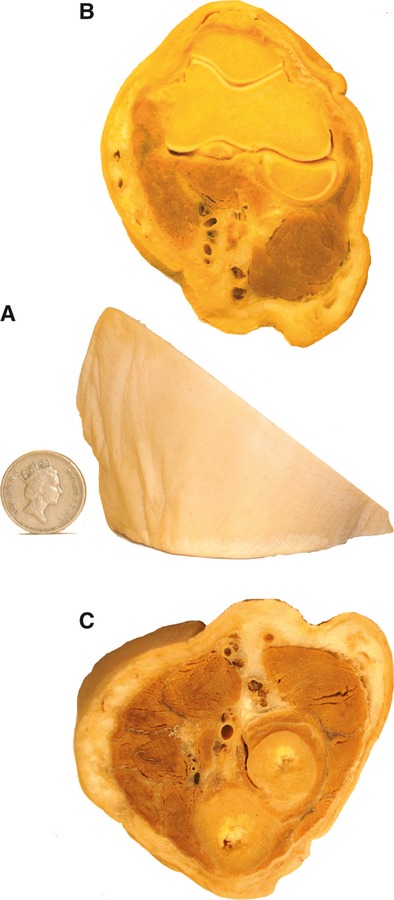
S10 plastinated left elbow with medial view (A), and superior (B) and inferior surfaces (C).
In Fig. 2, the elbow in the form of 6-mm-thick P40 polyester slices demonstrates the condylar aspect of the humero-radial joint (Fig. 2A) and the hinge joint between humerus and ulna (Fig. 2B). In particular, the olecranon, coronar processus of the ulna and the typical form of the radial head are seen and soft tissue is more transparent than with the S10 method.
Figure 2.
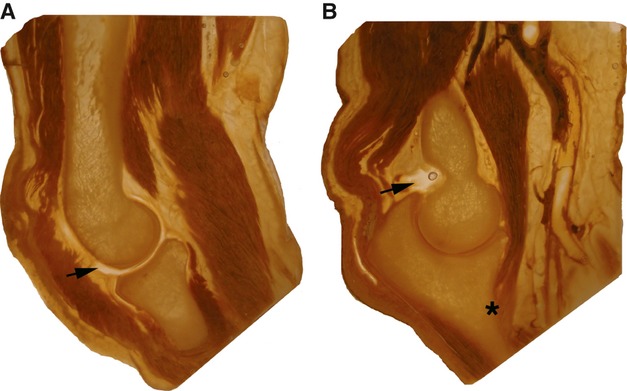
P40 slices of an elbow with humero-radial joint (A) and humero-ulnar joint (B). The arrows point to the capsular space. The asterisk indicates the insertion point on the ulna of the brachialis muscle.
In Fig. 3, two types of plastination, S10 and P40, are compared. Horizontal sections through the arm and forearm at corresponding levels present quite distinctive aspects in the representation of muscle tissue, blood vessels, and nerves. The S10 method demonstrates distinct features of blood vessels with thicker arteries, finer veins and nerves, often with many nerve bundles (Fig. 3A,C). Identification of nerve elements in the P40 slices is very difficult because soft tissue becomes transparent (Fig. 3B,D). Collagen fibers of tendons also become transparent, whereas muscle fibers or blood vessels with coagulated blood appear very dark, probably due to the myoglobin or hemoglobin present in tissue.
Figure 3.
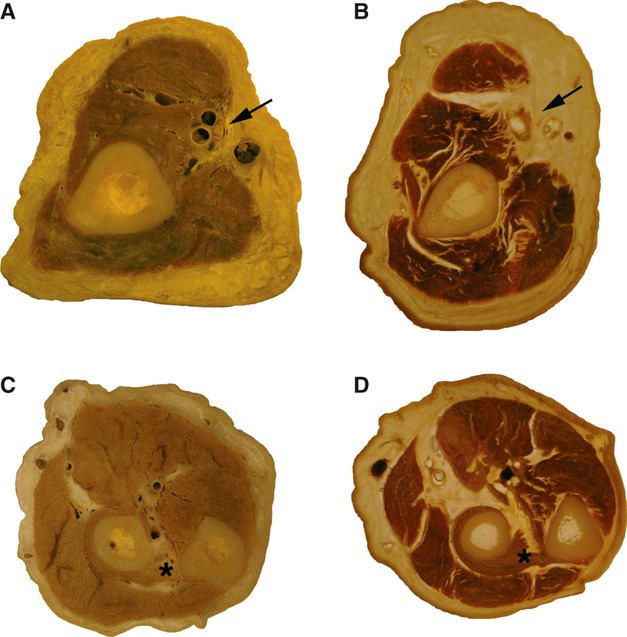
Slices of arm (A,B) and forearm (C,D) were prepared by the S10 (A,C) and P40 methods (B,D). Subcutaneous, tendinous tissue and nerves become quite transparent with the polyester method. The arrows point to brachial vessels and the median nerve. The asterisk indicates the supinator muscle surrounding the radius.
In Fig. 4, various aspects of the anatomy of the hand are shown. The S10 is ideal for a long-term preservation of delicate structures, which become quite resistant when plastinated, and the complex anatomy of innervations is seen (Fig. 4A). The P40 slice (Fig. 4B) demonstrates the different muscles, but soft tissue is transparent. The carpal tunnel S10 plastinate shows the different tendons, nerves and arteries (Fig. 4C). A P40 slice between phalanges shows the tiny joint space (Fig. 4D). A digit with its passage of tendons and short muscles allows the insertion of the different elements to be verified (Fig. 4E).
Figure 4.
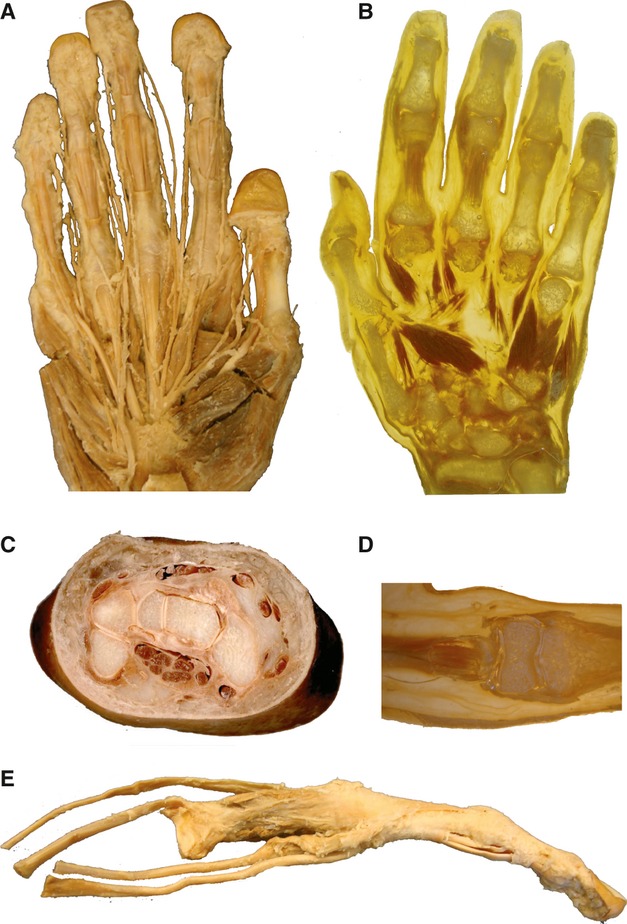
S10 plastinated hand (A), a slice through the carpal tunnel (C) and prepared digit (E). P40 slice plastination (B,D) provides complementary specimens of the hand to demonstrate innervation and insertion points of muscles, as well as the arrangement of carpal joints and the carpal tunnel. (D) Tendon sheaths and phalangeal joint are highlighted. (E) The third digit with entrance of extensor and flexor muscles.
In Fig. 5, various S10 plastinates of the knee joint illustrate that this joint is indeed the most complex joint of all. A frontal view (with patella removed) allows the transverse ligament identification of the two menisci (Fig. 5A). Figure 5B shows the posterior view with ramifications of semi-membranous tendon on the posterior capsule wall, forming the popliteus ligament. In the slice through the joint, the femur is still attached via collateral ligaments to the tibia/fibula; it can be tilted to show the tibial plateau with the intra-capsular trajectory of the popliteus tendon (Fig. 5C,D). The femur and tibial head that form the joint were kept in a jar before plastination (Fig. 5E,F). About 15 years ago, they were plastinated and since then have been used every year in practical and ex cathedra teaching.
Figure 5.
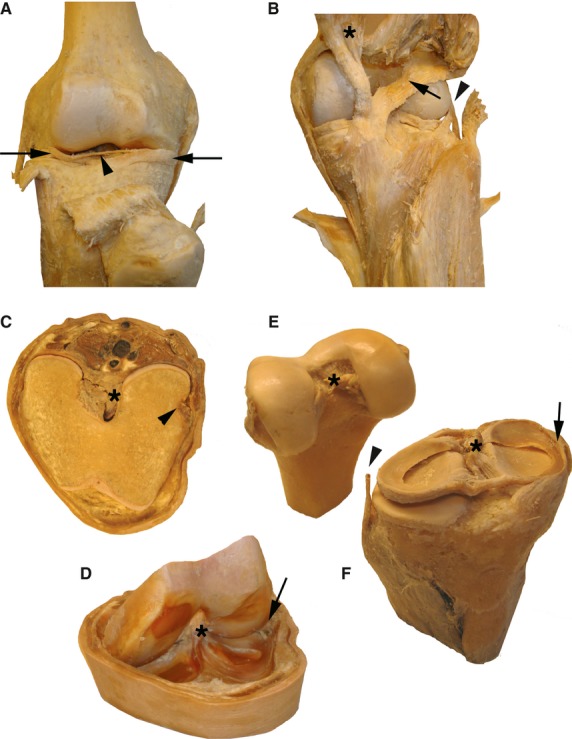
S10 plastinates of different preparations of the knee joint. (A,B) The anterior and posterior views, with lateral and medial menisci (arrows) and transverse ligament (arrowhead) indicated in (A). (B) Asterisk indicates the semi-membranous muscle, the arrow points to the popliteal ligament, and the arrowhead shows the passage of the popliteus tendon under the fibular collateral ligament. A slice through the knee joint is shown in (C,D), with the flat top surface (C) or in a lifted position of the femur (D) to view the cavity. The arrowhead indicates the insertion of popliteus muscle, and its intra-capsular passage (arrow). In both panels the anterior cruciate ligament is labeled with an asterisk. (E,F) Specimens are from the same joint, with cruciate ligaments labeled with an asterisk. The arrowhead points to the fibular collateral ligament separated from the lateral meniscus and the arrow indicates the tibial collateral ligament fused together with joint capsule and medial meniscus.
Figure 6 illustrates that gray and white matter of brain tissue are sometimes almost indistinguishable (Fig. 6A). These tissues cannot be distinguished by the S10 method alone. A variety of staining or impregnation procedures are therefore needed. Here, a combination of the Prussian blue or Berlin blue impregnation and S10 plastination for long-term preservation is ideal to document neuroanatomical features of thalamus, internal capsule and striatum, showing minute differences in blue staining (Fig. 6B,C). Observe the fine line surrounding the thalamus, representing the thalamic reticular nucleus. The P40 method is equally well suited for representing the fine structures of the human brain, as documented in a frontal section through the brain (Fig. 6D,E). White matter becomes nearly transparent, gray matter shows different levels of brownish color, and blood vessels are often dark due to the presence of hemoglobin and may indicate particularities in vascularization. The separations in the striatum, with putamen, medial and lateral pallidum, the subthalamic nucleus and many other structures such as mammilothalamic tract, internal capsule, anterior commissure and chiasm are distinctly seen.
Figure 6.
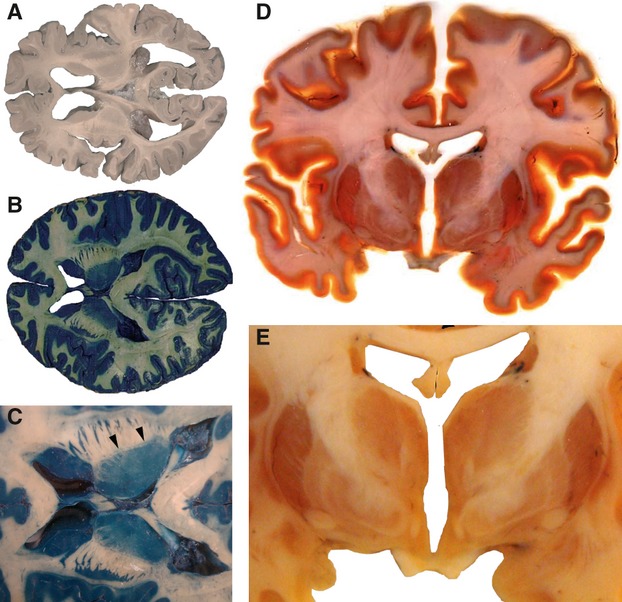
Horizontal brain slices plastinated with S10 (A-C) and a coronal brain slice preserved by P40 method (D,E) are very useful to preserve brain slices and to point out neuroanatomical structures. The Prussian blue impregnation (B, with higher magnified internal capsule panel C) with arrowheads pointing to the reticular thalamic nucleus. The P40 slice method indicates anatomical differences in the gray matter of thalamus and striatum.
Understanding pelvic floor anatomy is quite difficult due to its complexity and its three-dimensional arrangement. In Fig. 7, a well dissected plastinate may help to understand the different diaphragms and the arrangement of inner and outer sexual organs (Fig. 7A,B). This specimen is also a good example of a well prepared specimen that was kept in a pot for many years before plastination. Having specimens in a pot is not very practical and they are difficult to handle in practical courses. Since the specimen was plastinated, students have been able to study this region easily by ‘hands-on’ anatomy. In a sagittal section through a female pelvis, the arrangement of the bladder and urethra, the uterus and vagina, and the rectum and anal canal are seen (Fig. 7C).
Figure 7.
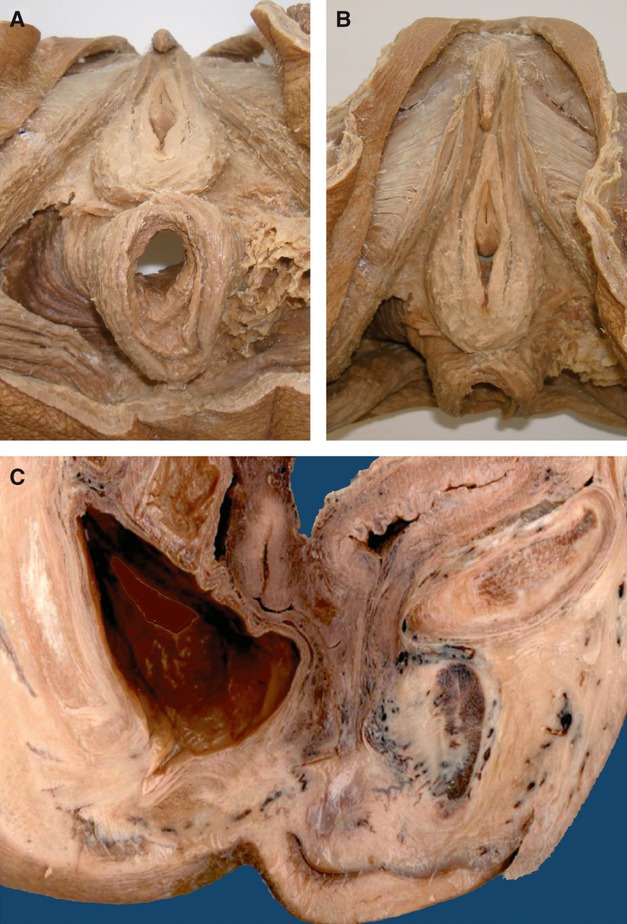
The S10 method is very useful for exquisite anatomical preparations. The female pelvic floor preparation was kept for a year in a pot before it was plastinated. (A) shows the pelvic diaphragm with anterior and posterior triangle. The same specimen was slightly tilted to show the uro-genital diaphragm. A sagittal section through a female pelvic floor shows the different organs (C).
Discussion
Ethical considerations and when to use plastination
Plastination allows a long-term preservation of tissue – sometimes for a very long period of time. All donors need to provide written consent for body donation and the use of their body for medical science. Although a donation agreement form indicates that some samples may be preserved for a long time and stipulates the right to use plastination as a means of preservation, this is not specifically mentioned in the form. At our institution we have established the following guideline; body parts may be used for preparation for demonstrations, for exams or for plastination only if families do not want to keep the remains of cremated bodies. Ethical and legal guidelines dictate how bodies are allowed to be used in medical teaching and research, and also prevent misconduct or abuse, such as commercialization of body parts or neglecting ethical aspects or disrespect of human remains. However, the legal conditions are far from satisfactory and do not prevent the export or import of body parts across countries, or ethically unacceptable exhibitions of plastinated cadavers. It is therefore even more important that the written consent form includes how a human body shall or shall not be used. One needs to realize that once tissue is plastinated, it is no longer offensive and can be handled without gloves. Thus, plastinates can be placed in any context, not necessarily related to anatomical teaching. It is therefore essential to have specific ethical and legal guidelines to define what is acceptable or prohibited (McHanwell et al. 2008; Riederer et al. 2012). A guide for good practice in body donation is currently in preparation.
Some advantages of different plastination techniques
The use of plastination has some advantages but also some disadvantages. Plastination provides an additional tool to complement regular dissection courses. We will not go into detail about the use of human cadavers for gross anatomy teaching. In Switzerland, all medical curricula comprise dissection courses in pre-and postgraduate anatomy teaching. A comparison of the S10 and P40 techniques clearly shows that plastination is a complementary teaching technique to demonstrate the different aspects of the human anatomy, the type of plastination being governed by the structure to be demonstrated. The S10 method is ideal for well dissected specimens and larger body slices. Fine structures become more resistant to damage but also become more rigid. This may influence exploratory anatomy using endoscopic procedures (Musumeci et al. 2003a,b2003b). The P40 method is excellent for fine slices because they become transparent; however, nervous structures are more difficult to identify. It should be borne in mind that badly prepared specimens do not become better by plastination. On the contrary, every time you see the specimen you are reminded that you should have spent more time and care on preparation.
Plastinated brain tissue is an essential tool for teaching neuroanatomy (Weiglein, 1997). During plastination of brain tissue, the tissue shrinks (loss of about 20% of its mass) and becomes more rigid. Therefore, hidden structures need to be specially prepared (e.g. demonstrating the gyri of Heschl is difficult). Visualization of gray and white matter can be enhanced by using staining or impregnation methods, such as the Prussian blue method (Thompsett, 1955), in combination with plastination (Suriyaprapadilok & Withyachumnarnkul, 1997). However, such brain slices become more rigid and break easily when handled roughly. Nonetheless, neuroanatomical structures are better highlighted, as shown for the thalamus.
Plastination as valorization of anatomical pot specimens in practical teaching
Plastination is also an essential method for a long-term preservation of nicely prepared specimens that have been kept for years/decades/centuries in jars and pots – and as such, are rather difficult to handle in practical anatomy teaching courses. Plastination is also the method of choice for preserving unique or pathological specimens. It should be borne in mind that the preservation liquid has to be removed first and specimens rinsed with 50% ethanol and then dehydrated by the usual protocol of the S10 method. Once plastinated, such specimens allow direct contact and three-dimensional vision of sometimes quite complex anatomical regions, such as the pelvic floor with the pelvic diaphragm, and its external and internal organs. Plastinates are useful in pre-and postgraduate teaching as well as continued training of surgeons to demonstrate critical points in innervation of the pelvic floor, compression points, nerve entrapments or how to avoid post-operative pain (Spinosa & Riederer, 2007; Riederer & Spinosa, 2011).
Conclusions
Plastination provides an ideal tool for long-term preservation of well dissected specimens and body slices, but there are some ethical and legal considerations involved. Different methods allow the different areas of the human body to be demonstrated and also highlight variations in human anatomy. The hands-on aspect is very important for the learning process, especially for complex structures where an understanding of the three-dimensional organization is required and plastinated specimens are highly appreciated by students and faculty (Roda-Murillo et al. 2006). Plastination is not a replacement for traditional guided dissection, but it does provide an additional learning tool to understand complex human anatomy.
Acknowledgments
This work was supported by the institution. We thank also all those who donated their bodies.
References
- Barnett RI, Lyons GW, Driscoll JD, et al. Improved sectioning and Berlin blue staining of whole human brain. Stain Technol. 1980;55:235–239. doi: 10.3109/10520298009067246. [DOI] [PubMed] [Google Scholar]
- DeJong K, Henry RW. Silicone plastination of biological tissue: cold-temperature technique Biodur S10/S15 technique and products. J Int Soc Plastination. 2007;22:2–14. [Google Scholar]
- von Hagens G. Heidelberg Plastination Folder. Heidelberg: Anatomisches Institut I, Universität Heidelberg; 1985. [Google Scholar]
- Latorre R, Henry RW. Polyester plastination of biological tissue: P40 technique for body slices. J Int Soc Plastination. 2007;22:69–77. [Google Scholar]
- McHanwell S, Brenner E, Chirculescu ARM, et al. The legal and ethical framework governing Body Donation in Europe – a review of current practice and recommendations for good practice. Eur J Anat. 2008;12:1–24. [Google Scholar]
- Musumeci E, Lang FJW, Duvoisin B, et al. Plastinated ethmoidal region: I. Preparation and applications in clinical teaching. J Int Soc Plastination. 2003a;18:23–28. [Google Scholar]
- Musumeci E, Lang FJW, Duvoisin B, et al. Plastinated ethmoidal region: II. The preparation and use of radio-opaque artery casts in clinical teaching. J Int Soc Plastination. 2003b;18:29–33. [Google Scholar]
- Riederer BM, Spinosa J-P. Teaching clinical anatomy of the female pelvic floor to undergraduate students: a critical review of neuralgic points. Anatomy. 2011;5:1–6. [Google Scholar]
- Riederer BM, Bolt S, Brenner E, et al. The legal and ethical framework governing body donation in Europe – 1st update on current practice. Eur J Anat. 2012;16:1–21. doi: 10.1016/j.aanat.2023.152195. [DOI] [PubMed] [Google Scholar]
- Roda-Murillo O, Lopez-Soler M, Roda-Murillo A, et al. Plastination in the teaching of neuroanatomy. Eur J Anat. 2006;10:85–89. [Google Scholar]
- SAMS, Swiss Academy of Medical Science. 2008. Verwendung von Leichen und Leichenteilen in der medizinischen Forschung sowie Aus-, Weiter-und Fortbildung http://www.samw.ch/de/Ethik/Richtlinien/Aktuell-gueltige-Richtlinien.html.
- SAMS, Swiss Academy of Medical Science. 2010. Research with Human Subjects; A manual for general practice http://www.samw.ch/en/News/News.html.
- Spinosa J-P, Riederer BM. De l'importance de l'anatomie. L'agenda Gynecol. 2007;49:6–8. [Google Scholar]
- Suriyaprapadilok L, Withyachumnarnkul B. Plastination of stained sections of the human brain: comparison between different staining methods. J Int Soc Plastination. 1997;12:27–32. [Google Scholar]
- Thompsett DH. Differential staining and mounting of human brain slices. Med Biol Illustr. 1955;5:29–34. [PubMed] [Google Scholar]
- Weiglein AH. Plastination in the Neurosciences. Acta Anat. 1997;158:6–9. doi: 10.1159/000147902. [DOI] [PubMed] [Google Scholar]


