Abstract
This review deals with the art of (anatomical) embalming. The first part contains a brief historical review of the history of embalming, starting with ancient cultures such as the Egyptians and the lesser known Chinchorro culture, then going down the centuries and describing the anatomical techniques developed over the last two centuries. The second part deals in detail with the chemicals used for embalming purposes. The third part deals with several approaches to evaluating embalming methods, their suitability for biomechanical testing, antimicrobial properties, histological appearance, and usability. The fourth and final part analyze the European Biocidal Products Directive (98/8/EC) in the light of embalming.
Keywords: anatomy/education, anatomy/history, anatomy/legislation and jurisprudence, anatomy/methods, anatomy/supply and distribution, education, embalming/education, embalming/history, embalming/legislation and jurisprudence, embalming/methods, embalming/standards, embalming/supply and distribution, medical/supply and distribution
Introduction
Within the framework of (undergraduate) medical education, anatomists use human bodies to teach students, either by demonstrating prosected specimens or by dissection done by the students themselves. The bodies are therefore used as educational tools. A comparison of educational tools (Brenner et al. 2003) revealed that human bodies have distinct properties and that there are no viable alternatives. The human cadaver has to be classified as a distinct educational tool as it is neither the student's ‘first patient’ nor a mere biological model. It is a non-vital, morbid and mortal, variable, and three-dimensional individual with a low health hazard and high quality of haptic experience, restricted availability and relatively moderate costs per student. It cannot be harmed by the student and its use is ethically sound.
In recent years, several concerns have arisen concerning this usage. The arguments against dissection include ethical and financial issues, fears of health hazards, and awareness of people's sensitivities and religious beliefs (Aziz et al. 2002). Dissection is seen as old-fashioned and outdated in the light of ‘virtualization’. On the other hand, there are also an increasing number of clinicians, most of them surgeons, arguing for re-enhancing anatomical education by dissection (Bergman et al. 2011).
One of the most important prerequisites for the use of human bodies in educational settings is the appropriate preservation of the cadaver. Preservation is considered appropriate when the cadaver is kept safe from harm, destruction or decomposition. This is achieved by treating the cadaver with special chemicals, i.e. embalming. One of the most important chemicals used for this purpose is formaldehyde.
Nowadays there is increasing opposition to this and other chemicals. There is also the threat that formaldehyde may be ruled out for embalming purposes by the Biocidal Products Directive 98/8/EC (European Parliament & Council, 1998).
The aim of this review is therefore to give a short overview of the history of embalming, summarize anatomical embalming procedures, identify and briefly describe the most important chemicals and finally clarify the relevant passages from the Biocidal Products Directive.
Definitions
When writing about human body preservation, the terminology has to be clarified. Merriam-Webster's dictionary (http://www.merriam-webster.com/) defines preservation as an action to keep something ‘safe from harm, destruction or decomposition’, conservation is defined as the process of ‘a careful preservation and protection of something’, and finally embalmment is defined as the ‘treatment (of a dead body) – with special chemicals – so as to protect from decay’. These definitions show that while the terms ‘preservation’ and ‘conservation’ may be interchangeable, different languages favour them differently. Whereas German-speaking countries rely more often on the term ‘conservation’ of a human body, in English the term ‘preservation’ is preferred. Nevertheless, ‘conservation’ and ‘preservation’ do cover more than the mere process of embalming, the use of chemicals on a body. One has also to consider appropriate storage, protection during use, and final disposal.
Means of preservation
Natural means of preservation
Natural means of preservation include freezing, desiccation/exsiccation either by dry cold or by dry heat, and the nature of the soil.
Artificial means of preservation
Artificial means of preservation comprise the application of simple heat or cold, powders, such as a sawdust bed mixed with zinc sulphate, evisceration combined with immersion, drying, local incision and immersion, arterial injections, cavity injections. Furthermore, simple immersion in alcohol, brine, etc., and sole arterial injection, which can be combined with cavity treatment and/or immersion, were used.
Periods of embalming
Ancient cultures
When summarizing the long history of embalming, one has to identify the main purposes for which cadavers were embalmed. One of the first and overall a very important motive was religious beliefs. In several ancient cultures, not only the Egyptian culture, eternal life was associated with a preserved body; those whose body decayed would be excluded from the afterlife. This was supported by the fact that bodies did not decompose when buried under certain circumstances in which natural preservation took place. These natural means of preservation comprise freezing, desiccation or exsiccation, either by dry heat or dry cold, or the specific nature of the soil at the burial site (Johnson et al. 2012). Coastal hunter-gatherers in the Atacama Desert of northern Chile and southern Peru, known as the Chinchorro culture, were among the first to perform artificial mummifications (Marquet et al. 2012). Under a scenario of increasing population size and extreme aridity (with little or no decomposition of corpses), dead individuals may have become a significant part of the landscape, creating the conditions for the manipulation of the dead that led to the emergence of complex mortuary practices as early as 5000–6000 BC (Marquet et al. 2012). Based on the empirical knowledge, the techniques of preservation were enhanced; in Egypt starting as early as in the first dynasty c. 3200 BC. Specialized persons were in charge of these activities; these were – or became therefore – members of the priest caste. Two major developments characterized the transition from the utilization of mere natural means of preservation to sophisticated embalming procedures performed by these priests: first of all the use of additional means such as natron, herbs, cedar oils, natural, tree-derived resins, incense and gums, pitch, and tar, and secondly the introduction of the exenteration or evisceration. This exenteration characterized the preservation of human remains for the next millennia. There are hints that also cadavers buried at the Royal Cemetery of Ur in the late Early Dynastic phase (c. 2500 BC) were preserved by means of heat and mercury (Baadsgaard et al. 2011).
Another method described was immersion in honey, which mainly descended from the Persians, with Alexander the Great being the most prominent cadaver treated in this way. The embalmment of Alexander reveals an additional purpose for body preservation: the necessity for a long-distance and long-term transportation, in Alexander's case, the transfer from Babylon to Alexandria. This technique was re-evaluated in 2004 (Sharquie & Najim, 2004). Whether the Ptolemaic scientists and ‘anatomists’ (first half of the third century BC), Herophilus of Chalcedon and Erasistratus of Ceos, used embalming techniques for their dissected cadavers is not known (Longrigg, 1988).
More or less sophisticated techniques of embalming are known from ancient Ethiopians, the Guanches of the Canary Islands, Peruvians, the Jivaro Indians of the Marano River in Ecuador, the Indians of Central America – Aztecs, Toltecs, and Mayans – and North America, and the inhabitants of the Aleutian Islands and the Kodiak Archipelago (Mayer, 2012), and also Tibetans and Nigerian tribes (Ezugworie et al. 2009). Ancient people of Ogoni, Nigeria, predominantly used large quantities of alcohol concentrate, potash, herbal leaf (Ocimum gratissimum, African basil) and kernel oil (Udoaka et al. 2009).
The hitherto oldest known form of artificial preservation in Europe has been found in the dolmenic burial ‘La Velilla’ in Osorno (Palencia, Spain; Martin-Gil et al. 1995). There, 5000-year-old human bones have been found, which were carefully covered by pulverized cinnabar (vermillion), which ensured their preservation. The authors believe that the vermillion was deliberately deposited for preservative purposes as no cinnabar mine is to be found within a range of 160 km and large amounts (hundreds of kilograms) were used, and as its composition, red mercuric sulphide, is similar to that of preparations used in technical embalming. Nevertheless, embalming remained unusual in Europe, with some reported exceptions during the time of the Roman Empire. The presence of chemical components, such as sesquiterpenes, triterpenoids, and diterpenoids, originating from coniferous and pistacia resins, myrrh, and other spices, found in a partially mummified body dating to AD 300 found in Northern Greece, confirm ancient information on preservation methods of the deceased in Greek and Roman times (Papageorgopoulou et al. 2009).
In China deceased people were obviously embalmed (Brown, 2002), with the main example of Xin Zhui, the Lady of Dai of the Western Han Dynasty, who died between 187 and 145 BC (Chunhong, 2004). Her corpse was found in 1971, when workers were digging an air raid shelter near the city of Changsha. Her remains were extraordinarily well preserved, to pave her way to immortality, but the methods of embalming, and especially the liquid in which Xin Zhui was immersed, are still unknown. To intensify the mystery, two other tombs containing bodies in a similar state of preservation have been found within a few hundred miles of Xin Zhui. One was a magistrate by the name of Sui and the other was Ling Huiping, the wife of a powerful Han Dynasty lord.
Several other well-preserved mummies such as the Iceman from the Similaun glacier (Seidler et al. 1992) or the bog bodies (Glob, 2004; Anonymous, 2012 (embalming essay)) cannot be accounted for as an intended preservation.
Period of anatomists
From those ancient cultures, embalming spread to Europe, where, in time, it became a widespread practice. Descriptions of methods used in Europe for almost 1200 years, starting at about AD 500, have been preserved in the writings of contemporary physicians, such as Peter Forestus (1522–1597) and Ambroise Paré (1510–1590; Table 1). Forestus described his procedure as follows: eviscerate the body, wash with cold water and aqua vita, fill cavities with consecutive layers of Aqua vita moistened cotton, and powder (Table 2), sew the corpse, and finally wrap the corpse in waxed cloth and other things.
Table 1.
Paré's components.
| Washing solution | Aromatic powder |
|---|---|
| Aqua vita | Radix pul rosar, Chamomile, Balsami, Methe, Anethi, Salvia, Lavendula, Rorismar, Marjoran, Thymi, Absinthi, Cyperi, Calami aromat, Gentiana, Irosflorent, Accavederata, Caryophyll, Nucis moschat, Cinamoni, Styracis calamita, Benjoini, Myrrha, Aloes, Santel |
| Strong vinegar, boiled with | |
| Wormewood (Artemisia absinthium) | |
| Aloes | |
| Coloquintida | |
| Common salt | |
| Alum |
Table 2.
Embalming powder used by Peter Forestius.
| Prepare a powder from |
| 2½ lbs aloes |
| 1½ lbs myrrh |
| 7 handsfull of ordinary wermut |
| 4 handsfull of rosemary |
| 1½ lbs pumice |
| 4 lbs majoran |
| 2 lot storacis calamata (≈ 1/16 lbs, 30 g) |
| ½ lot zeltlinalipta muscate |
Embalming during the Middle Ages included evisceration, immersion of the body in alcohol, insertion of preservative herbs into incisions previously made in the fleshy parts of the body, and wrapping the body in tarred or waxed sheets. Later on, in the renaissance period, embalming became influenced by scientific developments in medicine (Ezugworie et al. 2009). Bodies were needed for dissection purposes and preservation required more refined embalming techniques. Among these new techniques, there was the injection into hollow structures of the body, but normally not into the vascular system. Nevertheless, several attempts to inject the vascular system have been passed down; for example, Alessandro Giliani of Persiceto, who died in 1326, used an arterial injection of coloured solutions that later hardened (da Vinci & O'Malley, 1983). Leonardo da Vinci (1452–1519) described a method of preserving the cadavers that he studied. His embalming fluids were mixtures made from turpentine, camphor, oil of lavender, vermilion, wine, rosin, sodium nitrate, and potassium nitrate (McKone, 1999). Da Vinci also used an injection of wax to the ventricles, Jacobus Berengar (1470–1550) injected warm water into veins, Bartholomeo Eustachius (1520–1574) is said to have used injections of warm ink, Reinier de Graaf (1641–1673) injected different liquids and added mercury (de Graaf, 1668), and Jan Swammerdam (1637–1680) injected a wax-like material that later hardened (Mayer, 2012).
Another famous scientist known to embalm by injecting a prepared preservative chemical solution, liquor balsamicum, into the blood vessels was Frederik Ruysch (1638–1731), but his technique was unknown for a long time (Mayer, 2012). In 1717, Ruysch sold his ‘repository of curiosities’ to Peter the Great for 30 000 guilders, including the secret of the liquor, which, according to a recently published book, contained clotted pig's blood, Berlin blue and mercury oxide (Driessen-Van het Reve, 2006). After a first visit to Ruysch, Peter the Great wrote: ‘I saw boys and girls 4 years old, visibly well vascularized, with open eyes and soft little bodies, and they were not even in alcohol.’ (Driessen-Van het Reve, 2006). Another Dutch scientist, Stephen Blanchard (1650–1720), published his embalming method in 1688 (Mayer, 2012).
With the progress made in embalming by arterial injection, research for new preserving fluids opened up another possible way to extend this scientific field of expertise by means of chemistry (Trompette & Lemonnier, 2009). During the 19th century, British, French and Italian scientists perfected such techniques, thereby enabling them to reach every part of the cadaver. Among those British scientists were William Hunter (1718–1783), John Hunter (1728–1793) and Matthew Baillie (1761–1823), who all used an arterial injection of several oils, mainly oil of turpentine, to which they added Venice turpentine, oil of chamomile, and oil of lavender (Table 3). Vermillion was intentionally used a dye, but would have added additional preservative potential to the final solution (Mayer, 2012).
Table 3.
Solutions and powder used by W. Hunter.
| Arterial injection solution | Immersion solution |
|---|---|
| Oil of turpentine | Camphorated spirits of wine |
| Added Venice turpentine | |
| Oil of chamomile | |
| Oil of lavender | |
| Portion of vermilion dye | |
| Powder | Washing solution |
| Camphor | ‘Essential’ oils of rosemary and lavender |
| Resin | |
| Niter |
In France, several different approaches were developed and used. Cuvier (1769–1832) used pure alcohol, Chaussier (1746–1823) immersed eviscerated bodies in a solution of dichloride of mercury, Thenard (1777–1857) injected an alcoholic solution of dichloride of mercury, and Sucquet (1840–1870) used a 20% zinc chloride solution. Jean Nicolas Gannal (1721–1783) started his career as an apothecary's assistant and became the first to offer embalming to the general French public (Mayer, 2012). His research was not restricted to scientific and medical activities but also covered funeral embalming, using simplified methods that did not involve lacerating the corpse (Trompette & Lemonnier, 2009). In fact, he was the first embalmer to perform documented scientific studies in the field of embalming, which he published – almost completely – himself (Table 4; Gannal, 1840). The final formula was patented and secured, but his successful embalming fluid contained a solution of acetate of alumina (Mayer, 2012).
Table 4.
Gannal's experimental arsenal.
| Acids: acetic – arsenous – nitric – hydrochloric |
| Alkali salts of copper – mercury – alum |
| Tannin-creosote-alcohol |
| Various combinations: alum, sodium chloride, nitrate of potash, acetate of alumina, chloride of alumina |
In Italy, Guiseppe Tranchina (1797–1837) was a famous anatomist who openly advocated and successfully used arsenic solutions for arterial injection (Mayer, 2012). History has it that his technique was the very first documented method that did not involve evisceration. One of his successors, not as anatomist but as embalmer, was Alfredo Salafia (1869–1933; Piombino-Mascali, 2009). He embalmed several important persons, but his most prominent body was Rosalia Lombardo, an Italian child born in 1918 in Palermo, Sicily. She died of pneumonia on 6 December 1920. She was embalmed and her glass-covered coffin was admitted to the Capuchin catacombs of Palermo in Sicily. For a long time, it was suggested that his fluid might contain arsenic. The recent discovery of a hand-written manuscript by Salafia himself revealed that his solution was one of the very first formulas that included formaldehyde (Table 5; Salafia, c. 1927; Piombino-Mascali et al. 2009).
Table 5.
Salafia's solution (Salafia, ca. 1927).
| One part of glycerine |
| One part of a solution of formalin (40%) saturated with zinc-sulphate and 10% of dry zinc-chloride |
| One part of a solution of alcohol saturated of salicylic acid |
One of the last anatomists who openly published a report of an embalming fluid containing arsenic, was Edmond Souchon. His formula A contained 1.5 gallons of water, 1 gallon of arsenious acid (saturated solution) and 8 oz of 40% formaldehyde; this solution was mixed with formula B containing 16 oz of alcohol, 8 oz of carbolic acid (liquefied crystals), 16 oz of glycerine, and 2 oz of creosote (Souchon, 1908).
Funeral period
Modern embalming for mere funeral purposes is believed to have begun in 1861 in the American Civil War, mainly due to sentimental motives. The essential purposes of this type of embalming are the preservation of the body to permit burial without unseemly haste and the prevention of the spread of infection both before and after burial. Additionally, cosmetic work is used to restore injured facial features or for aesthetic reasons. Thus a separation of the fields of embalming by funeral directors and embalming for medical purposes occurred and schools of embalming, especially in the USA, were established. Embalming methods for funeral purposes now consist essentially of the removal of all blood and gases from the body and the insertion of a disinfecting fluid; the viscera might be removed and immersed in an embalming fluid and are then replaced in the body, in which they are covered with a preservative powder.
The Civil War embalmer experimented with a wide combination of arsenic, creosote, mercury, turpentine and various forms of alcohol. Thomas Holmes, who is said to have performed about 4000 procedures, had developed a fluid ‘free of poisons’ by the outbreak of the war. Arsenic-based solutions were the first generally accepted embalming fluid. In the 19th and early 20th centuries, arsenic was frequently used as an embalming fluid, but has since been supplanted by formaldehyde (Ezugworie et al. 2009).
Modern anatomical preservation
Prior to the introduction of carbolic acid, or phenol, and later of formaldehyde, the main preserving agents used in anatomies were alcoholic solutions of arsenic and/or alumina salts in different concentrations. Most of these ‘modern anatomical embalming fluids’ are summarized in Supporting Information Table S1. Table 6 gives a comparison of different embalming techniques in terms of advantages and disadvantages, long-term storage and usability for anatomical teaching.
Table 6.
Comparative table of different techniques.
| Technique | Advantages | Disadvantages | Long-term storage | Teaching (dissection) |
|---|---|---|---|---|
| Salafia (c. 1927) | Longterm storage | Toxic | Extremely well, when the coffin is sealed | Not tested |
| Kaiserling (Pulvertaft, 1950) | Good preservation of colour and form | Only for isolated specimens | Not applicable | Not applicable |
| Jores (1896, 1913) | Easy storage | No data available | Satisfactory | Satisfactory |
| Woodburne & Lawrence (1952) | Very active as fungicidal agent; soft and plastic; cheap | Medium brown colour | No data available | Highly satisfactory |
| Peters (1956) | Good preservation of intestines; does not affect the dissector's skin; odourless; objects sty smooth and elastic; colour-preserving | No data available | Possible | Satisfactory |
| Erskine (1961) | Soft and flexible, less exsiccation | No data available | Satisfactory | Satisfactory |
| Richins et al. (1963) | Decreased rigidity; increased bactercidity and fungicidity; less browning | No data available | Successful for 2 years | No data available |
| Dayton et al. (1965) | No data available | No data available | No data available | No data available |
| Beck (1966) | No data available | No data available | No data available | No data available |
| Tutsch (1975) | Cheap; odourless | No data available | No data available | Satisfactory |
| Bradbury & Hoshino (1978) | Moderate degrees of movability […] and adequate degree of hardness […] for dissection | No adequate fixation of brains | No data available | Satisfactory |
| Platzer et al. (1978) | Increased fungicidity; cheap | No data available | Almost unlimited, when vacuum packed | No data available |
| Logan (1983) | Soft preservation; obviates excessive noxious fumes | No data available | Satisfactory | Facilitates micro-dissection |
| Frølich et al. (1984) | Soft and flexible | Slight odour, headache, drowsiness; mild eye, nose and throat irritation | Up to 10 years | ‘Suitable’ |
| Frewein et al. (1987) | Smooth, colour-preserving | Fluid accumulations | No data available | Satisfactory |
| Ikeda et al. (1988) | ‘Well fixed’ | No data available | No data available | Satisfactory |
| O'Sullivan & Mitchell (1993) | Formaldehyde vapour levels below COSHH limits; improved tissue preservation; more nature coloration | No data available | Proved up to 2.5 years | Satisfactory |
| Macdonald & MacGregor (1997) | Less toxic | Grey hue of skin and muscles | No data available | Satisfactory up to 6 month |
| Coleman & Kogan (1998) | Excellent preservative properties; minimal structural distortion; tissue supple; little desiccation; natural colours | No data available | No data available | Satisfactory |
| Thiel (1992, 2002) | High colour preservation, smooth and flexible | Expensive; Disintegration of muscular tissue; limited time for dissection | No data available | High acceptance |
| Powers (2003) | No data available | No data available | No data available | No data available |
| Silva et al. (2007) | Laskowski: flexible Modified Larssen: good coloration, odourless, in vivo-like flexibility | Laskowski: dark, loss of tissue texture, skin desquamation, odour | No data available | Laskowski: less suitable for skin or oral cavity surgeries Modified Larssen: well accepted by students |
| Barton et al. (2009) | Smooth | No data available | No data available | High acceptance |
| Mills (2010) | High mould preventiong | No data available | No data available | No data available |
| Al-Hayani et al. (2011) | No structural distortion, not colour changes | Hardening outside the tank; > 2 days for re-softening | When waxed, possible | No data available |
| Anichkov et al. (2011) | Natural appearance, odourless | No data available | Up to 1.5 years | No data available |
| Janczyk et al. (2011a) | Neutral smell | Yellowish coloration; corrosion; Disintegration of abdominal organs | Up to 1 year | Limited usability |
| Hammer et al. (2012) | Flexible tissues, aesthetic appearance; less toxic | Expensive | Up to 3 years | No data available |
| Shi et al. (2012) | Less toxic, good preservative properties, low volatility | Up to 2 years | No data available | |
| Goyri-O'Neill et al. (2013) | Good coloration and flexibility | No data available | No data available (good short term preservation ≤ 6 month) | No data available |
Phenol was introduced to anatomical embalming by Laskowski (1886) in the mid-19th century. He initially used a mixture of phenol and glycerine as vehicle (one part phenol, 20 parts glycerine); later on he replaced parts of the glycerine with alcohol (one part phenol, one part boric acid, four parts alcohol, 20 parts glycerine). A similar formulation was developed some years later independently by Rüdinger in Munich (Grönroos, 1898). Alternatively, oxyquinoline (chinosol; 0.63%) was used as single chemical for injection purposes (Schiefferdecker, 1897).
A leap forward came with the discovery of formaldehyde by the German chemist August Wilhelm von Hofmann in 1869 (Hess, 1901). It was determined to be an excellent preservative (Trillat, 1892; Blum, 1893, 1894, 1896; Gerota, 1896) and became the foundation for modern methods of embalming (Ezugworie et al. 2009). Within a few years, until 1898, eight of 45 medical schools throughout Europe introduced formaldehyde for preservation purposes (Grönroos, 1898). Even at that time, there was discussion about the final concentration, with some authors advocating concentrations as low as 3%, others demanding 10%. In addition, the immediate adverse effects were already known: skin irritation, conjunctivitis, irritations of the respiratory system, and headache. Overall, Grönroos summarizes, formaldehyde is not appropriate as a solitary preservation agent.
Up to now, several modified formulae have been published in the scientific literature.
Kaiserling's method for the preservation of the colour and form of specimens, published in 1897, is still widely used (Supporting Information, Table S2); nevertheless, this method is mainly usable for isolated (organ) specimens and is not suitable for anatomical dissection, when the complete method is used (Pulvertaft, 1950). Specimens are fixed in Solution I for up to 2 weeks, depending on their size. Larger specimens should always be injected. In this solution the colour contrasts disappear and are to some extent restored by the ethyl alcohol, wherein the specimens should remain for periods of up to 1 h, but must be carefully watched to ensure that they are removed when the optimum stage is reached; if kept for longer periods, the colour fades (again) and cannot be restored. Solution III is the mounting fluid, which is obsolete for dissection purposes.
Another well-known fixative solution was developed by Jores, containing Karlsbad salts, chloral hydrate and formaldehyde (Supporting Information, Table S3; Jores, 1896, 1913; Bradbury & Hoshino, 1978).
Woodburne & Lawrence (1952) investigated an improved embalming fluid formulation, based on their usual alcohol-glycerine-phenol-formaldehyde embalming formula. Glucarine B (Glyco Products Company Inc., Brooklyn, NY, USA), a commerical sorbitol formulation, was found to be an entirely satisfactory replacement for glycerine. Isopropanol seemed to be the logical substitute for ethanol. Woodburne and Lawrence tested eight different fluids for their germicidal activity against Mycobacterium tuberculosis, Staphylococcus aureus, Eberthella typhosa, Pseudomonas aeruginosa, Proteus vulgaris, Bacillus anthracis, Clostridia tetani and novyi, β-haemolytic Streptococcus pyogenes, and for their fungicidal activity against Penicillium notatum, Aspergillus niger, Coccidioides immitis, Histoplasma capsulatum and Cryptococcus neoformans, with excellent results for the formulation given in Supporting Information, Table S4.
Peters described modifications of the Jores' solution (Peters, 1956). These immersion fluids are generally free of formaldehyde and phenol, which are replaced by choralhydrate (Supporting Information, Table S5); nevertheless, Peters adds 2% phenol for the preservation of pancreas, stomach and intestines.
Erskine described an embalming fluid used in Dublin (Supporting Information, Table S6) which is reported to provide excellent properties of embalmed cadavers for dissection over 3 years (Erskine, 1961). Besides the common shares of ethanol, formaldehyde, glycerine, and phenol, this fluid also contains sodium arsenate, salicylic acid and 6-chlorthymol, the latter to provide appropriate fungicide properties.
Richins et al. (1963) presented an improved embalming fluid, which uses potassium pyrophosphate and magnesium chloride to decrease the rigidity associated with formalin fixation (Supporting Information, Table S7). Furthermore, they substituted phenol with sodium pentachlorophenate, which improved colour relationships and eliminated most of the unpleasant cadaver odour. Finally, sorbitol replaced glycerine as a humectant with less browning of tissues, and a wetting agent was incorporated to facilitate distribution and penetration of the fluid.
Within their study on the influence of diet upon the composition of tissues and atheromata, Dayton et al. (1965) noted an embalming fluid consisting of sodium carbonate monohydrate 16 g, sodium borate 53 g, formaldehyde (37%) 200 mL, diethylene glycol 118 mL, eosin Y 0.16 g, Aquarome Special (unknown commercial product) 5.3 mL, Igepon 1.7 mL (sodium 2-sulphonatoethyl laurate, an anionic surfactant), and water to make 1 L. This fluid was used for embalming whole cadavers prior to pathological dissection.
Beck (1966) stated that the diffusing properties of arterial embalming fluids that contain formaldehyde as a prime preservative can be vastly improved when they also contain relatively small amounts of a substantially neutralized polyacrylic acid (0.005–0.5%). Furthermore, paradichlorobenzene and/or orthodichlorobenzene (0.025–5%) in embalming fluids and solutions should provide an unusual degree of penetration and outstanding preservation.
In 1975, Tutsch (1975) published an embalming fluid formula replaced phenol with Lysoformin® (Lysoform, Berlin, Germany). According to the maufacturer's product sheet, Lysoformin® contains 6.0 g of formaldehyde and 1.8 g of glutaraldehyde per 100 g (Lysoform Dr. Hans Rosemann GmbH, Berlin, Germany); thus, this embalming fluid is completely free of aromatic substances (Supporting Information, Table S8).
In 1978, two different embalming methods were published simultaneously. Bradbury & Hoshino (1978) published their ‘improved embalming procedure for long-lasting preservation of the cadaver for anatomical study’. Prior to the effective embalmment, they treated the cadavers by injecting a blood clot disperser (a diluted commercial product), and then injected 5–6 gal (22.730–27.277 L) of embalming fluid (Supporting Information, Table S9) together with draining of the blood from the internal jugular vein. They did not apply immersion, and the cadavers are stored in a walk-in cold room at 5°C, wrapped in plastic bags.
Platzer et al. (1978) described a preservation system with arterial injection of 3% phenolic acid and 4% formalin in deionised water (110–120 mL kg−1 cadaver weight) and immersion in 2% phenolic acid in deionised water for 1–3 month (Supporting Information, Table S10). Final storage is managed by sealing the fixed cadavers in plastic foils.
In 1983, Logan (1983) described a cadaver preservation procedure which differs in several important features from methods in common use. Fresh cadaver, deep-frozen at −35 °C, thawed for 2 days, then partial flushing of the venous system was effected by infusing a normal saline blood diluent. Arterial infusion and local injection of a preservative solution followed. His solution comprised alcohol, glycerine, phenol, and low formaldehyde, but no quantities were given.
Coleman & Kogan (1998) used almost the same chemicals (they replaced alcohol by isopropyl alcohol), but added a vast amount of sodium chloride (Supporting Information, Table S11). They argued that the high salt content retained in the tissues prevented any further significant desiccation. Salts have also been used in Basel (Supporting Information, Table S12; 4% of sodium choride, and 1% of anhydrous calcium chloride; Kurz, 1977/1978), and Bergen (Supporting Information, Table S13; 5% of potassium nitrate; Frølich et al. 1984).
In Zurich, Frewein et al. (1987) experimented with modifications of the basic recipe by Kurz. Their final modification contains formaldehyde, choral hydrate, calcium chloride, and Almudor® (ISS pest Control AG, Dietikon, Switzerland; apparently discontinued), a disinfecting mixture of formaldehyde, glyoxal and glutaraldehyde (Supporting Information, Table S14; Saupe et al. 2007).
Another embalming fluid, presented in a study of arterial patterns in the hand, consisted of 95% ethyl alcohol (7.6 L), 35% formalin (1.3 L) as a fixative, diethylene glycol (2.7 L) as a preservative, liquefied phenol (1.3 L) as a mould preventative, and water (8.0 L; Ikeda et al. 1988). It seems that this embalming fluid is, or at least was at that time, the common formulation used at Kawasaki Medical School in Kurashiki City, Okayama, Japan.
Thiel (1992, 2002) presented a delicate method for ‘the preservation of the whole corpse with natural colors’. This method has, as stated by the author, the advantage of meeting high standards of preservation without releasing harmful substances into the environment. Nevertheless, his method is quite complicated and includes several problematic and expensive substances during the process of preservation itself. In addition to the basic solutions, the infusion/visceral solution, and the storage solution (Supporting Information, Table S15), Thiel suggests injecting a mixture of 40 mL tap water, 45 mL ethanol and 15 mL formaldehyde to the ventricles of the brain.
To reduce the final formaldehyde concentration, phenoxyethanol can be used to wash out excessive formaldehyde from cadavers (Owen & Steedman, 1956, 1958; Spence, 1967; Frølich et al. 1984; Wineski & English, 1989). Nevertheless, there is no report using phenoxyethanol as primary agent in arterial injection solutions, but there are two US patents by Campbell & Margrave (1995, 1998). According to Campbell and Margrave, a preferred formulation should include glutaraldehyde from about 0.5% to about 2%, an aromatic ether of ethanol (e.g. phenoxyethanol) from about 1% to about 3%, a humectant (e.g. a polyhydric alcohol, 1,2-propanediol or hexylene glycol) from about 5% to about 9%, and an alcohol (e.g. ethanol) from about 27% to about 37% (Campbell & Margrave, 1995). In addition, a buffer and/or anti-oxidant may be included to maintain the stability of the glutaraldehyde. The buffer would adjust the pH in the range of pH 7–9. In addition, a biocide such as benzalkonium chloride or other quaternary ammonium compounds may be added further to deter microbial growth.
O'Sullivan & Mitchell (1993) examined the composition of the embalming fluids from 16 medical schools in the United Kingdom and found wide variation in the proportions, but not the identity, of the constituents of the embalming fluids. All of these medical schools in fact used formaldehyde, industrial methylated spirits1 water, phenol and glycerol, with the proportion of phenol appearing to be a constant feature in all formulae, reflecting its important disinfectant quality. In advance, the authors experimented with several concentrations of the same basic substances, either buffered with 0.075 m phosphate buffer (pH 7.4) or unbuffered (O'Sullivan & Mitchell, 1993). They found that the buffered solutions were ineffective because the pH changed from that of the original buffer to the pH of the embalming fluid itself. The concentrations of their suggested ‘new’ Southampton embalming fluid are given in Supporting Information, Table S16. Formaldehyde vapour level determination in their experimental fluid composition embalming was in all instances within the limits set by the ‘Control of Substances Hazardous to Health’ (COSHH) regulations.
To adopt the embalming fluids for purposes for plastination, Pretorius increased the contents of ethanol (28 L), formaldehyde (1.2 L) and glycerine (0.8 L), and reduced the phenol content (1.2 L); this mixture is diluted with 8 L of water (Pretorius, 1996).
The stock solution used at the Robert Wood Johnson Medical School (Piscataway, NJ, USA) contains three parts propylene glycol, three parts ethanol (95%), and one part phenol (90%) (Macdonald & MacGregor, 1997). The final embalming solution is prepared by adding 810 g potassium nitrate, 567 g sodium borate, and 3.8 g sodium lauryl sulphate to 25 L of hot tap water. Finally, 12.5 L of the stock solution is added to the dissolved salts. Sodium lauryl sulphate is used as a surfactant and should enable the embalming fluid to enter all areas of the cadaver.
At McMaster's University at Ontario (Canada), a complex set of solutions is used (Supporting Information, Table S17; Powers, 2003).
In 2007, Silva et al. compared a modified Laskowski-solution with a modified Larssen solution. The modified Laskowski solution was composed of 800 mL glycerine, 200 mL ethanol, 50 g ‘phenic acid’ (phenol) and 50 mg boric acid (Rodrigues, 1998; Silva et al. 2007). Their modified Larssen solution included 100 mL of 10% formalin, 400 mL glycerine, 200 g chloral hydrate, 200 g sodium sulphate, 200 g sodium bicarbonate, 180 g sodium chloride and (in the final working solution) 9.5 L of distilled water (Guimarães Da Silva et al. 2004). The original solution formula of Larssen from the Hospital Cochim, Paris, was reported by Sampaio to be composed of 500 g sodium chloride, 900 g sodium bicarbonate, 1000 g chloral hydrate, 1100 g sodium sulphate and 500 mL of a solution of 10% formalin and 1 L distilled water (Sampaio, 1989). Sampaio used one part of this solution with five parts of distilled water. We could find no further evidence of this Larssen solution.
In the same year, Constantinescu et al. (2007) noted another formulation of well known ingredients: 1200 mL formaldehyde, 400 mL propylene-or ethylene-glycol, 1000 mL phenol, and water added to 20 L.
Barton et al. (2009) described a ‘soft-preservation fluid’ containing 2 L of phenol (80% aqueous solution), 8 L of industrial methylated spirits,1 8 L water, and 4 L glycerol, for arterial injection.
Investigating a fixation–preservation salt solution containing 23% of nitrite pickling salt, 30% ethanol and 20% Pluriol® E 400 (a mixture of polyethylene glycols), Janczyk et al. (2011a) found it suitable for the preservation of (animal) cadavers with opened abdominal cavity, but not for cadavers, which had a closed abdominal cavity. In these cadavers, the abdominal organs changed their consistency and colour dramatically.
The Anatomy Department of the University of Sydney, Australia, reported in 2010 that two distinct formulations of embalming fluids were being used (Supporting Information, Table S18; Mills, 2010). Formula (A) is routinely used for preserving cadavers destined for the dissecting room or prosected specimens. The combination of pine oil, phenol and particularly di-(2-hydroxyethoxy)-methane in formula (A) has almost completely eradicated the problem of mould growth, particularly Penicillium simplicissimum and Penicillium waksmanii. Vigilance is only required for areas of poor fixation, such as gangrenous extremities. Formula (B) is a modified Kaiserling solution and is used for embalming cadavers destined for cross-section and plastination. In both of these applications, the initial high formaldehyde concentration is removed from the finished product. The absence of alcohol makes it easier to freeze the specimen prior to sectioning. With both formulae, at least 20 L of embalming fluid is injected into each body. After injection, the cadaver is washed down with tap water and then sprayed with surface disinfectant (70% alcohol, 5% Dettol, 25% water) and placed in a cold room at 4–6 °C for 12 months prior to use. For moistening purposes, the cadavers or specimens are sprayed intermittently via soaker hoses installed on the walls and roofs of the cabinets with a preservative fluid comprising 1% di-(2-hydroxyethoxy) methane, 1% 2-phenoxyethanol, 30% methylated spirit and 65% water.
Recently, Hammer et al. (2012) described a formaldehyde-free system which comprises ethanol (0.7 L kg−1 body weight), glycerine (5%) and thymol. The ethanol–glycerine fluid is injected arterially; afterwards the bodies are immersed in ethanol (65%). A thymol-ethanol solution (thymol 30.044 g L−1; 10% ethanol in aqueous solution) as moistening solution is used for keeping the state of fixation at room temperature.
Polyhexamethyleneguanidine hydrochloride was used as embalming agent and was compared with the efficiency of formalin fixation by Anichkov et al. (2010, 2011). They used this fixation method to obtain anatomical and histological preparations from human organs and chick embryos at 12 days of development. The anatomical preparations had external appearances similar to those of freshly prepared organs; nevertheless, only organs – not whole bodies – were embalmed.
Another replacement for formaldehyde has been suggested by Shi et al. (2012). Their preservative is a blend of acid, buffer solution and cross-linking agent, Tetrakis(hydroxymethyl)phosphonium chloride, which acts as fungicide, stabilizer and fixative, respectively.
Recently, Al-Hayani et al. (2011; Bedir, 2009), suggested the use of shellac, a complex mixture of aliphatic and alicyclic hydroxyl acids and their polyesters, derived from the hardened secretion of the lac insect(s). The use of shellac had previously been proposed by Pate (1938) for preserving anatomical specimens for museum and teaching purposes. Shellac is soluble in alcohol and alkaline solutions but insoluble in water. It is widely used in the food industry, and in the pharmaceutical industry as an enteric coating material. For their purposes, the authors solved the dry shellac (80 kg) in diluted ethanol (200 L; 58%). Defrosted cadavers were immersed in this solution at a pressure of 15 kPa for 3 days. The authors found that the cadavers could be used in the open air for a long time; however, if kept out of the tank for a period of more than 1 week, they may harden due purely to the hardening of resin. Long-term storage of the cadavers was achieved by spraying the cadaver with a waxed solution. This led to hardening within 2 days. Such cadavers could be stored easily in room conditions. For dissection/examination, re-softening was done by replacing the cadaver inside the softening tank for a couple of days. The gross anatomy of tissues and organs showed neither structural distortion nor colour changes, with tissues remaining supple and easy to dissect; only the skin exhibited brownish glistening discoloration with no colour changes in the subcutaneous structures, even over an extended period.
Goyri-O'Neill et al. (2013) have recently reported an embalming fluid containing nine parts diethylene glycol and one part monoethylene glycol. They used this clear liquid, which they described as practically odourless and colourless, to inject on average 7 L arterially, using a pulsed infusion at a rate of 60–70 pulses per minute. The pressures used are not given but should have been quite high, as the whole injection was performed within 30–45 min. Whereas the authors provide appropriate information on the pre-treatment (external washing with chorhexidine soap and cooling during transport to 4–6 °C), there is no information on the conditions in which the cadavers were stored after injection of the embalming fluid. Macroscopically, this embalming fluid results in good short-term preservation quality (up to 6 months). A histological evaluation 1 month after injection revealed the best results for a striated thigh muscle; the skin was also well preserved, whereas the quality of preservation of the buccal mucosa was not as good.
There are also several proprietary mixes, such as the Dodge and Genelyn solutions, whose exact composition is not available. However, it is known that the Dodge and Genelyn solutions both contain methanol and formaldehyde and dyes but no phenol (Jaung et al. 2011). The AnubiFiX™ method was recently introduced in the Netherlands (Kleinrensink, 2011). This embalming technique is based on a new prerinsing method combined with a normal 4% formaldehyde fixation solution. In contrast to conventional embalming methods, AnubiFiX™ embalming should result in a very small decrease in flexibility and plasticity (Slieker et al. 2012).
Embalming fluids – fundamental properties
Aims of embalming – chemicals
Embalming fluids should ensure that there is no risk or fear of infection on contact with the dead body; they should ensure preservation of the body and the prevention of putrefaction changes and disturbances, and prevent contamination with insects and maggots. Attempts have also been made to produce, without mutilation, a natural colour and effect of the body (Ajmani, 1998). In the words of Edmond Souchon (1908), the aims of embalming for anatomical purposes are:
The thorough and complete preservation.
The softness of the tissues, as they are found in the unembalmed subjects.
The colour of the muscles and organs, the securing at least of a brown dark colour for the muscles.
The distension – and the colouring – of the arteries […].
Embalming fluids used in anatomical preservation, despite their chemical properties, should provide a good long-term structural preservation of organs and tissues together with a prevention of over-hardening and a retention of colour of tissues and organs (Coleman & Kogan, 1998). They should also prevent desiccation, and fungal or bacterial growth. Reduction of both potential biohazards and environmental chemical hazards is also necessary (Supporting Information, see also Table S19).
Therefore embalming fluids can be grouped as preservatives, germicides (disinfectants), modifying agents [buffers, surfactants (wetting agents), anticoagulants], dyes, (other) vehicles and, finally, perfuming agents (Mayer, 2012).
For the following paragraphs, information was derived from the PubChem Compound Database (Bolton et al. 2008); where applicable, the appropriate database-entry for a substance is referenced in the literature.
The final sentence for each substance will state whether a decision was made not to include the respective substance into Annex I or Ia of the Directive 98/8/EC of the European Parliament and of the European Council concerning the placing of biocidal products on the market (European Parliament & Council, 1998). A decision of non-inclusion in Annex I or Ia would mean that this respective substance is, or will be, phased out. Details on Directive 98/8/EC will be given in the final chapter. None of the substances has been included in Annex I or Ia.
Preservatives or fixatives
Aldehydes
Formaldehyde
The first documented embalming of a human cadaver with formaldehyde is believed to have occurred in 1899 (Fig. 1). Over 100 years later, very little is fundamentally changed in basic chemistry or technique of formaldehyde preservation of human cadavers. By around 1906–1910, formaldehyde had supplanted the dangerous and toxic concoctions of heavy metal salts that had been used previously. Formaldehyde became the chemical of choice for human cadaver embalming (Bedino, 2003).
Figure 1.
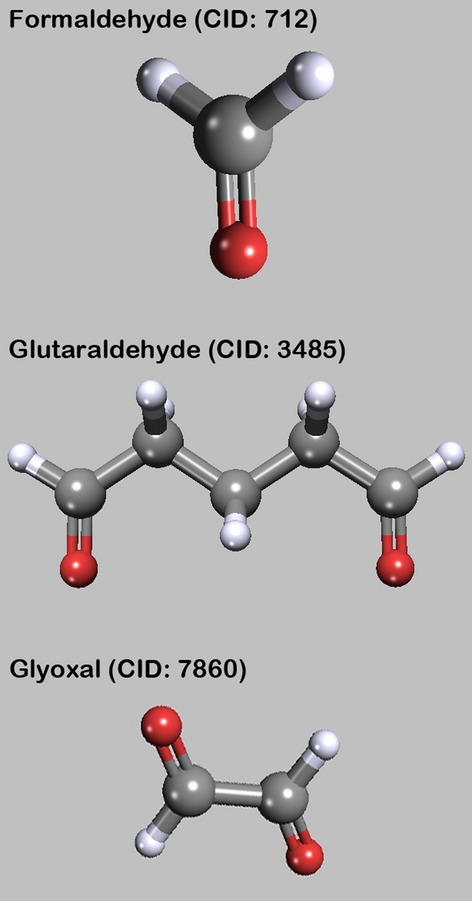
Aldehydes. The images of the molecules were created with PubChem3D Viewer (v2.0;Bolton et al. 2011), data were derived from PubChem Compound (Bolton et al. 2008).
Although formaldehyde is an excellent tissue fixative, its use is generally associated with extreme rigidity. It is possible, however, to modify this effect by adding 0.025 m sodium pyrophosphate, with or without additional 0.001 m magnesium chloride. The muscles remain pliable and the joints freely movable (Richins et al. 1963).
Formaldehyde is bactericidal, fungicidal and insecticidal (in descending efficiency). The extensive use of formaldehyde as a curing and preserving agent is based on the fact that formaldehyde has excellent antiseptic properties and thus prevents the entry of decay organisms, and it tans tissues without destroying their delicate structure (Hess, 1901). Nevertheless, although formaldehyde is an excellent tissue fixative, its use is generally associated with extreme rigidity (Richins et al. 1963). It is classified as a high level (8% formaldehyde in 70% alcohol) or intermediate-to-high level (4–8% formaldehyde in water) disinfectant (Mayer, 2012). It has a broad spectrum of action on microorganisms. It destroys putrefactive organisms when carried by a proper vehicle that permits it to penetrate these organisms; furthermore, by reacting with proteins it forms new chemical compounds (resins), which are stable and unfit as food for organisms.
Besides hardening, formaldehyde has several other disadvantages for embalming purposes (Mayer, 2012). It rapidly coagulates the blood, converts the tissues to a grey hue when it mixes with blood, fixes discolorations, dehydrates tissues, constricts capillaries, deteriorates with age, and has an unpleasant odour. Too much formalin tends to create moulding when the embalmed cadaver is left exposed for a protracted period of time in the dissecting laboratory (Bradbury & Hoshino, 1978).
Formaldehyde, CH2O, is a highly reactive aldehyde gas formed by oxidation or incomplete combustion of hydrocarbons. Formaldehyde gas is also created from the combustion of organic material and can be produced secondarily in air from photochemical reactions involving virtually all classes of hydrocarbon pollutants (National Toxicology Program, 2010). Formaldehyde is rapidly metabolized; it is produced endogenously in humans and animals and is also formed through the metabolism of many xenobiotic agents (National Toxicology Program, 2010). Because of these issues, typical biological indices of exposure, such as levels of formaldehyde or its metabolites in blood or urine, have proven to be ineffective measures of exposure.
In fact formaldehyde in formalin does not even exist as an aldehyde; 99.9% of formalin solutions exist as methylene glycol and its various polymers, with the true monomeric form present at only 0.1% (Bedino, 2003).
Formaldehyde is also used in the production of industrial resins (mainly urea-formaldehyde, phenol-formaldehyde, polyacetal, and melamine-formaldehyde resins) (National Toxicology Program, 2010). In the form of Bakelite, they are the earliest commercial synthetic resin (Hesse, 2000). This may be important inasmuch as several embalming fluids combine both formaldehyde and phenol (Woodburne & Lawrence, 1952; Erskine, 1961; Bradbury & Hoshino, 1978; Platzer et al. 1978; Frølich et al. 1984; Coleman & Kogan, 1998; Powers, 2003). It is not known whether these chemicals react within the fluid itself or within the corpse to form such a resin. As this resin formation can take place either using acid-catalysis (e.g. oxalic acid, hydrochloric acid or sulphonate acids) or base-catalysis, such resin formation may take place when considering the long storage-times of the cadavers.
Formaldehyde is known to react with proteins, lipids and nucleic acids (Hopwood, 1969). Formaldehyde acts by crosslinking several proteins chemically by inserting a methylene bridge (-CH2-) between the nitrogens of adjacent proteins, amines and related nucleophiles resulting in fixation or tanning-type action. The initially reversible hydroxymethyls in protein reaction, therefore, reduce by condensation reaction to hydrophobic methyls or N-formyls with formic acid formation. Methylene bridging occurs most often between lysine and various other moieties: lysine-arginine, lysine-cysteine, lysine-asparagine and lysine-glutamine and is strongly sterically controlled, occurring only when favourable proximities exist. In addition to the hydroxymethyl derivatives of the amine functions, guanidine, other hydroxyls, indoles and imidazoles being very unstable, certain other bridgings are also somewhat susceptible. The lysine-cysteine couplings are relatively stable, but reversible. Lysine-arginine, lysine-asparagine and lysine-glutamine are stable but susceptible to acid hydrolysis. Lysine-tyrosine links appear to be very stable and are acid-resistant. It seems in general that weaker, reversible links are generated during mild treatment, whereas strong formaldehyde treatment during fixation results in a significant amount of acid-resistant linkages (Bedino, 2003). Not all proteins are cross-linked similarly; for instance, the solubility of lact-and ovalbumins is even enhanced (Blum, 1896). Formaldehyde can bind covalently to single-stranded DNA and protein to form cross-links, or with human serum albumin or the N-terminal valine of hemoglobin to form molecular adducts (National Toxicology Program, 2010).
When formalin reacts with protein, it requires about 4.0–4.8 g of formaldehyde to totally react with and fix 100 g of a soluble protein; nonsoluble proteins require even more preservative (Fredrick, 1968; reprinted in Mayer, 2012). Brožek et al. (1963) defined an average protein-content of 164.4 g kg−1 body weight, or 16.4%. From these data one can calculate the protein content of a human and therefore the amount of formaldehyde needed. For example, an 80-kg corpse should contain about 13.12 kg of proteins and these would need 0.52–0.63 kg of pure formaldehyde or 1.4–1.7 L of a common formaldehyde solution (37%). Based on an average amount of 10 L injected, the final formaldehyde concentration of the embalming fluid would be about 5.2–6.3%. That amount of formaldehyde will harden a corpse vigorously, not really viable for a student's dissection course. On the other hand, 10 L of a 10% formaldehyde fluid would be appropriate for a corpse weighing 126.7–152.1 kg. Using such high concentrations for medium-weighted corpses would result in excess free formaldehyde, which will evaporate whenever possible, especially in the dissection lab. Anatomical Departments using low formaldehyde contents do not have problems with vapourous formaldehyde in their dissection labs (e.g. E. Brenner, personal communication, Innsbruck; B. Moxham, T. Wilkinson, personal communicaton, Cardiff).
The reaction of formaldehyde with lipids is less well known. Formaldehyde reacts with the double bonds in the presence of an acid catalyst. This eventually gives 1 : 3-glycols and 1 : 3-dioxanes. After fixation there was a decrease in the number of unsaturated bonds (Hopwood, 1969).
The reaction of formaldehyde with nucleic acids has been well investigated, as it forms the basis for attenuating viruses. The reaction between formaldehyde and adenosine forms two compounds. One of these is rapidly formed and labile, the reaction being reversible by dilution. The final product was a methylene-bis-adenosine, which was stable (Hopwood, 1969).
Formaldehyde solution (formalin; 37% formaldehyde gas by mass in water or 40% by volume in water) is considered a hazardous compound, and its vapour toxic (National Toxicology Program, 2010). For an extensive review of studies of the biological effects and toxicity see the ‘Final Report on Carcinogens Background Document for Formaldehyde’ (National Toxicology Program, 2010). Among the pathologies addressed here are sinonasal cancers, nasopharyngeal cancers, other head and neck cancers, respiratory cancer, lymphohematopoietic cancers, and brain and central nervous system cancers. For most of them, data cannot show clear increased risks, although there are trends towards higher risks.
In the anatomical context of the dissection laboratory, the adverse effects of formaldehyde have been studied extensively (Ohmichi et al. 2006; Takahashi et al. 2007; Wei et al. 2007; Khaliq & Tripathi, 2009; Lakchayapakorn & Watchalayarn, 2010; Ahmed, 2011; Mirabelli et al. 2011; Vohra, 2011; Raja & Sultana, 2012). At air levels of 0.5–2 ppm, formaldehyde may function as an irritant and cause mild eye and mucous membrane complaints. Acute exposure to formaldehyde may reversibly diminish the sense of smell. Acute and chronic skin exposure may produce irritation and peeling, as well as an allergic contact dermatitis (Suruda, 2003).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Glutaraldehyde
The first successful synthesis of glutaraldehyde is credited to Harries & Tank in 1908 (Fig. 1). Glutaraldehyde was catalogued as a typically reactive dialdehyde and was used for various chemical syntheses of more complex chemicals in laboratories and its properties were moderately investigated. By the 1940s and 1950s, it had become obvious that glutaraldehyde exhibited properties that were superior in many ways to formaldehyde in protein fixation chemistry and the early field of disinfection/sterilization. Interest in glutaraldehyde peaked in the early 1960s, when several investigations found it to have outstanding disinfection and sterilization capabilities, even surpassing formaldehyde (Bedino, 2003).
In reaction with proteins the aldol polymers of glutaraldehyde react to form α,β-unsaturated imino type reaction products that are highly resonance-stabilized and very resistant to acid hydrolysis and rehydration (Bedino, 2003). Glutaraldehyde appears to react chiefly with the amino groups of lysine, but also tyrosine, tryptophan and phenylalanine (Hopwood, 1969). The reactions of glutaraldehyde with lipids appear to be slight (Hopwood, 1969). Glutaraldehyde occasionally cross-reacts with formaldehyde, but in the literature it has not been found to be a formaldehyde releaser (De Groot et al. 2009).
Glutaraldehyde reaction with lipids and nucleic acids is as expected based on aldehyde chemistry and is similar to that of formaldehyde (Bedino, 2003).
An interesting feature of glutaraldehyde is that, unlike other aldehydes, it is capable of reacting with protein structures over a wide pH range. In addition, glutaraldehyde used as a disinfectant agent is effective against most microorganisms including viruses and spores, making it many times more effective as a disinfectant than formaldehyde (Mayer, 2012).
In embalming settings, glutaraldehyde, in contrast to formaldehyde, is a slow diffuser but delivers a rapid and irreversible final reaction with proteins. Therefore glutaraldehyde is expected to deliver more endpoint permanent fixation but perfuse the tissues slowly, whereas formaldehyde perfuses tissues rapidly but only forms irreversible fixation at a very slow rate (Bedino, 2003).
Although glutaraldehyde is a weak allergen, the vapours from glutaraldehyde (< 1 ppm) may act as an irritant to bronchial and laryngeal mucous membranes, and prolonged exposure could produce localized oedema and other symptoms suggestive of an allergic response.
Glutaraldehyde should have been phased out for Product Type 22 ‘Embalming and taxidermist fluids’ by 9 February 2011 (European Commission, 2010).
Glyoxal
Glyoxal (oxaldehyde) is a slimicide, a pesticide designed to kill organisms that produce slime (Fig. 1). Because it contains a chromophore group, glyoxal solutions tends to stain tissues yellow (Mayer, 2012). Several US-patented embalming fluids contain glyoxal. In this review, glyoxal is only a minor component, used in an unknown proportion by adding commercial disinfectant to the embalming solution (Frewein et al. 1987).
Glyoxal attacks the amino groups of proteins, nucleotides and lipids with its highly reactive carbonyl groups. A sequence of non-enzymatic reactions, called glycation, yields stable advanced glycation end products, which alter protein function and inactivates enzymes, resulting in disturbance of cellular metabolism, impaired proteolysis, and inhibition of cell proliferation and protein synthesis.
High-molecular-weight aldehydes such as glyoxal appear to be less toxic than formaldehyde, although studies of these compounds are incomplete (National Research Council (US) Committee on Aldehydes, 1981). Contact dermatitis has also been described for glyoxal (Uter et al. 2001; Aalto-Korte et al. 2005).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Tetrakis(hydroxymethyl)phosphonium chloride
Tetrakis(hydroxymethyl)phosphonium chloride (tetramethylolphosphonium chloride) is synthesized by a reaction of phosphine, formaldehyde and hydrochloric acid (Fig. 5). It can be absorbed through the skin. In general, this substance is used as flame retardant in cotton fabrics. A formaldehyde-free embalming fluid using this substance was recently presented by Shi et al. (2012). It contains a 15% solution of tetrakis(hydroxymethyl)phosphonium chloride as cross-linking agent in an 85% acidic buffer solution.
Figure 5.
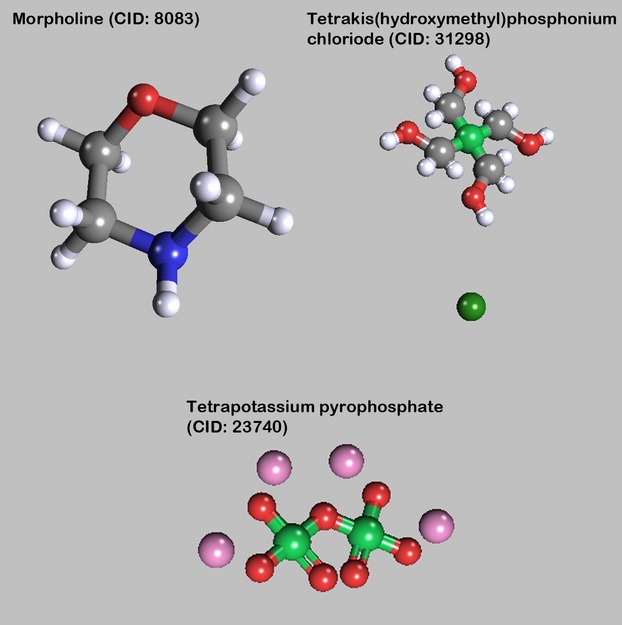
Other substances. The images of the molecules were created with PubChem3D Viewer (v2.0;Bolton et al. 2011), data were derived from PubChem Compound (Bolton et al. 2008).
Tetrakis(hydroxymethyl)phosphonium chloride can be absorbed through the skin; orally administered, it can affect the liver. No epidemiological data relevant to the carcinogenicity of tetrakis(hydroxymethyl)phosphonium salts were available.
Again, so far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
1-Methyl-3-octyloxymethylimidazolium tetrafluoroborate
1-Methyl-3-octyloxymethylimidazolium tetrafluoroborate, [(C8H17OCH2)MIM]+[BF4]−, is an ionic liquid that was used as a substitute for formaldehyde by Majewski et al. (2003). Ionic liquids are a class of organic salts that are liquid at room temperature in their pure form. Some of them are composed of organic cations such as quaternary ammonium cations, imidazolium cations, and heterocyclic aromatic and non-aromatic compounds. Its density is close to the density of water, but it is immiscible with water (Majewski et al. 2003).
In contrast to formaldehyde, ionic liquids do not bind to amino or imide groups in proteins, but they (i) can form ionic pairs with DNA and RNA, (ii) restrict access of water to tissues (this is particularly true in the case of 1-methyl-3-octyloxymethylimidazolium tetrafluoroborate, which resembles paraffin in its characteristics), and (iii) ionic liquids with a long alkoxymethyl substituent kill bacteria and fungi and in this way inhibit biological decomposition of tissues (Majewski et al. 2003). Nevertheless, the basic preserving effects of the borate component have to be considered.
Ionic liquids are considered advantageous not only because of their versatility but also for their ‘green’ credentials, although it is important to remember that not all ionic liquids are environmentally benign (Rogers & Seddon, 2003).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Alcohols
As a group, the alcohols have a pronounced bactericidal as well as some bacteriostatic action against vegetative forms, the specific effect depending on concentration and condition. They have a wide range of antiviral, antifungal and antimycosal effects. The predominant mode of action appears to come from protein coagulation/denaturation, with the fact that proteins are not denaturated as readily in the absence of water as by mixtures of alcohol and water (Ali et al. 2001).
Methanol
Methyl alcohol is toxic to organisms and also has disinfectant properties (Bradbury & Hoshino, 1978; Fig. 3). In addition, methyl alcohol has several advantages as an embalming chemical because it prevents polymerization of formaldehyde in the embalming fluid (additive), acts as an antirefrigerant, helps to establish the proper density of the solution, and coagulates albumin (Bradbury & Hoshino, 1978).
Figure 3.
Polyols. The images of the molecules were created with PubChem3D Viewer (v2.0;Bolton et al. 2011), data were derived from PubChem Compound (Bolton et al. 2008).
Methanol is metabolized primarily in the liver by sequential oxidative steps to formaldehyde, formic acid and carbon dioxide. Formic acid, the toxic metabolite of methanol, has been hypothesized to produce retinal and optic nerve toxicity by disrupting mitochondrial energy production. It has been shown in vitro to inhibit the activity of cytochrome oxidase, a vital component of the mitochondrial electron transport chain involved in ATP synthesis (Treichel et al. 2004).
Humans (and non-human primates) are uniquely sensitive to methanol poisoning and the toxic effects in these species are characterized by formic acidemia, metabolic acidosis, ocular toxicity, nervous system depression, blindness, coma and death. Nearly all of the available information on methanol toxicity in humans relates to the consequences of acute rather than chronic exposures.
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Ethanol
In embalming settings, ethanol is widely used as alcoholic solvent and anti-infective agent (Fig. 2). Furthermore, several authors suggest washing out (excessive) formaldehyde with ethanol (e.g. Bjorkman et al. 1986). There is almost no specific literature on the action of ethanol as preserving fluid. Ethanol, at least when combined with glycerine, denatures the proteins reversibly, affecting the hydrate coating of the tertiary structures. Hydrogen bridge bonds are disrupted (Hammer et al. 2012).
Figure 2.
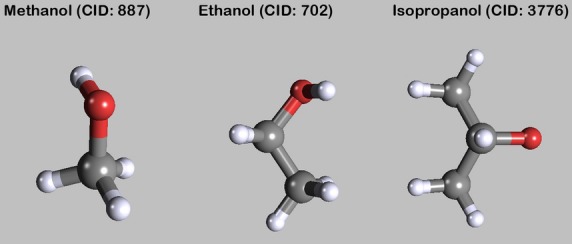
Alcohols. The images of the molecules were created with PubChem3D Viewer (v2.0;Bolton et al. 2011), data were derived from PubChem Compound (Bolton et al. 2008).
Ethanol tends to reduce the activity of the central nervous system.
Ethanol ought to have been phased out for Product Type 22 ‘Embalming and taxidermist fluids’ by 1 September 2006 (European Commission, 2005).
Isopropanol
Isopropanol, which is readily available, is considered a better germicidal and antiseptic agent than ethanol (Fig. 2). Isopropanol has a distinctive odour, but not an objectionable one (Woodburne & Lawrence, 1952).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Phenoxyethanol
Phenoxyethanol (PE) is a non-toxic lightly scented, common chemical often used in cosmetic and first-aid products (Wineski & English, 1989). Additionally, dilute phenoxyethanol is relatively inexpensive, non-flammable, slow to evaporate, effectively antimicrobial and an excellent tissue preservative and softener. Phenoxyethanol is merely a preservative, not a fixative.
Phenoxyethanol (ethylene glycol monophenyl-ether) is not used as embalming agent itself in most cases but it is used to wash out excessive formaldehyde from cadavers (Owen & Steedman, 1956, 1958; Spence, 1967; Frølich et al. 1984; Wineski & English, 1989). Nevertheless, phenoxyethanol appears to be an effective bactericide at a 1% concentration in creams (Lovell et al. 1984). It has a broad spectrum of antimicrobial activity and is particularly effective against strains of P. aeruginosa (Lovell et al. 1984).
The only description we found using phenoxyethanol in an embalming fluid comes from Nicholson et al. (2005). Among those embalming fluids they compared, there was a phenoxyethanol mix containing phenoxyethanol (7%), ethanol (61%), water (15%), glycerine (15%) and formaldehyde (1.9%). They found the tissues from cadavers embalmed with the phenoxyethanol fluid to produce good quality histological sections.
Despite its widespread use for many years, contact allergy to PE has been very rarely described (Lovell et al. 1984). A case of an immediate hypersensitivity reaction has been reported (Bohn & Bircher, 2001).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Sodium nitrate
Sodium nitrate is well known as a preservative (‘curing salt’; Macdonald & MacGregor, 1997). Besides its use in ancient embalming methods, it is purported to have been one component of Leonardo da Vinci's embalming fluid (McKone, 1999). Furthermore, it is mentioned as a component in several patents of embalming fluids.
As an additive, sodium nitrate (similar to the sodium nitrite) serves to inhibit the growth of bacteria, specifically Clostridium botulinum in an effort to prevent botulism, and helps preserve the colour of cured meat (Sárraga et al. 1989). It does not affect cathepsin D activity, inhibit cathepsin L activity at very high concentrations, and even enhance Ca-dependent proteolytic activity.
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Boric acid/sodium borate
Boric acid or its salts, borates, were used for embalming purposes already in pharaonic Egypt (Kaup et al. 2003; Buckley et al. 2004). Borates were used for anatomical preservation by Lakowski, Thiel and Majewski (Laskowski, 1886; Thiel, 2002; Majewski et al. 2003). It was estimated that borates forms borate complex with the carbohydrate residues of glycoproteins, especially with the functionally active alkaline phosphatase (Kaup et al. 2003). According to Peters (1956), boric acid has to be classified as preserving agent. It works as insecticide, has been used as a mild antiseptic or bacteriostatic in eyewashes, mouthwashes, burn dressings, and diaper rash powders; however, the effectiveness of boric acid has largely been discredited (Seiler et al. 1988).
Benkhadra et al. (2011) assume that the boric acid content in Thiel's embalming fluid is responsible for a distinct major modification of the integrity and the alignment of muscle fibres. The muscle fibres had a cut-up ‘minced’ appearance, but remained aligned; the conjunctive collagen fibrils were undisturbed. Benkhadra et al. argue that the acids are well known to have very corrosive effects on proteins and, in this special case, muscle proteins. The only acid present in Thiel's mixture is boric acid, thus they suspected it to be the reason for the observed damage, as the other chemicals of the Thiel's embalming solution could not be involved in this very singular destruction of the muscles.
Human borate exposures, even in the highest exposed cohorts, are too low to reach the blood (and target tissue) concentrations that would be required to exert adverse effects on reproductive functions (Bolt et al. 2012).
Boric acid should have been phased out for Product Type 22 ‘Embalming and taxidermist fluids’ by 1 February 2013 (European Commission, 2012).
Disinfectants
Phenol
Phenol, or carbolic acid, is a colourless or white crystalline solid with a relatively low melting point (Fig. 4). The majority of phenol and phenol derivates are used in resins and resin-based products such as formaldehyde and bisphenol resions from acetones, smaller portions as general disinfectant and, finally, in the production of organic dyes. The disinfective properties of phenol have been known throughout most of history. The first documented and widely publicized use of phenol as a disinfectant in the medical field was by Lister in 1867 (Bedino, 1994).
Figure 4.
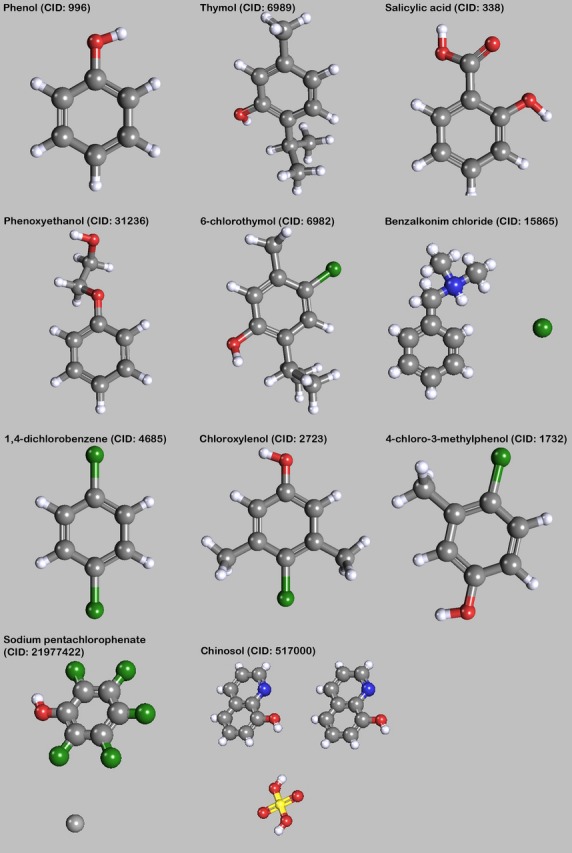
Aromatic substances. The images of the molecules were created with PubChem3D Viewer (v2.0;Bolton et al. 2011), data were derived from PubChem Compound (Bolton et al. 2008).
Phenol is bacteriostatic in as small a concentration as 0.2% by virtue of its ability to deactivate enzymes within the cell and affect cell permeability. It becomes bactericidal/fungicidal at concentrations of 1.0–1.5% and actually destroys cell walls. There is a marked increase in bactericidal activity with halogenation or alkylation of the basic phenol molecule. The mode of action of phenol and its derivates against various bacteria, fungi and viruses is due to its ability to denaturate and precipitate protein and proteinaceous products and its ability effectively to attack and destroy the cell wall due to its lipophilic character (Bedino, 1994).
Liquefied phenol has proved to prevent moulding effectively (Bradbury & Hoshino, 1978). Phenol is an excellent fungicide and bacteriocide but it denatures proteins, with resultant drying and discoloration of tissues, and has an unpleasant odour (Richins et al. 1963). On the other hand, phenol will actually reverse the greying effects of formaldehyde embalming (Bedino, 1994).
Phenol is used in embalming as a medium to lower preservative strength, which has superior penetration ability. In addition, its use results in superior disinfection (Bedino, 1994).
As an exposure hazard, phenol is corrosive to the throat and stomach, causing nausea, vomiting, cyanosis, loss of blood pressure, convulsion and pulmonary oedema (Bedino, 1994). Furthermore, it desensitizes the skin. Other adverse effects described are eczema, headache and faintness (Lischka et al. 1981).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Phenolic derivates
Salicylic acid
Salicylic acid, chemically 2-carboxyphenol, was used in the formulations by Salafia, Peters and Erskine (Salafia, c. 1927; Peters, 1956; Erskine, 1961; Fig. 4). The major aim of adding salicylic acid would be its action as antioxidant, but according to Peters (1956), salicylic acid can be classified as preserving agent. Pharmacologically, salicylic acid acts as anti-infective, antifungal and keratolytic agent; at high concentrations (e.g. 20%) it has a caustic effect. Salicylic acid itself should not be administered systemically because of its severe irritating effect on gastrointestinal mucosa and other tissues.
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Sodium pentachlorophenate
The use of sodium pentachlorophenate diminished the undesirable side effects of phenol, and is stated to be even more effective as a bacteriocide and fungicide (Richins et al. 1963; Fig. 4). Also, the visual appearance of the organs and tissues is better than with phenol. Fascia, tendons and aponeuroses are white in colour. Fat remains yellow and can easily be seen even in small amounts. Muscle is tan to brown; higher concentrations of the sodium pentachlorophenate have been found to produce darker muscle colour. Nevertheless, some methanol should be added to keep the pentachlorophenate in solution, particularly in the concentrated stock solutions.
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Thymol
Thymol (2-isopropyl-5-methylphenol) is a naturally occurring, oxygenated monoterpene phenol derivative of p-cymene that is found in thyme oil (Bisht et al. 2011; Fig. 4).
Hammer et al. (2012) use thymol in an alcoholic solution for moistening the specimens externally at the end of every dissection course, at least once a week. At McMaster's University, a thymol content in their moistening fluid is also used (Powers, 2003). Thymol has no known carcinogenic or other harmful effects to health besides skin irritation, and it is well known for its bactericidal and fungicidal effects. The lipophilic character of thymol disturbs the aqueous phase and, therefore, the integrity of bacterial and fungal membranes. Proteins related to bacterial metabolism become inactivated.
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
4-Chloro-3-methylphenol (parol; 4-chloro-m-cresol, PCMC)
In general, 4-chloro-3-methylphenol is used as an antiseptic and preservative agent (Fig. 4). It is a compound in Thiel's basic solution II. PCMC has a considerably high solubility (4 g L−1 at 20 °C), being higher than other chlorophenols, and remains active over a wide pH range (4–8), where, compared with other phenolic derivates, only PCMC remains sufficiently soluble (Goddard & McCue, 2001).
An antagonistic interaction in toxicity occurred between phenol and p-chloro-m-cresol (Babich & Stotzky, 1985). Nevertheless, systemic effects are presumably like those of phenol. It is known to be a moderate allergen for sensitive skin.
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
1,4-Dichlorobenzene
Adding para-and/or orthodichlorobenzene in concentrations from 0.025% to 5% to formaldehyde containing embalming fluids should provide an unusual degree of penetration and outstanding preservation (Beck, 1966; Fig. 4).
1,4-Dichlorobenzene is used as moth repellent.
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Chinosol/oxyquinoline
Chinosol was used as a single medium for injection by Schiefferdecker (1897) (Fig. 4). Chemically, it is oxyquinoline sulphate. In vitro, although a powerful antiseptic, it is only very slightly germicidal; a 2% solution did not kill S. aureus in 24 h (Lusk, 1919).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Quaternary ammonium compounds
Some quaternary ammonium compounds, di-C8-10-alkyldimethyl chlorides, should have been phased out for Product Type 22 ‘Embalming and taxidermist fluids’ by 9 February 2011 (European Commission).
Benzalkonium chloride
Benzalkonium chloride, a quaternary ammonium compound, chemically known as alkyldimethylbenzylammonium chloride, has been found to have definite germicidal and fungicidal properties; it is used mainly as a mould inhibitor (Woodburne & Lawrence, 1952; Macdonald & MacGregor, 1997; Fig. 4). It is a component of many patented embalming solutions. One can ‘paint’ the interior of cadaver tanks and crates with benzalkonium chloride (pure, 50% aqueous solution) before putting the embalmed cadavers or their parts in. After putting the specimens in, some of this chemical solution is poured over the surface of the main preservative solution (Buch, 2013).
Tetradecylamine
Tetradecylamine, also know as myristylamine, is also a quaternary ammonium compound.
Polyhexamethylene guanidine hydrochloride
Polyhexamethylene guanidine hydrochloride (PHMGH) is an antimicrobial biocide of the guanidine family (Oulé et al. 2008).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Modifying agents
Buffers
Target pH: 7.38–7.40.
Sodium borate (Borax): Sodium borate acts to buffer the embalming mixture at pH 9 and affords protection against mould growth and bacterial decomposition (Macdonald & MacGregor, 1997).
Sodium bicarbonate.
Sodium carbonate.
Magnesium carbonate.
Humectants and wetting agents
Glycerine
Spriggs (1963) referred to an Austrian scientist who discovered that glycerine, although not a disinfectant in itself, so increased the efficiency of formaldehyde as to render a small amount of formalin and glycerine just as powerful a disinfectant as a much larger amount of formalin alone (Bradbury & Hoshino, 1978; Fig. 3).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Chloral hydrate
Chloral hydrate belongs to the group of glycol derivates and is a major metabolite of trichloroethylene (Fig. 3). It is used as component of Sihler's whole mount nerve staining technique that renders other soft tissue translucent or transparent while staining the nerves (Mu & Sanders, 2010). In anatomical embalming fluids, chloral hydrate has been used by several authors (Jores, 1913; Peters, 1956; Kurz, 1977/1978; Frewein et al. 1987; Sampaio, 1989; Guimarães Da Silva et al. 2004).
Chloral hydrate is commonly known as an outdated sedative and hypnotic. Overdosage produces symptoms that are similar to those of barbiturate overdosage and may include coma, hypotension, hypothermia, respiratory depression and cardiac arrhythmias. Miosis, vomiting, areflexia, and muscle flaccidity may also occur. Oesophageal stricture, gastric necrosis and perforation, and gastrointestinal haemorrhage have also been reported.
Chloral hydrate probably reacts with protein in a similar manner to formaldehyde but irreversibly (Hopwood, 1969). Furthermore, this substance is used for colour preservation (dye; Jimenez Collado et al. 1999).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Mono-, di-and poly-ethylene glycol
(Mono-)ethylene glycol, like glycerine, serves to preserve moisture in the embalmed body, acts as anti-refrigerant, and also mixes well with other chemicals in the embalming fluid (Bradbury & Hoshino, 1978; Fig. 3). Ethylene glycol, at least in combination with glycerol and/or ethanol, decreases the opacity of muscles, with the muscle fibres becoming more spatially separated (Oliveira et al. 2007, 2010). Ethylene glycol also decreases the sample pH due to water loss inside tissue (Oliveira et al. 2007). (Mono-)ethylene glycol is toxic (Friedman et al. 1962; Brent et al. 1999); the same is also true for di-ethylene glycol (O'Brien et al. 1998; Schep et al. 2009). The mean estimated fatal dose in an adult has been defined as 1 mL kg−1 of pure di--ethylene glycol (DEG; Schep et al. 2009). Polyethylene glycol is superior to glycerine as a solubilizer and is an inhibitor of mould growth (Macdonald & MacGregor, 1997).
So far there has been no ‘decision of non-inclusion’ of mono-, di-or polyethylene glycols in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Sorbitol
Sorbitol can be used as a replacement for glycerine (Richins et al. 1963; Fig. 3). It is a better humectant, and there is less generalized darkening or ‘browning’ of tissues.
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Sodium lauryl sulphate
Sodium lauryl sulphate, a non-ionizing surfactant, enables embalming fluid to enter all areas of the cadaver (Macdonald & MacGregor, 1997).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Sodium 2-sulphonatoethyl laurate
Sodium 2-sulphonatoethyl laurate was used as anionic surfactant in an embalming fluid described by Dayton et al. (1965).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Phosphorated higher alcohols
Phosphorated higher alcohols can be used as wetting agents (Richins et al. 1963).
Softener
Tetrapotassium pyrophosphate decreases the tension which has developed in isolated glycerinated muscle (Richins et al. 1963).
So far there has been no ‘decision of non-inclusion’ in Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Anticoagulants
Sodium citrate (also buffer; Mayer, 2012).
Sodium oxalate.
Salts
Several preservative mixtures have added salts. It was found that various anions had a denaturing effect on DNA. These included the sodium salts of trichloracetate, thiocyanate, perchlorate and iodide. Cations had only a small denaturing effect. This sort of effect has also been described for various proteins. Calcium chloride and potassium thiocyanide were shown to be potent structural destabilizers and denaturants. Salts such as ammonium sulphate and potassium dihydrogen phosphate strongly stabilized the native conformation of the proteins. The results were not explained other than that salts do react with proteins and this may alter their ordered stability (Hopwood, 1969).
Green or natural embalming fluids
Natural/green burial demands a new and enlightened definition, approach and procedure drastically different from traditional toxic formaldehyde embalming. Ecobalming does not and will not create a long-term preserved anatomical specimen. If this result is required, or demanded, then the embalmer must resort to a traditional style of embalming (Bedino, 2009).
These green or natural fluids contain some disinfectant and preservative components. They are composed of oil ingredients derived from gums and plant resins, a variety of spices, and certain alcohols used as vehicles for these preservatives; similar to the formulations prior to the discovery of formaldehyde. These embalming fluids do provide good preservation for 3–5 days, or possibly a week or longer but do not meet the need for anatomical preservation (Mayer, 2012).
‘Green’ or natural embalming fluids contain synergistic mixes of essential, plant-based oils and their purified or synthesized/derivatized active aldehyde or phenolic-like components in a near-anhydrous carrier. The active chemical components of ecobalming fluids include vanillic aldehyde, guaiacol and eugenol as the active essential oil ingredients and propylene glycol as the near-anhydrous carrier. Vanillic aldehyde (vanillin) is both a derivitized phenolic and an aldehyde and is the active aldehyde-driver precipitant/reactant. Notable protein precipitation/coagulation is effected with both the aldehyde moiety and phenolic group. The pH of vanillic aldehyde in 5% water is acidic (pH 4.3) and thus contributes to precipitation. Vanillin presents little or no exposure impact when in liquid solution and only commonsense precautions need be taken. Guaiacol (2-methoxyphenol) is an additional phenolic precipitant/reactant. Eugenol [2-methoxy-4-(2-propenyl)phenol] is the principal essential oil from cloves and is also found in nutmeg, cinnamon and bay leaf. It is an active, substituted protein precipitant/reactant (Bedino, 2009).
Analyses of embalming quality
Of course, many of the embalming chemicals have been tested for their individual toxicity, their suitability as bacteriostatic agent, etc., but only a few embalming solutions have been tested for the embalming purposes.
One of the best investigated embalming methods is Thiel's method (Thiel, 1992, 2002). This method is praised for resulting in soft and flexible cadavers with almost natural colours. The flexibility might be due to a considerable fragmentation of muscle proteins, where the muscle fibres show a cut-up ‘minced’ appearance with the surrounding collagenous fibrils being undisturbed (Benkhadra et al. 2011). These authors relate these changes in muscular tissue to the content of boric acid in Thiel's basic solution I (and of course in the storage solution). Whereas they could not find major changes in collagenous tissues, others found that tendons from cadavers embalmed with Thiel's method showed statistically lower failure stress compared with fresh frozen samples and trended to a decreased tangential modulus (Fessel et al. 2011). They argue that Thiel-preserved samples demonstrated altered failure characteristics, indicating a different collagen fibre/collagen network failure mechanism, again most likely due to partial denaturing by boric acid in Thiel's solution. Finally, they conclude that Thiel-embalmed tendons did not faithfully represent the biomechanical characteristics of fresh frozen tendons. Compared with formalin-fixated specimens which become approximately five times stiffer and completely lose their non-linear load-deformation characteristic, Thiel fixation maintains the non-linear load-deformation characteristic but increases the range of motion in biomechanical tests of L1–L2 spinal segments (Wilke et al. 2011). Differences are also quite low for testing the human middle ear mechanics in Thiel-embalmed cadavers compared with living subjects and fresh temporal bones; however, significant differences in some frequencies, particularly at the round window, have to be considered (Stieger et al. 2012). Chest radiographs of Thiel-embalmed cadavers with inflated lungs are of high quality (De Crop et al. 2012).
Nevertheless, Thiel-embalmed cadavers have been widely appraised for post-graduate hands-on workshops for several medical disciplines (Peuker et al. 2001; Baca et al. 2006; Feigl et al. 2008; Giger et al. 2008; Wolff et al. 2008; Eisma et al. 2011; Prasad Rai et al. 2012; Eisma et al. 2013). Testing four criteria, one Thiel-embalmed cadaver showed a joint flexibility comparable to fresh tissue, a tissue pliability also like fresh tissue, a colour that was most akin to fresh tissue, and did not grow mould (Jaung et al. 2011).
Biomechanical testing
Other important studies tested the suitability of different embalming methods for biomechanical testing. Thiel's embalming method shows significant changes in biomechanical properties, as described above (Benkhadra et al. 2011; Fessel et al. 2011; Wilke et al. 2011). In early investigations, embalming itself seems not to alter the density of either compact or cancellous bone (Blanton & Biggs, 1968). Later studies have found that formalin storage caused a 50% reduction in energy absorption and increased the brittleness of the bones (Goh et al. 1989), a significant decrease in impact strength in even short formalin-fixed specimens (< 3 h of embalming; Currey et al. 1995), a decrease in mechanical integrity after embalming (Ohman et al. 2008), perhaps due to the occurrence of bone demineralization (Fonseca et al. 2008), and an alteration of visco-elastic properties by reducing its ability to dissipate viscous energy (Nazarian et al. 2009).
Comparing three different preservation methods – formalin fixation (according to Platzer et al. 1978), Thiel fixation (according to Thiel, 2002), and alcohol–glycerine fixation (96% ethanol, 3% glycerine and 1% phenol)] – on the elastic and postyield mechanical properties of cortical bone with fresh-frozen specimens, the bone mineral density as well as the initial Young's modulus showed no significant differences between the four test groups (Unger et al. 2010). After 6 months in fixative solution, the Young's modulus was significantly lowered in human Thiel specimens and only showed minor changes in formalin-and alcohol–glycerine-treated specimens. The plastic energy absorption of human and bovine specimens was altered significantly. Formalin as well as alcohol–glycerine fixation yielded a significant decrease in plastic energy absorption, whereas Thiel fixation significantly increased the plastic energy absorption.
Antimicrobial testing
Present embalming practices reduce the hazard of transmission of potentially infectious microbial agents within the immediate environment of embalmed human remains (Burke & Sheffner, 1976). In their study, the administration of arterial and cavity embalming chemicals resulted in a 99% reduction of the postmortem microbial population after 2 h of contact. This level of disinfection was maintained for the 24-test period. Topical disinfection of the body orifices was also observed.
Woodburne & Lawrence (1952) developed their ‘improved embalming fluid formula’ by testing eight different fluids for their germicidal activity against M. tuberculosis, S. aureus, E. typhosa, P. aeruginosa, P. vulgaris, B. anthracis, C. tetani and novyi, β-haemolytic S. pyogenes, and for their fungicidal activity against P. notatum, A. niger, C. immitis, and C. neoformans.
On the other hand, Putz et al. (1974) tested tubs, tanks and crates, tables, and cadavers for fungi. The main species found were Aspergillus vesicolor, Penicillium cyclopium, Penicillium frequentans, and Cladosporium sphaerospermum, all of them being human and animal saprophytes. Aspergillus vesicolor as well as P. frequentans may also attack softeners, which might be important for the storage of embalmed cadavers in plastic materials. These investigations resulted in the embalming method described by Platzer et al. (1978), which solved the mould problem described earlier. In a series of papers, Lischka and Wewalka and colleagues analyzed the microbial fauna on cadavers during dissection (Lischka et al. 1979a,b1979b; Wewalka et al. 1979). They found mainly aerobic sporulates and Staphylococcus epidermidis, with occasional occurrences of S. aureus, Streptococcus sp., and moulds. The authors argue that these microbes did not originate from the cadavers but are ubiquitous dermal germs. Testing prior to embalming revealed a different microbial vegetation, consisting of S. aureus, Staphylococcus epidermidis, Enterococcae, β-haemolytic Streptococcae, Streptococcus viridans, Corynebacteriae, some aerobic sporulates, and a high number of enterobacteriaceae, such a Escherichia coli, Proteus sp., Enterobacter cloaecae, Klebsiella sp., and P. aeruginosa, Acinetobacter sp. and several fungi. All of the three embalming fluids, a mixture of 4.1% phenolic acid and 2.1% formaldehyde, a mixture according to Tutsch (1975), and Jores embalming fluid (1913), reduced the initial amount of microbia fast and effectively, with only some S. epidermidis and some aerobic sporulates surviving the first day of embalmment. Among these three tested embalming techniques, the Jores solution was revealed as being the least effective one.
Janczyk et al. (2011b) tested their ethanol-polyethylene glycol-formalin embalming fluid also for microbes and found single colonies of Pseudomonas oryzihabitans, Chryseobacterium sp., Acinetobacter sp. in the lungs, and Micrococcus sp. and Bacillus sp. isolated from one muscle sample.
When using only formalin in a morphological laboratory, a microbiocenosis consisting of the bacteria Bacillus sp., Providencia alcalifaciens, and S. aureus, the micromycetes Scopulariopsis brevicaulis, Aspergillus sp., Hormodendron sp., and the mites Dermatophagoides pterronissimus were found (Svidovyi et al. 2011). These authors state that there is experimental evidence that these organisms are resistant to formalin.
Testing for histological appearance
Thiel-embalmed specimens present with considerable changes in their histological appearance (Benkhadra et al. 2011; Fessel et al. 2011). Other embalming methods were tested with better results (Coleman & Kogan, 1998), with the latter presenting samples of an excellent long-term microanatomical preservation of various tissues from cadavers treated with their embalming solution. Nicholson et al. (2005) showed that embalmed cadaveric tissue can be used for routine histology, although it may not be suitable for all histological studies. Frølich et al. (1984) reported satisfactory preservation of cadavers embalmed between 8 and 48 h post-mortem. When embalming is usually undertaken within 24 h of death and embalment uses formalin, this results in adequate preservation of tissues for dissection and satisfactory quality of histological sections (Nicholson et al. 2005). Skin samples taken from mono-and diethylene glycol-preserved cadavers 1 month after embalming showed mainly excellent or good histological conservation, surpassing that of striated muscle, whereas the buccal mucosa showed worse results (Goyri-O'Neill et al. 2013).
Usability testing
Besides the usability test for Thiel-embalmed cadavers, especially with respect to their usage in post-graduate education (see above), only a few more embalming solutions were tested. Cadavers, preserved either with a modified Laskowski solution or modified Larssen solution, were analyzed for the following aspects: (i) colour and texture of tissues, (ii) flexibility of joints and skin, (iii) odour and (iv) suitability to specific operative procedures (Silva et al. 2007). Although these fixatives maintain body flexibility, the Laskowskic solution failed to keep an ordinary tissue coloration (cadavers were intensely red) and tissue preservation was not adequate. By contrast, the modified Larssen solution did not alter the coloration of cadavers. A remarkable characteristic was a very strong and unpleasant sugary odour in Laskowski-preserved cadavers and therefore the modified Larssen solution was the elected method to preserve cadavers for surgical technique classes. The students' feedback to the use of Larssen-preserved cadavers was very satisfactory (96.6% of students in favour).
Biocidal Products Directive (98/8/EC)
The Biocidal Product Directive has the aim to harmonize the European market for biocidal products and their active substances.2 At the same time, it aims to provide a high level of protection for humans, animals and the environment.
The Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market should be the framework of rules to provide that biocidal products should not be placed on the market for use unless they have complied with the relevant procedures of this Directive (European Parliament & Council, 1998). Among these regulations, the directive states that ‘it is appropriate that the applicant submit dossiers which contain information which is necessary to evaluate the risks that will arise from proposed uses of the product’, and concludes that it is necessary to establish a Community list of active substances permitted for inclusion in biocidal products. It will be replaced by Regulation (EU) No 528/2012 (European Parliament & Council, 2012) as of 1 September 2013. This new regulation aims to improve the functioning of the internal (European) market in biocidal products while ensuring a high level of environmental and human health protection.
There are two important Annexes to the Biocidal Products Directive (98/8/EC): (i) Annex I, which covers active substances with requirements agreed at community level for inclusion in biocidal products, and (ii) Annex Ia, which comprises a list of active substances with requirements agreed at community level for inclusion in low-risk biocidal products. An active substance cannot be included in Annex Ia if it is classified as carcinogenic, mutagenic, toxic for reproduction, sensitizing, or bioaccumulative and does not readily degrade. There are two measures to be taken, either a decision of inclusion in Annex I or Ia, or a decision of non-inclusion in Annex I or Ia.
The Biocidal Products Directive (98/8/EC) contains an exhaustive list of 23 product types with an indicative set of descriptions within each type in Annex V (Supporting Information, Table S20). The content covers disinfectants and general biocidal products, non-food preservatives, products for pest control, preservatives for food or feedstocks, antifouling products, and products used for control of other vertebrates. Chemicals used for embalming are covered by Product-type 22 (PT22): Embalming and taxidermist fluids [no change in Regulation (EU) No 528/2012].
The EU review programme for biocidal substances comprise 25 substances in PT22. The most important of these (in terms of production tonnage) are 2-butenone peroxide, dodecylguanadine monohydrochloride and methylene dithiocyanate (J. Kjølholt, unpublished data). It should be mentioned that no quantitative data on the uses of formaldehyde was obtained. The main biocide used in embalming fluids is formaldehyde. Yet in some cases glutaraldehyde is preferred (Tissier & Migné, 2001).
Product type 22: exposure of humans
Exposure may take place when mixing the fluid in a fume cupboard, during decantation of the fluid. When opening the lid/boxes containing the cadaver and by dissection of the embalmed cadaver the students and lecturer may be exposed. The funeral undertakers may be exposed when the preserved corpses are prepared for the funeral.
Product type 22: exposure of the environment
Emissions of the biocides by cremation are presumed to be insignificant as the organic compounds are degraded. If the embalmed corpses are buried, release of the agents to soil may occur.
Product type 22: assessment of risk
The production tonnage in PT22 is low; most likely below 100 tons per year in the EU based on extrapolation of production data for only about 16% of the notified substances. The use of products in PT22 must be characterized as non-dispersive, as application is performed either by professional users or by specialist non-professionals, and the exposure of humans and the environment during the service life of the products (the preserved corpses or animals) is considered to be insignificant. Some of the active substances within PT22 appear to be sensitizing and significantly toxic to humans but the information about the environmental toxicity is too limited to allow an assessment. Overall, the risk to humans and the environment from the use of PT22 substances is considered to be low to moderate.
Product type 22: substances phased out
Among those substances identified for anatomical embalming purposes, it has actually been decided nor to include ethanol, glutaraldehyde, sodium sulphite, boric acid, or polyhexamethylene guanidine hydrochloride in Annex I or Ia of Directive 98/8/EC (Table 7). Concerning PT22, no decision has been made to include any substance in Annex I or Ia.
Table 7.
Existing active substances for which a decision of non-inclusion into Annex I or Ia of Directive 98/8/EC, for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’, has been adopted (http://ec.europa.eu/environment/biocides/pdf/list_dates_product_2.pdf; last accessed 13 May 2013).
| Name (EINECS and/or others) | EC number | CAS number | Products to be phased-out by | Decision reference |
|---|---|---|---|---|
| Ethanol | 200-578-6 | 64-17-5 | 1 September 2006 | Commission Regulation (EC) 1048/2005 |
| Glutaral | 203-856-5 | 111-30-8 | 9 February 2011 | Commission Decision 2010/72/EU |
| N,N-diethyl-m-toluamide | 205-149-7 | 134-62-3 | 9 February 2011 | Commission Decision 2010/72/EU |
| 2-Butanone, peroxide | 215-661-2 | 1338-23-4 | 1 November 2011 | Commission Decision 2010/675/EU |
| 1,2-Benzisothiazol-3(2H)-one | 220-120-9 | 2634-33-5 | 9 February 2011 | Commission Decision 2010/72/EU |
| 2-Methyl-2H-isothiazol-3-one | 220-239-6 | 2682-20-4 | 9 February 2011 | Commission Decision 2010/72/EU |
| Bis(trichloromethyl) sulphone | 221-310-4 | 3064-70-8 | 9 February 2011 | Commission Decision 2010/72/EU |
| Methylene dithiocyanate | 228-652-3 | 6317-18-6 | 9 February 2011 | Commission Decision 2010/72/EU |
| Sulphur dioxide | 231-195-2 | 7446-09-5 | 9 February 2011 | Commission Decision 2010/72/EU |
| Sodium hydrogensulphite | 231-548-0 | 7631-90-5 | 9 February 2011 | Commission Decision 2010/72/EU |
| Disodium disulphite | 231-673-0 | 7681-57-4 | 9 February 2011 | Commission Decision 2010/72/EU |
| Sodium sulphite | 231-821-4 | 7757-83-7 | 9 February 2011 | Commission Decision 2010/72/EU |
| Boric acid | 233-139-2 | 10043-35-3 | 1 February 2013 | Commission Decision 2012/78/EU |
| Potassium sulphite | 233-321-1 | 10117-38-1 | 9 February 2011 | Commission Decision 2010/72/EU |
| Dodecylguanidine monohydrochloride | 237-030-0 | 13590-97-1 | 9 February 2011 | Commission Decision 2010/72/EU |
| Dipotassium disulphite | 240-795-3 | 16731-55-8 | 9 February 2011 | Commission Decision 2010/72/EU |
| m-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate/permethrin | 258-067-9 | 52645-53-1 | 9 February 2011 | Commission Decision 2010/72/EU |
| Quaternary ammonium compounds, di-C8-10-alkyldimethyl, chlorides | 270-331-5 | 68424-95-3 | 9 February 2011 | Commission Decision 2010/72/EU |
| Monohydro chloride of polymer of N,N′′′-1,6-hexanediylbis[N′-cyanoguanidine] (EINECS 240-032-4) and hexamethylenediamine (EINECS 204-679-6)/Polyhexamethylene biguanide (monomer: 1,5-bis(trimethylen)-guanylguanidinium monohydrochloride) | Polymer | 27083-27-8/32289-58-0 | 9 February 2011 | Commission Decision 2010/72/EU |
In accordance with Article 4(2) of Regulation (EC) No 2032/2003, biocidal products containing active substances for which a non-inclusion decision was taken shall be removed from the market within 12 months of the entering into force of such decision; unless otherwise stipulated in that non-inclusion decision. Dates by which products containing these active substances shall no longer be placed on the market for the relevant product-type PT 22 ‘Embalming and taxidermist fluids’.
Substances addressed within this review are indicated italic and bold.
Biocide regulations in other countries
Biocidal products are governed not only by EU regulations, but also by international conventions, for instance the Rotterdam Convention on the prior informed consent procedure for certain hazardous chemicals and pesticides in international trade (Rotterdam Convention Secretariat). Particularly relevant with regard to risk assessment and communication of active substances and biocidal products are the efforts at United Nations Economic Commission for Europe (UNECE) level based on the Globally Harmonized System of classification and labelling of chemicals (UNECE).
In recent years, a working group under the auspices of the OECD Task Force on Biocides made valuable contributions to issues of content regulation in the field of biocides. Of these contributions, the Report of the Survey of OECD Member Countries' Approaches to the Regulation of Biocides is – to the best of our knowledge – hitherto the first and only comparison of regulations in several different countries (OECD, 1999). This survey comprises data from 18 countries (Australia, Austria, Belgium, Canada, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, New Zealand, the Netherlands, Portugal, Sweden, Switzerland, the UK, the US) and the European Commission (with some minor exemptions, the EC data will be used for all member states). Substances used for preservation and disinfection of human and animal corpses (embalming fluids) are labelled as Use Category 11 (UC11). According to this survey, embalming fluids had to be approved only in Belgium and Switzerland; a notification (a system in which a manufacturer/supplier must inform the appropriate national authority about their intention of placing a chemical on the market) was necessary in Ireland and New Zealand. In Australia, these products were regulated by laws relating to therapeutic goods, dangerous goods, and chemical substances; these laws are administered by the Department of Health and Family Services, Worksafe Australia and various State Government Departments. In Canada, embalming fluids were not regulated as pesticides by the Pest Management Regulatory Agency (PMRA), nor were they regulated as drugs under the Food and Drugs Act. It is possible that they were regulated as workspace substances or commercial chemicals and there would have been any data requirements other than a material safety data sheet (MSDS). In New Zealand, embalming fluids were regulated by the Toxic Substance Act 1979 as the responsibility of The Toxic Substance Board, a statutory decision-making board served by the Ministry of Health; notification was necessary only for the end-use biocide but not for the active ingredient. Switzerland regulated embalming fluids by the more or less general federal law on the trade in toxic substances, where the end-use biocide needed approval. Within the US, mortuary embalming fluids were explicitly exempted from regulation as pesticides issued by the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA).
Acknowledgments
This paper is based on an oral presentation at the Joint Winter Meeting of the Anatomical Society, the British Association of Clinical Anatomists and the Institute of Anatomical Sciences held at the University of Cardiff on 19–21 December 2011 (Brenner, 2012).
Footnotes
Methylated spirits are ethanol that has additives to make it inedible (poisonous) to prevent human consumption. The main additive has traditionally been 10% methanol, giving rise to the term ‘methylated spirit’ (http://en.wikipedia.org/wiki/Denatured_alcohol).
For extensive information visit the EC-Website at http://ec.europa.eu/environment/biocides/index.htm.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Summative table of substances used in modern anatomical embalming.
Table S2. Kaiserling's solutions for color and form preservation (Pulvertaft, 1950).
Table S3. Jores' fixative solution (Bradbury & Hoshino, 1978).
Table S4. Enhanced embalming fluid by Woodburne & Lawrence (1952).
Table S5. Peters' salt solutions (Peters, 1956).
Table S6. Dublin embalming fluid (Erskine, 1961).
Table S7. Richins' solutions (Richins et al. 1963).
Table S8. Tüubingen embalming fluid (Tutsch, 1975).
Table S9. Bradbury and Hoshino's embalming fluid (Bradbury & Hoshino, 1978).
Table S10. Solutions published by Platzer et al. (1978).
Table S11. Coleman and Kogan's preservation (Coleman & Kogan, 1998).
Table S12. ‘New Basler solution’ (Kurz, 1977/1978; Frølich et al. 1984).
Table S13. Bergen solution, used until 1979 (Frølich et al. 1984).
Table S14. Modified Kurz arterial embalming fluid (Frewein et al. 1987).
Table S15. Thiel's solutions (either in millilitres for liquids or grams for solids; Thiel, 2002).
Table S16. Proposed ‘new‘ Southampton embalming fluid (O'Sullivan & Mitchell, 1993).
Table S17. McMaster's solutions (Powers, 2003).
Table S18. Anatomy Institute of Sidney University's embalming fluids (Mills, 2010).
Table S19. Table of hazards of substances used in modern anatomical embalming.
Table S20. Product types of the Biocidal Products Directive (98/8/EC).
References
- Anonymous 2012. Embalming essay. Available at: http://www.freeessays.cc/db/40/rfh153.shtml (accessed 2 May 2012)
- Aalto-Korte K, Mäkelä EA, Huttunen M, et al. Occupational contact allergy to glyoxal. Contact Dermatitis. 2005;52:276–281. doi: 10.1111/j.0105-1873.2005.00580.x. [DOI] [PubMed] [Google Scholar]
- Ahmed HO. Preliminary study: formaldehyde exposure in laboratories of Sharjah University in UAE. Ind J Occup Environ Med. 2011;15:33. doi: 10.4103/0019-5278.82997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmani ML. Embalming: Principles and Legal Aspects. Daryagani: Jaypee Brothers Publishers; 1998. [Google Scholar]
- Al-Hayani AA, Hamdy RM, El-Aziz GSA. Shellac: a non-toxic preservative for human embalming techniques. J Anim Vet Adv. 2011;10:1561–1567. [Google Scholar]
- Ali Y, Dolan MJ, Fendler EJ. Alcophls. In: Block SS, et al., editors. Disinfection, Sterilization, and Preservation. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 229–254. [Google Scholar]
- Anichkov NM, Danilova IA, Riabinin IA, et al. [Application of polyguanidine solution for fixation of biological and anatomical specimens] Morfologiia. 2010;137:58–61. [PubMed] [Google Scholar]
- Anichkov N, Danilova I, Vasiliev O, et al. Use of a polyguanidine solution for fixing biological and anatomical specimens. Neurosci Behav Physiol. 2011;41:18–21. [Google Scholar]
- Aziz MA, McKenzie JC, Wilson JS, et al. The human cadaver in the age of biomedical informatics. Anat Rec. 2002;269:20–32. doi: 10.1002/ar.10046. [DOI] [PubMed] [Google Scholar]
- Baadsgaard A, Monge J, Cox S, et al. Human sacrifice and intentional corpse preservation in the Royal Cemetery of Ur. Antiquity. 2011;85:27–42. [Google Scholar]
- Babich H, Stotzky G. A microbial assay for determining the influence of physicochemical environmental factors on the toxicity of organics: phenol. Arch Environ Contam Toxicol. 1985;14:409–415. doi: 10.1007/BF01055526. [DOI] [PubMed] [Google Scholar]
- Baca V, Doubkova A, Kachlik D, et al. [Teaching arthroscopy techniques at the Educational Center for Clinical Anatomy and Endoscopy (ECAE), Department of Anatomy, 3rd Faculty of Medicine, Charles University in Prague] Acta Chir Orthop Traumatol Cech. 2006;73:356–358. [PubMed] [Google Scholar]
- Barton DPJ, Davies DC, Mahadevan V, et al. Dissection of soft-preserved cadavers in the training of gynaecological oncologists: report of the first UK workshop. Gynecol Oncol. 2009;113:352–356. doi: 10.1016/j.ygyno.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Beck JB. USA: Gold Crest Chemical Corporation Inc; 1966. Arterial embalming fluid and method for embalming therewith. US Patent Office US Patent 3.293.127. [Google Scholar]
- Bedino JH. Phenol exposure in embalming rooms: part 1. Champion Expanding Encyclopedia of Mortuary Practices. 1994;621:2498–2500. [Google Scholar]
- Bedino JH. Embalming chemistry: glutaraldehyde versus formaldehyde. Champion Expanding Encyclopedia of Mortuary Practices. 2003;649:2614–2632. [Google Scholar]
- Bedino JH. ENIGMA: champion's fourth generation chemostasis infusion chemicals: embalming redefined for the 21st century. Champion Expanding Encyclopedia of Mortuary Practices. 2009;657:2709–2717. [Google Scholar]
- Bedir Y. 2009. Technique of mummies processing. EP Patent 1,790,222.
- Benkhadra M, Bouchot A, Gerard J, et al. Flexibility of Thiel's embalmed cadavers: the explanation is probably in the muscles. Surg Radiol Anat. 2011;33:365–368. doi: 10.1007/s00276-010-0703-8. [DOI] [PubMed] [Google Scholar]
- Bergman EM, van der Vleuten CP, Scherpbier AJ. Why don't they know enough about anatomy? A narrative review. Med Teach. 2011;33:403–409. doi: 10.3109/0142159X.2010.536276. [DOI] [PubMed] [Google Scholar]
- Bisht D, Pal A, Chanotiya C, et al. Terpenoid composition and antifungal activity of three commercially important essential oils against Aspergillus flavus and Aspergillus niger. Nat Prod Res. 2011;25:1993–1998. doi: 10.1080/14786419.2010.521926. [DOI] [PubMed] [Google Scholar]
- Bjorkman N, Nielsen P, Hornshoj Moller V. Removing formaldehyde from embalmed cadavers by percolating the body cavities with dilute ethanol. Acta Anat (Basel) 1986;126:78–83. [PubMed] [Google Scholar]
- Blanton PL, Biggs NL. Density of fresh and embalmed human compact and cancellous bone. Am J Phys Anthropol. 1968;29:39–44. doi: 10.1002/ajpa.1330290113. [DOI] [PubMed] [Google Scholar]
- Blum J. Formol als Conservierungsflüssigkeit. Zool Anz. 1893;16:450–452. [Google Scholar]
- Blum F. Notiz über die Anwendung des Formaldehyds (Formol) als Härtungs und Konservierungsmittel. Anat Anz. 1894;9:229–231. [Google Scholar]
- Blum F. Über Wesen und Wert der Formolhärtung. Ann Anat. 1896;11:718–727. [Google Scholar]
- Bohn S, Bircher AJ. Phenoxyethanol-induced urticaria. Allergy. 2001;56:922–923. doi: 10.1034/j.1398-9995.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- Bolt HM, Basaran N, Duydu Y. Human environmental and occupational exposures to boric acid: reconciliation with experimental reproductive toxicity data. J Toxicol Environ Health A. 2012;75:508–514. doi: 10.1080/15287394.2012.675301. [DOI] [PubMed] [Google Scholar]
- Bolton EE, Wang Y, Thiessen PA. Chapter 12 PubChem: integrated platform of small molecules and biological activities. In: Ralph AW, David CS, et al., editors. Annual Reports in Computational Chemistry. Amsterdam: Elsevier; 2008. pp. 217–241. [Google Scholar]
- Bolton EE, Chen J, Kim S, et al. PubChem3D: a new resource for scientists. J Cheminform. 2011;3:32. doi: 10.1186/1758-2946-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury SA, Hoshino K. An improved embalming procedure for long-lasting preservation of the cadaver for anatomical study. Acta Anat (Basel) 1978;101:97–103. doi: 10.1159/000144954. [DOI] [PubMed] [Google Scholar]
- Brenner E. Human body preservation – old and new techniques. J Anat. 2012;221:76–77. doi: 10.1111/joa.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner E, Maurer H, Moriggl B, et al. The human cadaver as an educational tool – classification and comparison with other educational tools. Ann Anat. 2003;185:229–230. [Google Scholar]
- Brent J, McMartin K, Phillips S, et al. Fomepizole for the treatment of ethylene glycol poisoning. N Engl J Med. 1999;340:832–838. doi: 10.1056/NEJM199903183401102. [DOI] [PubMed] [Google Scholar]
- Brown M. Did the early Chinese preserve corpses? A reconsideration of elite conceptions of death. J East Asian Archaeol. 2002;4:201–223. [Google Scholar]
- Brožek J, Grande F, Anderson JT, et al. Densitometric analysis of body composition: revision of some quantitative assumptions*. Ann N Y Acad Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- Buch HA. [AACA] Mold Inhibition with Benzalkonium Chloride. AACA; 2013. AACA@lists.aecom.yu.edu. [Google Scholar]
- Buckley SA, Clark KA, Evershed RP. Complex organic chemical balms of pharaonic animal mummies. Nature. 2004;431:294–299. doi: 10.1038/nature02849. [DOI] [PubMed] [Google Scholar]
- Burke PA, Sheffner AL. The antimicrobial activity of embalming chemicals and topical disinfectants on the microbial flora of human remains. Health Lab Sci. 1976;13:267–270. [PubMed] [Google Scholar]
- Campbell JW, Margrave JL. Embalming Composition and Method. Houston, TX: EFH, Inc; 1995. [Google Scholar]
- Campbell JW, Margrave JL. 1998. Preservative and embalming fluid. Google Patents.
- Chunhong Y. 2004. Chinese lady Dai leaves Egyptian mummies for dead. CHINAdaily. Available at: http://www.chinadaily.com.cn/english/doc/2004-08/25/content_368631.htm (accessed 18 February 2013)
- Coleman R, Kogan I. An improved low-formaldehyde embalming fluid to preserve cadavers for anatomy teaching. J Anat. 1998;192(Pt 3):443–446. doi: 10.1046/j.1469-7580.1998.19230443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu GM, Beittenmiller MR, Mann F, et al. Clinical anatomy of the prepubic tendon in the dog and a comparison with the cat. J Exper Med Surg Res. 2007;14:79–83. [Google Scholar]
- Currey JD, Brear K, Zioupos P, et al. Effect of formaldehyde fixation on some mechanical properties of bovine bone. Biomaterials. 1995;16:1267–1271. doi: 10.1016/0142-9612(95)98135-2. [DOI] [PubMed] [Google Scholar]
- Dayton S, Hashimoto S, Pearce ML. Influence of a diet high in unsaturated fat upon composition of arterial tissue and atheromata in man. Circulation. 1965;32:911–924. doi: 10.1161/01.cir.32.6.911. [DOI] [PubMed] [Google Scholar]
- De Crop A, Bacher K, Van Hoof T, et al. Correlation of contrast-detail analysis and clinical image quality assessment in chest radiography with a human cadaver study. Radiology. 2012;262:298–304. doi: 10.1148/radiol.11110447. [DOI] [PubMed] [Google Scholar]
- De Groot AC, Flyvholm M-A, Lensen G, et al. Formaldehyde-releasers: relationship to formaldehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde-releasers. Contact Dermatitis. 2009;61:63–85. doi: 10.1111/j.1600-0536.2009.01582.x. [DOI] [PubMed] [Google Scholar]
- Driessen-Van het Reve JJ. De Kunstkamera van Peter de Grote: de Hollandse inbreng, gereconstrueerd uit brieven van Albert Seba en Johann Daniel Schumacher uit de jaren 1711–1752. Hilversum: Uitgeverij Verloren; 2006. [Google Scholar]
- Eisma R, Mahendran S, Majumdar S, et al. A comparison of Thiel and formalin embalmed cadavers for thyroid surgery training. Surgeon. 2011;9:142–146. doi: 10.1016/j.surge.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Eisma R, Lamb C, Soames RW. From formalin to thiel embalming: what changes? One anatomy department's experiences. Clin Anat. 2013;26:564–571. doi: 10.1002/ca.22222. [DOI] [PubMed] [Google Scholar]
- Erskine CA. [The results of 10 years of experimental research on the anatomical preservative solutions for the prevention of the drving out of tissues and fungus infections] Anat Anz. 1961;109:348–350. [PubMed] [Google Scholar]
- European Commission. Brussels: 2005. Commission Regulation (EC) No 1048/2005 of 13 June 2005 amending Regulation (EC) No 2032/2003 on the second phase of the 10-year work programme referred to in Article 16(2) of Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market Commission Regulation (EC) 1048/2005. [Google Scholar]
- European Commission. Brussels: 2010. Commission Decision of 8 February 2010 concerning the non-inclusion of certain substances in Annex I, IA or IB to Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market 2010/72/EU. [Google Scholar]
- European Commission. Brussels: 2012. Commission Decision of 9 February 2012 concerning the non-inclusion of certain substances in Annex I, IA or IB to Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market 2012/78/EU. [Google Scholar]
- European Parliament and Council. Brussels: 1998. Directive 98/8/EC of the European Parliament and of the Council of 16 February 1998 concerning the placing of biocidal products on the market 98/8/EC. [Google Scholar]
- European Parliament and Council. Brussels: 2012. Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products EU/528/2012. [Google Scholar]
- Ezugworie J, Anibeze C, Ozoemena R. Trends in the development of embalming methods. Internet J Altern Med. 2009;7 doi: 10.5580/29b. [Google Scholar]
- Feigl G, Kos I, Anderhuber F, et al. Development of surgical skill with singular neurectomy using human cadaveric temporal bones. Ann Anat. 2008;190:316–323. doi: 10.1016/j.aanat.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Fessel G, Frey K, Schweizer A, et al. Suitability of Thiel embalmed tendons for biomechanical investigation. Ann Anat. 2011;193:237–241. doi: 10.1016/j.aanat.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Fonseca A, Cherubini K, Veeck E, et al. Effect of 10% formalin on radiographic optical density of bone specimens. Dentomaxillofac Radiol. 2008;37:137. doi: 10.1259/dmfr/18109064. [DOI] [PubMed] [Google Scholar]
- Fredrick JF. The mathematics of embalming chemistry: part I. A critical evaluation of “one-bottle” embalming chemical claims. De-Ce-Co Magazine. 1968;60:622–624. [reprinted in Mayer, 2012] [Google Scholar]
- Frewein J, Steinmann W, Muller U. [Experiences with reduced formalin fixation of specimens and formalin-free preservation of anatomic samples] Anat Histol Embryol. 1987;16:250–253. [PubMed] [Google Scholar]
- Friedman EA, Greenberg JB, Merrill JP, et al. Consequences of ethylene glycol poisoning: report of four cases and review of the literature. Am J Med. 1962;32:891–902. doi: 10.1016/0002-9343(62)90035-9. [DOI] [PubMed] [Google Scholar]
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271–278. doi: 10.1002/ar.1092080214. [DOI] [PubMed] [Google Scholar]
- Gannal JN. History of Embalming: And of Preparations in Anatomy, Pathology, and Natural History; Including an Account of a New Process for Embalming. Philadelphia: J. Dobson; 1840. [Google Scholar]
- Gerota D. Ueber die Anwendung des Formols in der topographischen Anatomie. Anat Anz. 1896;11:417–420. [Google Scholar]
- Giger U, Frésard I, Häfliger A, et al. Laparoscopic training on Thiel human cadavers: a model to teach advanced laparoscopic procedures. Surg Endosc. 2008;22:901–906. doi: 10.1007/s00464-007-9502-7. [DOI] [PubMed] [Google Scholar]
- Glob PV. The Bog People: Iron-Age Man Preserved. New York: NYRB Classics; 2004. [Google Scholar]
- Goddard PA, McCue KA. Phenolic compounds. In: Block SS, editor. Disinfection, Sterilization, and Preservation. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 255–281. [Google Scholar]
- Goh JCH, Ang EJ, Bose K. Effect of preservation medium on the mechanical properties of cat bones. Acta Orthop. 1989;60:465–467. doi: 10.3109/17453678909149321. [DOI] [PubMed] [Google Scholar]
- Goyri-O'Neill J, Pais D, Freire de Andrade F, et al. Improvement of the embalming perfusion method: the innovation and the results by light and scanning electron microscopy. Acta Méd Port. 2013;26:188–194. [PubMed] [Google Scholar]
- de Graaf R. Tractatulus de usu siphonis in anatomia. In: de Graaf R, editor. De virorum organis generationi inservientibus, de clijsteribus et de usu siphonis in anatomia. Lugdunum Batavorum: Hackian; 1668. pp. 215–234. [Google Scholar]
- Grönroos H. Zusammenstellung der üblichen Konservierungsmethoden für Präpariersaalzwecke. Anat Anz. 1898;15:61–84. [Google Scholar]
- Guimarães Da Silva RM, Matera JM, Coppi Maciel Ribeiro AA. Preservation of cadavers for surgical technique training. Vet Surg. 2004;33:606–608. doi: 10.1111/j.1532-950x.2004.04083.x. [DOI] [PubMed] [Google Scholar]
- Hammer N, Loffler S, Feja C, et al. Ethanol-glycerin fixation with thymol conservation: a potential alternative to formaldehyde and phenol embalming. Anat Sci Educ. 2012;5:225–233. doi: 10.1002/ase.1270. [DOI] [PubMed] [Google Scholar]
- Hess O. Der Formaldehyd; seine Darstellung, Eigenschaften und seine Verwendung als Conservierungs-, therapeutisches und Desinfectionsmittel: mit besonderer Berücksichtigung der Wohnungsdesinfection. Marburg: Elwert; 1901. [Google Scholar]
- Hesse W. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2000. Phenolic resins. [Google Scholar]
- Hopwood D. Fixatives and fixation: a review. Histochem J. 1969;1:323–360. doi: 10.1007/BF01003278. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Ugawa A, Kazihara Y, et al. Arterial patterns in the hand based on a three-dimensional analysis of 220 cadaver hands. J Hand Surg. 1988;13:501–509. doi: 10.1016/s0363-5023(88)80085-6. [DOI] [PubMed] [Google Scholar]
- Janczyk P, Weigner J, Luebke-Becker A, et al. Nitrite pickling salt as an alternative to formaldehyde for embalming in veterinary anatomy – a study based on histo-and microbiological analyses. Ann Anat. 2011a;193:71–75. doi: 10.1016/j.aanat.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Janczyk P, Weigner J, Luebke-Becker A, et al. A pilot study on ethanol-polyethylene glycol-formalin fixation of farm animal cadavers. Berl Munch Tierarztl Wochenschr. 2011b;124:225–227. [PubMed] [Google Scholar]
- Jaung R, Cook P, Blyth P. A comparison of embalming fluids for use in surgical workshops. Clin Anat. 2011;24:155–161. doi: 10.1002/ca.21118. [DOI] [PubMed] [Google Scholar]
- Jimenez Collado J, Arene Rada E, Chavez Inzunza R. Compositions Containing Dialkyl (C 1-C 6)-Ketone Peroxide for the Preservation of Animal and Human Dead Tissues. Madrid: Universidad, Complutense De Madrid; 1999. [Google Scholar]
- Johnson EC, Johnson GR, Johnson M. The origin and history of embalming. In: Mayer RG, editor. Embalming – History, Theory, and Practice. New York: McGraw-Hill; 2012. pp. 467–509. [Google Scholar]
- Jores L. Die Conservirung anatomischer Präparate in Blutfarbe mittelst Formalin. Centralbl f allgemeine Path und Path Anat. 1896;7:134. [Google Scholar]
- Jores L. Ueber eine verbesserte Methode der Konservierung anatomischer Objekte. Münch Med Wschr. 1913;60:976. [Google Scholar]
- Kaup Y, Schmid M, Middleton A, et al. Borate in mummification salts and bones from Pharaonic Egypt. J Inorg Biochem. 2003;94:214–220. doi: 10.1016/s0162-0134(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Khaliq F, Tripathi P. Acute effects of formalin on pulmonary functions in gross anatomy laboratory. Indian J Physiol Pharmacol. 2009;53:93–96. [PubMed] [Google Scholar]
- Kleinrensink GJ. Anatomieonderwijs in Rotterdam: van eikenbast in wijnazijn tot operaties zonder pijn. Rotterdam: Kleinrensink GJ; 2011. [Google Scholar]
- Kurz H. Die Entwicklung moderner Konservierungsmethoden. Der Präparator. 1977;24:180–187. /1978) [Google Scholar]
- Lakchayapakorn K, Watchalayarn P. Formaldehyde exposure of medical students and instructors and clinical symptoms during gross anatomy laboratory in Thammasat University. J Med Assoc Thai. 2010;93(Suppl. 7):S92–S98. [PubMed] [Google Scholar]
- Laskowski S. L'embaumement, la conservation des sujets et les préparations anatomiques. H. Georg: Genève-Bâle-Lyon; 1886. [Google Scholar]
- Lischka MF, Wewalka G, Stanek G. Methoden der Qualitätskontrolle bei Studienleichen. Verh Anat Ges. 1979a;73:1213–1215. [Google Scholar]
- Lischka MF, Wewalka G, Stanek G, et al. Vergleichsuntersuchungen der Desinfektionswirkung verschiedener Konservierungslösungen für Anatomieleichen. Anat Anz. 1979b;146:295–306. [PubMed] [Google Scholar]
- Lischka MF, Krammer EB, Egger TP, et al. Zur Präparatequalität von anatomischen Studienleichen: Vergleich verschiedener phenolfreier Rezepturen mit der klassischen Phenol-Formalin-Methode. Anat Anz. 1981;150:226–234. [PubMed] [Google Scholar]
- Logan B. The long-term preservation of whole human cadavers destined for anatomical study. Ann R Coll Surg Engl. 1983;65:333. [Google Scholar]
- Longrigg J. Anatomy in Alexandria in the third century B.C. Br J Hist Sci. 1988;21:455–488. doi: 10.1017/s000708740002536x. [DOI] [PubMed] [Google Scholar]
- Lovell CR, White IR, Boyle J. Contact dermatitis from phenoxyethanol in aqueous cream BP. Contact Dermatitis. 1984;11:187. doi: 10.1111/j.1600-0536.1984.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Lusk WC. The disinfection of vitalized tissues and the healing of wounds with chinosol and salt. Proc Soc Exp Biol Med. 1919;16:104–110. doi: 10.1097/00000658-191905000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald GJ, MacGregor DB. Procedures for embalming cadavers for the dissecting laboratory. Proc Soc Exp Biol Med. 1997;215:363–365. doi: 10.3181/00379727-215-44144. [DOI] [PubMed] [Google Scholar]
- Majewski P, Pernak A, Grzymislawski M, et al. Ionic liquids in embalming and tissue preservation. Can traditional formalin-fixation be replaced safely? Acta Histochem. 2003;105:135–142. doi: 10.1078/0065-1281-00707. [DOI] [PubMed] [Google Scholar]
- Manzoli FA, Maraldi NM, Capitani S, et al. In memoriam of Prof. Giovanni Mazzotti. Eur J Histochem. 2011;55:175. [Google Scholar]
- Marquet PA, Santoro CM, Latorre C, et al. Emergence of social complexity among coastal hunter-gatherers in the Atacama Desert of northern Chile. Proc Natl Acad Sci. 2012;109:14754–14760. doi: 10.1073/pnas.1116724109. doi: 10.1073/pnas.11164724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Gil J, Martin-Gil FJ, Delibes-de-Castro G, et al. The first known use of vermillion. Experientia. 1995;51:759–761. doi: 10.1007/BF01922425. [DOI] [PubMed] [Google Scholar]
- Mayer RG. Embalming: History, Theory, and Practice. New York: McGraw-Hill; 2012. [Google Scholar]
- McKone HT. Embalming – chemistry for eternity. Chem Matters. 1999;17:12–13. [Google Scholar]
- Mills PR. Preparation and Presentation of Anatomical Specimens at the University of Sydney. Sydney, NSE: Anatomy Department, University of Sydney; 2010. [Google Scholar]
- Mirabelli MC, Holt SM, Cope JM. Anatomy laboratory instruction and occupational exposure to formaldehyde. Occup Environ Med. 2011;68:375–378. doi: 10.1136/oem.2010.059352. [DOI] [PubMed] [Google Scholar]
- Mu L, Sanders I. Sihler's whole mount nerve staining technique: a review. Biotech Histochem. 2010;85:19–42. doi: 10.1080/10520290903048384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. 1,4-dichlorobenzene: CID=4685. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=4685 (accessed on 23 August 2013)
- National Center for Biotechnology Information. 4-chloro-3-methylphenol: CID=1732. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1732 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Benzalkonium chloride: CID=15865. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=15865 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Boric acid: CID=7628. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=7628 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Chinosol: CID=517000. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=517000 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Chloral Hydrate: CID=2707. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=2707 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Diethylene glycol: CID=8117. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8117 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Ethanol: CID=702. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=702 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Eugenol: CID=3314. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3314 (accessed on 27 August 2013)
- National Center for Biotechnology Information. Formaldehyde: CID=712. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=712 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Glutaraldehyde: CID=3485. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3485 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Glycerine: CID=753. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=753 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Guaiacol: CID=460. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=460 (accessed on 27 August 2013)
- National Center for Biotechnology Information. Isopropanol: CID=3776. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3776 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Methanol: CID=887. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=887 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Monoethylene glycol: CID=174. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=174 (accessed on 23 August 2013)
- National Center for Biotechnology Information. N-tetradecylamine: CID=16217. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=16217 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Pentachlorphenol: CID=21977422. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=21977422 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Phenol: CID=996. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=996 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Phenoxyethanol: CID=31236. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=31236 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Salicylic acid: CID=338. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=338 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Sodium 2-sulphonatoethyl laurate: CID=23668826. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=23668826 (accessed on 27 August 2013)
- National Center for Biotechnology Information. Sodium lauryl sulfate: CID=3423265. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3423265 (accessed on 27 August 2013)
- National Center for Biotechnology Information. Sodium nitrate: CID=24268. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=24268 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Sorbitol: CID=5780. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5780 (accessed on 27 August 2013)
- National Center for Biotechnology Information. Tetrakis(hydroxymethyl)phosphonium chloride: CID=31298. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=31298 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Thymol: CID=6989. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6989 (accessed on 23 August 2013)
- National Center for Biotechnology Information. Vanillic aldehyde: CID=1183. PubChem Compound Database. Available at: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1183 (accessed on 27 August 2013)
- National Research Council (US) Committee on Aldehydes. Formaldehyde and Other Aldehydes. Washington, DC: Natl Academy Press; 1981. [Google Scholar]
- National Toxicology Program. Final report on carcinogens background document for formaldehyde. Rep Carcinog Backgr Doc. 2010;10-5981:i-512. [PubMed] [Google Scholar]
- Nazarian A, Hermannsson BJ, Muller J, et al. Effects of tissue preservation on murine bone mechanical properties. J Biomech. 2009;42:82–86. doi: 10.1016/j.jbiomech.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Nicholson HD, Samalia L, Gould M, et al. A comparison of different embalming fluids on the quality of histological preservation in human cadavers. Eur J Morphol. 2005;42:178–184. doi: 10.1080/09243860500473306. [DOI] [PubMed] [Google Scholar]
- O'Brien KL, Selanikio JD, Hecdivert C, et al. Epidemic of pediatric deaths from acute renal failure caused by diethylene glycol poisoning. JAMA. 1998;279:1175–1180. doi: 10.1001/jama.279.15.1175. [DOI] [PubMed] [Google Scholar]
- OECD. Series on Pesticides No. 9. Paris: Organisation for Economic Co-operation and Development, Environment Directorate; 1999. Report of the survey of OECD Member Countries' approaches to the regulation of biocides; pp. 1–202. OECD Environmental Health and Safety Publications. [Google Scholar]
- Ohman C, Dall'Ara E, Baleani M, et al. The effects of embalming using a 4% formalin solution on the compressive mechanical properties of human cortical bone. Clin Biomech (Bristol, Avon) 2008;23:1294–1298. doi: 10.1016/j.clinbiomech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Ohmichi K, Komiyama M, Matsuno Y, et al. Formaldehyde exposure in a gross anatomy laboratory–personal exposure level is higher than indoor concentration. Environ Sci Pollut Res Int. 2006;13:120–124. doi: 10.1065/espr2005.06.265. [DOI] [PubMed] [Google Scholar]
- Oliveira L, Lage A, Clemente MP, et al. Saratov Fall Meeting 2006: Optical Technologies in Biophysics and Medicine VIII. International Society for Optics and Photonics; 2007. Concentration dependence of the optical clearing effect created in muscle immersed in glycerol and ethylene glycol. pp. 653511–653511-12, [Google Scholar]
- Oliveira L, Lage A, Clemente MP, et al. Rat muscle opacity decrease due to the osmosis of a simple mixture. J Biomed Opt. 2010;15 doi: 10.1117/1.3486539. 055004–055004-9. [DOI] [PubMed] [Google Scholar]
- O'Sullivan E, Mitchell BS. An improved composition for embalming fluid to preserve cadavers for anatomy teaching in the United Kingdom. J Anat. 1993;182(Pt 2):295–297. [PMC free article] [PubMed] [Google Scholar]
- Oulé MK, Azinwi R, Bernier AM, et al. Polyhexamethylene guanidine hydrochloride-based disinfectant: a novel tool to fight meticillin-resistant Staphylococcus aureus and nosocomial infections. J Med Microbiol. 2008;57:1523–1528. doi: 10.1099/jmm.0.2008/003350-0. [DOI] [PubMed] [Google Scholar]
- Owen G, Steedman HF. Preservation of animal tissue, with a note on staining solutions. Q J Microsc Sci. 1956;97:319–321. [Google Scholar]
- Owen G, Steedman HF. Preservation of molluscs. Proc Malac Soc (London) 1958;33:101–103. [Google Scholar]
- Papageorgopoulou C, Xirotiris NI, Iten PX, et al. Indications of embalming in Roman Greece by physical, chemical and histological analysis. J Archaeol Sci. 2009;36:35–42. [Google Scholar]
- Pate JR. Preservation of anatomical specimens. Science. 1938;87:586. doi: 10.1126/science.87.2269.586. [DOI] [PubMed] [Google Scholar]
- Peters E. Eine verbilligte, farberhaltende Konservierungsmethode für Präparationsmaterial zu Lehr-und Übungszwecken. Anat Anz. 1956;103:89–91. [PubMed] [Google Scholar]
- Peuker E, Werkmeister R, Pera F, et al. [Surgical procedures in mouth, jaw and facial surgery in Thiel embalmed body donors] Mund-, Kiefer-und Gesichtschirurgie. 2001;5:141. doi: 10.1007/PL00010796. [DOI] [PubMed] [Google Scholar]
- Piombino-Mascali D. Il Maestro del sonno eterno. Palermo: La Zisa; 2009. [Google Scholar]
- Piombino-Mascali D, Aufderheide AC, Johnson-Williams M, et al. The Salafia method rediscovered. Virchows Arch. 2009;454:355–357. doi: 10.1007/s00428-009-0738-6. [DOI] [PubMed] [Google Scholar]
- Platzer W, Putz R, Poisel S. Ein neues Konservierungs-und Aufbewahrungssystem für anatomisches Material. Acta Anat (Basel) 1978;102:60–67. [PubMed] [Google Scholar]
- Powers RF. McMaster's Embalming Fluid. Hamilton, ON: American Association of Clinical Anatomists; 2003. p. 1. [Google Scholar]
- Prasad Rai B, Tang B, Eisma R, et al. A qualitative assessment of human cadavers embalmed by Thiel's method used in laparoscopic training for renal resection. Anat Sci Educ. 2012;5:182–186. doi: 10.1002/ase.1267. [DOI] [PubMed] [Google Scholar]
- Pretorius W. Formula for embalming of cadavers for student dissection and the modification thereof for plastination. J Int Soc Plastination. 1996;10:35–36. [Google Scholar]
- Pulvertaft RJ. Museum techniques; a review. J Clin Pathol. 1950;3:1–23. doi: 10.1136/jcp.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz R, Poisel S, Tiefenbrunner F. Probleme mit Konservierungsflüssigkeiten im anatomischen Präpariersaalbetrieb. Acta Anat (Basel) 1974;90:394–402. [PubMed] [Google Scholar]
- Raja DS, Sultana B. Potential health hazards for students exposed to formaldehyde in the gross anatomy laboratory. J Environ Health. 2012;74:36–40. [PubMed] [Google Scholar]
- Richins CA, Roberts EC, Zeilmann JA. Improved fluids for anatomical embalming and storage. Anat Rec. 1963;146:241–243. doi: 10.1002/ar.1091460309. [DOI] [PubMed] [Google Scholar]
- Rodrigues H. Técnicas anatômicas. Vitória, Brazil: Arte Visual; 1998. [Google Scholar]
- Rogers RD, Seddon KR. Ionic liquids–solvents of the future? Science. 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- Salafia A. Nuovo metodo speciale per la conservazione del cadavere umano intero allo stato permanentemente fresco. Palermo: Institute for Mummies and the Iceman, EURAC, Bolzano; 1927. (ca. –1933) [Google Scholar]
- Sampaio F. Estudo do Crescimento do Rim Humano Durante o Período Fetal (Study of the Growth of the Human Kidney During the Fetal Period) São Paulo, Brazil: Escola Paulista de Medicina; 1989. [Google Scholar]
- Sárraga C, Gil M, Arnau J, et al. Effect of curing salt and phosphate on the activity of porcine muscle proteases. Meat Sci. 1989;25:241–249. doi: 10.1016/0309-1740(89)90042-9. [DOI] [PubMed] [Google Scholar]
- Saupe N, Pfirrmann CWA, Schmid MR, et al. MR imaging of cartilage in cadaveric wrists: comparison between imaging at 1.5 and 3.0 T and gross pathologic inspection 1. Radiology. 2007;243:180–187. doi: 10.1148/radiol.2431060294. [DOI] [PubMed] [Google Scholar]
- Schep LJ, Slaughter RJ, Temple WA, et al. Diethylene glycol poisoning. Clin Toxicol. 2009;47:525–535. doi: 10.1080/15563650903086444. [DOI] [PubMed] [Google Scholar]
- Schiefferdecker P. Verwendung von Chinosol zur Conservierung der Leichen auf dem Secirsaale. Sitzungsberichte der Niederrheinischen Gesellschaft für Natur-und Heilkunde. 1897;47:11. [Google Scholar]
- Seidler H, Bernhard W, Teschler-Nicola M, et al. Some anthropological aspects of the prehistoric Tyrolean ice man. Science. 1992;258:455. doi: 10.1126/science.1411539. [DOI] [PubMed] [Google Scholar]
- Seiler HG, Sigel H, Sigel A. Handbook on Toxicity of Inorganic Compounds. New York, NY: Marcel Dekker Inc; 1988. [Google Scholar]
- Sharquie KE, Najim RA. Embalming with honey. Saudi Med J. 2004;25:1755–1756. [PubMed] [Google Scholar]
- Shi KQ, Shao SX, Yin WG. An improved non-formaldehyde tissue preservative. Adv Mat Res. 2012;356:360–363. [Google Scholar]
- Silva RMG, Matera JM, Ribeiro AACM. New alternative methods to teach surgical techniques for veterinary medicine students despite the absence of living animals. Is that an academic paradox? Anat Histol Embryol. 2007;36:220–224. doi: 10.1111/j.1439-0264.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- Slieker JC, Theeuwes HP, Rooijen GL, et al. Training in laparoscopic colorectal surgery: a new educational model using specially embalmed human anatomical specimen. Surg Endosc. 2012;26:2189–2194. doi: 10.1007/s00464-012-2158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchon E. Embalming of bodies for teaching purposes. Anat Rec. 1908;2:244–247. [Google Scholar]
- Spence TF. Teaching and Display Techniques in Anatomy and Zoology. Oxford: Pergamon Press; 1967. [Google Scholar]
- Spriggs A. The Art and Science of Embalming. Springfield, OH: Champion Co; 1963. [Google Scholar]
- Stieger C, Candreia C, Kompis M, et al. Laser Doppler vibrometric assessment of middle ear motion in Thiel-embalmed heads. Otol Neurotol. 2012;33:311–318. doi: 10.1097/MAO.0b013e3182487de0. [DOI] [PubMed] [Google Scholar]
- Suruda A. Morticians. In: Greenberg MI, Hamilton RJ, Phillips SD, McCluskey GJ, editors. Occupational, Industrial, and Environmental Toxicology. Philadelphia, PA: Mosby Inc; 2003. pp. 274–283. [Google Scholar]
- Svidovyi VI, Riabinin IA, Vasil'ev OD. [The microflora associated with anatomical embalming as a harmful factor of a working process] Gig Sanit. 2011:51–53. Available at: http://www.medlit.ru/en/journal/291 [accessed on 15 January 2014] [PubMed] [Google Scholar]
- Takahashi S, Tsuji K, Fujii K, et al. Prospective study of clinical symptoms and skin test reactions in medical students exposed to formaldehyde gas. J Dermatol. 2007;34:283–289. doi: 10.1111/j.1346-8138.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Thiel W. Die Konservierung ganzer Leichen in natürlichen Farben. Ann Anat. 1992;174:185–195. [PubMed] [Google Scholar]
- Thiel W. Ergänzung für die Konservierung ganzer Leichen nach W. Thiel. Ann Anat. 2002;184:267–269. doi: 10.1016/s0940-9602(02)80121-2. [DOI] [PubMed] [Google Scholar]
- Tissier C, Migné V. 2001. Emission scenario document for biocides used in taxidermy and embalming processes. Report developed in the context of the EU project entitled “Gathering, review and development of environmental emission scenarios for biocides” (EUBEES 2)
- Treichel JL, Henry MM, Skumatz CMB, et al. Antioxidants and ocular cell type differences in cytoprotection from formic acid toxicity in vitro. Toxicol Sci. 2004;82:183–192. doi: 10.1093/toxsci/kfh256. [DOI] [PubMed] [Google Scholar]
- Trillat A. Sur les propriétés antiseptiques de la formaldéhyde. Compt Rend Acad Sci Paris. 1892;114:1278. [Google Scholar]
- Trompette P, Lemonnier M. Funeral embalming: the transformation of a medical innovation. Sci Stud. 2009;22:9–30. [Google Scholar]
- Tutsch H. Eine geruchsfreie, gut konservierende Lösung für Kursleichen. Ann Anat. 1975;138:126–128. [PubMed] [Google Scholar]
- Udoaka AI, Oghenemavw L, Ebenezer T. Ancient embalming techniques amongst the Ogoni Tribe in Southern Nigeria. J Exp Clin Anat. 2009;8 doi: 10.4314/jeca.v8i2.55635. [Google Scholar]
- UNECE. Globally harmonized system of classification and labelling of chemicals (GHS). Available at: http://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html (accessed on 27 August 2013)
- Unger S, Blauth M, Schmoelz W. Effects of three different preservation methods on the mechanical properties of human and bovine cortical bone. Bone. 2010;47:1048–1053. doi: 10.1016/j.bone.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Uter W, Schwanitz HJ, Lessmann H, et al. Glyoxal is an important allergen for (medical care) cleaning staff. Int J Hyg Environ Health. 2001;204:251–253. doi: 10.1078/1438-4639-00099. [DOI] [PubMed] [Google Scholar]
- da Vinci L, O'Malley CD. Leonardo on the Human Body. New York: Dover Publ; 1983. [Google Scholar]
- Vohra MS. Personal formaldehyde exposure level in the gross anatomy dissecting room at College of Medicine King Saud University Riyadh. Int J Occup Med Environ Health. 2011;24:108–113. doi: 10.2478/s13382-011-0004-4. [DOI] [PubMed] [Google Scholar]
- Wei CN, Harada K, Ohmori S, et al. Subjective symptoms of medical students exposed to formaldehyde during a gross anatomy dissection course. Int J Immunopathol Pharmacol. 2007;20:23–25. doi: 10.1177/03946320070200S205. [DOI] [PubMed] [Google Scholar]
- Wewalka G, Lischka MF, Krammer EB, et al. Mikrobiologische Überwachung von konservierten Studienleichen während des anatomischen Praparierkurses. Anat Anz. 1979;146:285–294. [PubMed] [Google Scholar]
- Wilke HJ, Werner K, Haussler K, et al. Thiel-fixation preserves the non-linear load-deformation characteristic of spinal motion segments, but increases their flexibility. J Mech Behav Biomed Mater. 2011;4:2133–2137. doi: 10.1016/j.jmbbm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Wineski LE, English AW. Phenoxyethanol as a nontoxic preservative in the dissection laboratory. Acta Anat (Basel) 1989;136:155–158. doi: 10.1159/000146816. [DOI] [PubMed] [Google Scholar]
- Wolff KD, Kesting M, Mücke T, et al. Thiel embalming technique: a valuable method for microvascular exercise and teaching of flap raising. Microsurgery. 2008;28:273–278. doi: 10.1002/micr.20484. [DOI] [PubMed] [Google Scholar]
- Woodburne RT, Lawrence CA. An improved embalming fluid formula. Anat Rec. 1952;114:507–514. doi: 10.1002/ar.1091140309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summative table of substances used in modern anatomical embalming.
Table S2. Kaiserling's solutions for color and form preservation (Pulvertaft, 1950).
Table S3. Jores' fixative solution (Bradbury & Hoshino, 1978).
Table S4. Enhanced embalming fluid by Woodburne & Lawrence (1952).
Table S5. Peters' salt solutions (Peters, 1956).
Table S6. Dublin embalming fluid (Erskine, 1961).
Table S7. Richins' solutions (Richins et al. 1963).
Table S8. Tüubingen embalming fluid (Tutsch, 1975).
Table S9. Bradbury and Hoshino's embalming fluid (Bradbury & Hoshino, 1978).
Table S10. Solutions published by Platzer et al. (1978).
Table S11. Coleman and Kogan's preservation (Coleman & Kogan, 1998).
Table S12. ‘New Basler solution’ (Kurz, 1977/1978; Frølich et al. 1984).
Table S13. Bergen solution, used until 1979 (Frølich et al. 1984).
Table S14. Modified Kurz arterial embalming fluid (Frewein et al. 1987).
Table S15. Thiel's solutions (either in millilitres for liquids or grams for solids; Thiel, 2002).
Table S16. Proposed ‘new‘ Southampton embalming fluid (O'Sullivan & Mitchell, 1993).
Table S17. McMaster's solutions (Powers, 2003).
Table S18. Anatomy Institute of Sidney University's embalming fluids (Mills, 2010).
Table S19. Table of hazards of substances used in modern anatomical embalming.
Table S20. Product types of the Biocidal Products Directive (98/8/EC).


