Abstract
We sought to examine, via Phosphorus-31 magnetic resonance spectroscopy (31P-MRS) in a case-control design, whether bioenergetic deficits in autism spectrum disorders extend to brain and muscle. Six cases with autism spectrum disorder with suspected mitochondrial dysfunction (age 6–18 years) and six age/sex-matched controls underwent 31P-MRS. The outcomes of focus were muscle resting phosphocreatine and intracellular pH; as well as post-exercise phosphocreatine-recovery-time-constant; and frontal brain phosphocreatine. Intracellular muscle pH was lower in each autism spectrum disorder case than their matched control (6/6, p=0.03; p=0.0048 paired t-test). Muscle phosphocreatine (5/6), brain phosphocreatine (3/4), and muscle phosphocreatine-recovery-time-constant (3/3) trends were in the predicted direction (not all participants completed each). This study introduces 31P-MRS as a noninvasive tool for assessment of mitochondrial function in autism spectrum disorder enabling bioenergetic assessment in brain; and provides preliminary evidence suggesting bioenergetic defects in cases with autism spectrum disorder are present in muscle and may extend to brain.
Keywords: Autism Spectrum Disorder, 31P-MRS, brain, muscle, bioenergetics
Introduction
There is mounting theoretical and empirical support for mitochondrial predisposition and dysfunction, as well as energetic impairment, in cases of autism spectrum disorders1–5.
With the striking upsurge in autism spectrum disorder, efforts to understand this condition have assumed new urgency and noninvasive tools are needed to further examine mitochondrial function and energetics in autism spectrum disorder. Noninvasive assessments are desired, ideally accommodating investigation of brain (the organ of primary interest in autism spectrum disorder) and muscle (the other organ most classically affected in mitochondrial pathology) - both are postmitotic tissues with high energetic demand6, 7.
We sought to evaluate muscle as well as brain energetics in a pilot study of autism spectrum disorder cases versus controls using in vivo Phosphorus-31 magnetic resonance spectroscopy (31P-MRS). Phosphocreatine levels have been characterized as an index of energy reserve8, and low phosphocreatine in brain and muscle are reported in mitochondrial pathology8, 9. Reduced phosphocreatine can in principle reflect reduced supply or increased demand (as can reduced pH). However, in autism spectrum disorder to date, mounting evidence for mitochondrial defects reinforces prospects for supply-side problems2–5. Phosphocreatine was the sole 31P-MRS brain marker described as “markedly” differing in patients with diagnosed mitochondrial cytopathies, suggesting it would likely be the most sensitive brain index10. The preference for frontal brain phosphocreatine assessment is based on a prior report, in which prefrontal brain phosphocreatine on 31P-MRS was reported to be lower in (older) participants with autism spectrum disorder, though the findings were not placed in a bioenergetic context1. Phosphocreatine recovery following exercise is considered a reliable index of mitochondrial function on 31P-MRS11–14. Muscle lactic acid may be elevated in mitochondrial dysfunction due to increased dependence on anaerobic energy sources which may reduce pH15, and relative elevations in lactic acid in those with autism spectrum disorder have been reported16, 17, providing foundation for emphasis on pH. Thus, we sought to assess phosphocreatine and intracellular pH in resting muscle, phosphocreatine recovery (time constant) following exercise (recognizing that some autism spectrum disorder cases may be unable to complete exercise testing), and phosphocreatine in brain, in autism spectrum disorder cases and matched controls.
To our knowledge, no previous study has endeavored to use 31P-MRS to assess bioenergetics including muscle intracellular pH and phosphocreatine recovery following exercise, in persons with autism spectrum disorder.
Method
Participants
Twelve participants included 4 boys and 2 girls from 6 to 18 years with autism spectrum disorder and suspected mitochondrial dysfunction, and six age- and sex-matched controls (matched 1:1 to cases) from the San Diego area. Recruitment for the study occurred from August 2009 to May 2010, with participants seen from December 2009 through May 2010. Controls had no self-reported past family history of Autism or autism spectrum disorder and were not currently taking any prescription or over-the-counter medications (one took a multivitamin). Cases and controls were referred from University of California, San Diego (Dr. Haas’ group) and University of California, Irvine (Dr. Wallace’s group). Cases were chosen (under supervision of mitochondrial-experts Haas and Wallace) to have factors that raise suspicion for mitochondrial involvement. These included family history of autism spectrum disorder, history of developmental regression, or history of hypotonia. Participants underwent the Autism Diagnostic Interview-Revised18 and Autism Diagnostic Observation Schedule19 to ensure conformity to autism spectrum disorder and non-autism spectrum disorder criteria (by Dr. Lincoln’s group). Participants’ legal guardian gave University of California, San Diego Human Research Protections Program-approved informed consent; and participants gave University of California, San Diego Human Research Protections Program-approved assent, with age-appropriate assent forms.
31P-MRS method (performed by Dr. Hamilton)
31P-MR spectra were acquired on a 3 Tesla GE Signa EXCITE HD scanner (GE Healthcare, Waukesha, WI). The 1H signal was acquired using the body coil for collection of multiplanar localization images and for shimming. Participants were scanned in the supine position. The 31P-MR spectra were collected with a 5-inch diameter surface coil, using a slice selective free induction decay sequence with a repetition time of 3 seconds. Spectra had a sampling interval of 0.2 msec; 2048 data points were collected. For the frontal brain spectra, the coil was placed at the front of the head. Brain spectra were collected at rest with 128 signal averages. For muscle spectra, the coil was placed under the calf. The free induction decay sequence excited a thick slice (60 mm) parallel to the coil. The slice was positioned close to the coil to maximize signal-to-noise whilst attempting to minimize inclusion of surface features in the region close to the coil. The intrinsic weighting of the coil provided the remaining localization. For participants able to complete the exercise protocol, a spectrum was collected every 3 seconds during 2 minutes of rest (providing resting muscle spectra), then 5 minutes of exercise (repetitively pushing a pedal, similar to depressing a car pedal) and 6 minutes of recovery. Resting muscle spectra were secured for participants unable to follow the exercise protocol20.
Figure 1 shows an example of resting brain and muscle spectra; and phosphocreatine rest-exercise-recovery profile.
Figure 1.
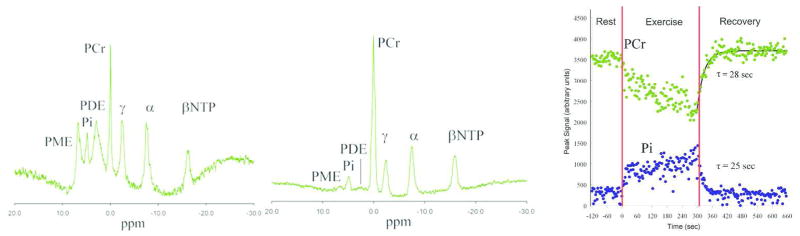
Left: Example of frontal brain 31P-MR Spectra. Middle: Example of resting muscle 31P-MR Spectra. Right: Example of rest-exercise-recovery phosphocreatine response curve (rest −120 to 0 sec, exercise 0 to 300 sec, and recovery 300 to 660 sec) (Control 3).
Movies, music, and weighted blankets were used to reduce anxiety and increase participant comfort. A range of other approaches to foster successful awake, nonsedated scanning were used and have been reported elsewhere20.
31P-MRS analysis
The raw spectra files were transferred and analyzed off-line. All spectroscopy analyses were carried out by a single observer (Dr. Hamilton) who monitored spectral quality during the analysis process, to confirm quality of acquisition. The inherently low signal-to-noise ratio of brain 31P-MRS spectra and the complex overlapping peak structure of the spectra were addressed via AMARES algorithm21 included in the magnetic resonance user interface software program22, using prior knowledge adapted from an approach previously used by Dr. Hamilton at 1.5T23. The first 2ms of the signal was truncated to remove the broad component to the residual, which is the difference between the full signal and the fit of the truncated signal. This broad component corresponds to signals from motion-restricted phospholipid in cell membrane and vesicle bilayers24. For brain and rest muscle spectra, the areas of the peak were expressed as a fraction of the total 31P-MRS signal (sum of the areas of all visible peaks). Post-exercise phosphocreatine recovery time constant (relative change in the area of phosphocreatine) was assessed. For the brain and resting muscle spectra, the phosphocreatine-Pi chemical shift was accurately measured to allow the intracellular pH to be calculated1, 25, 26. The following standard formula was used to calculate the pH:
where δ=chemical shift difference in ppm between Pi and phosphocreatine27. The chemical shift difference was carefully measured manually rather than taking the values from the magnetic resonance user interface fit, as manual measurement produces a more consistent pH value25.
Statistical Analyses
Due to the lack of prior information regarding distribution, effect size and variance, we prespecified use of nonparametric sign tests, examining the sign (direction) of difference between each case and their corresponding control. This simple test has high statistical authority28. No adjustment for multiple comparisons was performed. The predicted direction (sign) of difference between autism spectrum disorder cases and their respective controls were, lower phosphocreatine and lower intracellular pH in autism spectrum disorder; and prolonged time constant of recovery for phosphocreatine recovery following exercise. Sample size for this pilot assessment was constrained by funding. For the sign test, two-sided significance for a variable could be achieved only if all six pairs had data, for each member of the pair, and all were in the predicted direction. Missing data on any outcome from either member of a case-control pair prevented that case-control pair from being included in assessment for that outcome. (Autism spectrum disorder cases had challenges that could preclude completion of all assessments.) Paired t-tests often have greater power for continuous data, and were also assessed.
Results
Participant Characteristics
Participant characteristics are shown in Supplement Table A. Supplement Table B shows features that contributed to referral of the cases and subsequent test findings. The designation of certainty of mitochondrial disease diagnosis is based on Bernier criteria29. Autism Diagnostic Observation Schedule19 and Autism Diagnostic Interview-Revised18 scores are shown in Supplement Table C/D: cases met criteria for autism spectrum disorder, while controls did not. Results of other mitochondrial testing on these participants will be reported separately (by the Haas/Naviaux and Wallace groups).
Data Acquisition
Resting muscle parameters (including phosphocreatine and intracellular pH) were completed for all participants. Exercise and recovery testing was completed in all controls, but only successfully completed in three cases, providing three comparisons.
Frontal phosphocreatine was completed for four case-control pairs. For two pairs, no comparison was possible: One case could not complete the procedure, and one control (for a different case) had extensive braces precluding interpretation of the frontal scan.
Because frontal brain and muscle recovery profiles had fewer than five evaluable comparisons, precluding sign-test significance irrespective of findings, primary outcomes were revised to comprise muscle resting phosphocreatine and intracellular pH (for which data were available); other assessments were designated exploratory.
Magnetic Resonance Spectroscopy
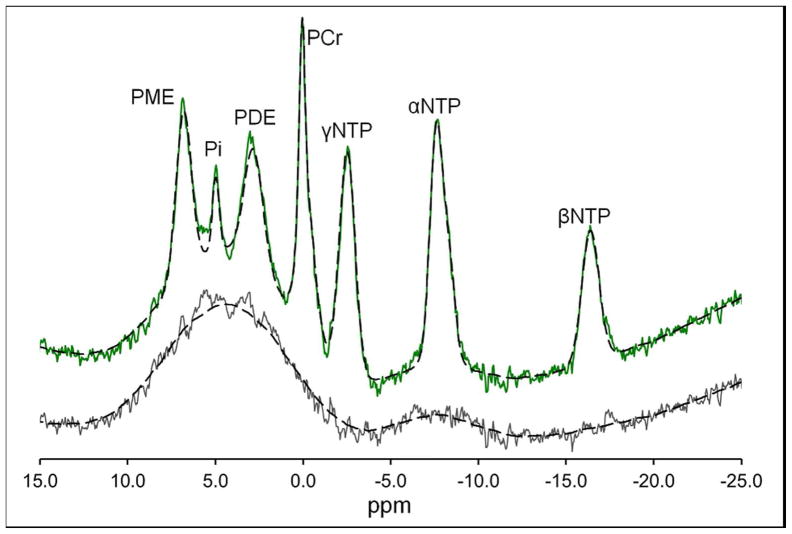
Figure 1 illustrates 31P-MRS muscle resting spectrum as well as recovery profile after exercise; and spectrum in frontal brain. The fit of the same spectra is shown in Figure 2. The residue shows the underlying broad component from signals from motion-restricted phospholipid. Also shown is the smoothed fit (100 point moving average) of the residue and the sum of the smoothed fit and magnetic resonance user interface fit. The smoothed fit is added to the magnetic resonance user interface fit to allow comparison of the raw spectrum and the fitted spectra. The muscle spectra showed a far smaller broad component.
Figure 2.
Frontal brain 31P MR Spectrum and residue from Control 3. The residue shows the broad resonance underling the spectrum. Also shown are the fit of the residue (100 point moving average) and the magnetic resonance user interface fit added to the residue fit.
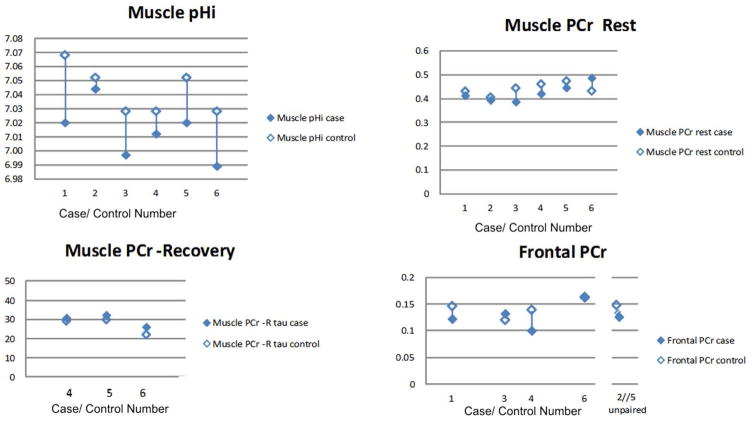
Table 1 and Figure 3 show the primary and secondary outcome findings for 31P-MRS in cases and controls. Muscle intracellular pH was lower in cases than in controls (predicted) for each of the six pairs (p=0.032 sign-test, p=0.0048 paired t-test). The intracellular pH for each case (with only one exception) fell below that for any control. Resting muscle phosphocreatine was lower in cases than controls for five of six pairs (provisionally positive but not significant).
Table 1.
31P-MRS Results
| Muscle pHi | Muscle PCr (rest) | Muscle PCr-R (τ) | Frontal PCr | |
|---|---|---|---|---|
| Case 1 | 7.020 | 0.415 | Not interpretable | 0.122 |
| Control 1 | 7.068 | 0.433 | 43.2 | 0.146 |
| Case 2 | 7.044 | 0.396 | Not completed | Not completed |
| Control 2 | 7.052 | 0.408 | 19.8 | 0.148 |
| Case 3 | 6.997 | 0.389 | Not completed | 0.132 |
| Control 3 | 7.028 | 0.446 | 28.1 | 0.120 |
| Case 4 | 7.012 | 0.423 | 30.9 | 0.100 |
| Control 4 | 7.028 | 0.463 | 29.3 | 0.139 |
| Case 5 | 7.020 | 0.448 | 32.4 | 0.126 |
| Control 5 | 7.052 | 0.476 | 30.0 | Not interpretable |
| Case 6 | 6.989 | 0.489 | 26.2 | 0.162 |
| Control 6 | 7.028 | 0.434 | 22.2 | 0.163 |
| #Expected: #Contrary (Sign of case-control difference) | 6:0 | 5:1 | 3:0 | 3:1 |
| 1-sided p (sign test) | 0.016 | 0.11 | 0.125 | 0.31 |
| 1-sided p (paired t-test) | 0.0024 | 0.17 | 0.031 | 0.17 |
PCr = phosphocreatine; PCr-R = phosphocreatine recovery following exercise; pHi = intracellular pH.
Phosphocreatine is in ratio to total peaks (a fraction of total signal)
Except for case 2, intracellular pH of each case is lower than that of any control.
Figure 3.
Case-control comparison for 31P-MRS assessments
Three cases could not complete the exercise testing. Of note, the case with the aberrantly high phosphocreatine value for both brain and muscle weighed approximately 300lbs (at the limit of acceptability for the magnetic resonance machine).
Frontal brain phosphocreatine was lower in cases than controls (predicted) for three of the four pairs for whom both a case and a control were successfully tested (nonsignificant). Of note, the case for the sixth pair, who had anomalously elevated frontal as well as resting muscle phosphocreatine, was a 300lb male. One control had extensive dental braces creating artifact, leaving their case unpaired; and one case did not complete the test, leaving their control unpaired. Their unpaired partners appeared to fit the overall theme: phosphocreatine of the unpaired control was higher than phosphocreatine for all cases except one (the 300lb male who had elevated frontal and resting muscle phosphocreatine); while phosphocreatine of the unpaired case was lower than phosphocreatine for all but one control.
Muscle phosphocreatine recovery time constant was longer in cases than controls in all three of the pairs where this measure was successfully obtained (nonsignificant with sign test, p=0.03 with one-sided paired t-test, with the caveat that the sample is very small).
Discussion
31P-MRS at rest in muscle and brain of autism spectrum disorder participants, as young as six years of age, show findings provisionally consistent with impaired energetics in brain and muscle in autism spectrum disorder. Muscle intracellular pH was lower in cases for all six comparisons (significant), with phosphocreatine in muscle lower in 5 of 6 pairs, provisionally compatible with prediction. Frontal phosphocreatine bore too few comparisons to enable significance testing, as did phosphocreatine recovery following exercise (though one-sided significance was present on paired t-test), but findings were congruent in direction with prediction, given low phosphocreatine10 and prolonged phosphocreatine recovery following exercise30 in mitochondriopathies. Participants typically had suspicion for possible mitochondrial problems (though mitochondrial defects assessed by other modalities had not been confirmed at the time of referral), and are not necessarily representative of all autism spectrum disorder participants.
We identified one previous study in older autism spectrum disorder participants and controls that used 31P-MRS to examine dorsal prefrontal cortex in older autism spectrum disorder cases and controls, without bioenergetics or mitochondrial function in mind. The study did show significantly lower phosphocreatine in prefrontal brain of adolescent and young adult autism spectrum disorder participants than their matched controls1. Our data conform to those in the direction of phosphocreatine findings in frontal brain, do so with a priori consideration of bioenergetics, add assessment of muscle phosphocreatine and intracellular pH – and show feasibility of phosphocreatine recovery following exercise assessment in some children with autism spectrum disorder. We emphasize that the relatively shorter scan length of brain scanning, and unconstrained (i.e., without a birdcage coil) nature of 31P-MRS, make this technique particularly attractive for investigations of brain physiology in individuals with autism spectrum disorder across a large range of ages and abilities.
This pilot assessment is limited by small sample size. Cases were selected for suspicion of energetic impairment, and are not presumed to reflect expectation for all cases with autism spectrum disorder.
In summary, bioenergetic defects shown (resting muscle intracellular pH) and suggested (muscle phosphocreatine with suggestive evidence also from frontal brain phosphocreatine and muscle phosphocreatine recovery following exercise), extend evidence consistent with mitochondrial or bioenergetic defects in autism spectrum disorder, or subsets of autism spectrum disorder, to end organs including muscle – though autism spectrum disorder is defined in relation to the brain. It supports 31P-MRS as a noninvasive approach to studying bioenergetics in autism spectrum disorder that can be extended to the brain, and provides provisional data suggestive of bioenergetic defects in the brain itself, the organ of primary interest in study of autism. There is need for replication of bioenergetic findings in similar autism spectrum disorder cases versus controls in a larger sample, extension to a sample not selected for suspicion of bioenergetic impairment, with attention to different organs and tissues, and assessment of the relation of bioenergetic findings to phenotypic manifestations.
Supplementary Material
Acknowledgments
We thank Julia Platt MS, CGC and Mariella Simon MS, CGC in the Wallace group for the referrals of our participant population. We thank John Firebaugh, Richard Znamirowski, Lilly Pacheco and Dr. Graeme Bydder from the UCSD Bydder MR Center for their gracious technical help, support, and contributions to this project. We acknowledge the excellent work of Rebecca McNally Keehn MA, BCABA, from the Center for Autism Research and Evaluation and Service for her expertise and evaluations of our participant population. From the Golomb Group, we thank Juliet Parrish MA and Julie Broadwin PhD, MPH, whose contributions in early phases of this study were central; and Janis Ritchie BSN, and Stephanie Cham BS each of whom assisted in this effort. We specially thank the children and parents who graciously gave of their time to participate. It is with particular gratitude that we acknowledge the invaluable contribution of Autism Speaks and particularly Dr. Leanne Chukoskie who made this work possible.
Funding: This study was funded by Autism Speaks High Risk High Impact Grant entitled Mitochondria and Autism #7278 (Dr. Golomb), #7277 (Dr. Haas), and #5668 (Dr. Wallace) as well as NIH Grant NS070298, DCW PI and the University of California Foundation Fund No. 3923 (Dr. Golomb). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Ethical approval: This study was approved by the University of California, San Diego Human Research Protections Program. Participants’ legal guardian gave University of California, San Diego Human Research Protections Program-approved informed consent; and participants gave University of California, San Diego Human Research Protections Program-approved assent, with age-appropriate assent forms.
Author Contributions: BG had the idea for the study and wrote the initial draft. LE saw participants. GR assisted with recruitment and served as a liaison between groups. Haas’ and Wallace’s group conducted recruitment. Haas’ group (authors RH, RN, and GR) provided detailed tables on mitochondrial function status. AL conducted autism spectrum disorder assessment. AS supervised MRS visits and provided expertise in conducting MRS assessments in children with autism spectrum disorder. GH conducted 31P-MRS assessment and conducted analysis of MRS data. SK provided administrative, editorial and some data management support. All authors participated in revision of the manuscript of scholarly content and approved the manuscript.
Declaration of conflicting of interests: The authors have no financial interests that may be relevant to the submitted work. Investigators received funding from Autism Speaks for this Study.
References
- 1.Minshew NJ, Goldstein G, Dombrowski SM, Panchalingam K, Pettegrew JW. A preliminary 31P MRS study of autism: evidence for undersynthesis and increased degradation of brain membranes. Biol Psychiatry. 1993 Jun 1–15;33(11–12):762–773. doi: 10.1016/0006-3223(93)90017-8. [DOI] [PubMed] [Google Scholar]
- 2.Giulivi C, Zhang Y-F, Omanska-Klusck A, et al. Mitochondrial dysfunction in autism. jama. 2010;304(21):2389–2396. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipek PA, Juranek J, Smith M, et al. Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Ann Neurol. 2003 Jun;53(6):801–804. doi: 10.1002/ana.10596. [DOI] [PubMed] [Google Scholar]
- 4.Pons R, Andreu AL, Checcarelli N, et al. Mitochondrial DNA abnormalities and autistic spectrum disorders. J Pediatr. 2004 Jan;144(1):81–85. doi: 10.1016/j.jpeds.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Poling JS, Frye RE, Shoffner J, Zimmerman AW. Developmental regression and mitochondrial dysfunction in a child with autism. J Child Neurol. 2006 Feb;21(2):170–172. doi: 10.2310/7010.2006.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehm HL, Kern W, Peters A. The selfish brain: competition for energy resources. Prog Brain Res. 2006;153:129–140. doi: 10.1016/S0079-6123(06)53007-9. [DOI] [PubMed] [Google Scholar]
- 7.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004 Aug;27(8):489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Lodi R, Iotti S, Scorolli L, et al. The use of phosphorus magnetic resonance spectroscopy to study in vivo the effect of coenzyme Q10 treatment in retinitis pigmentosa. Mol Aspects Med. 1994;15(Suppl):s221–230. doi: 10.1016/0098-2997(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 9.Barbiroli B, Frassineti C, Martinelli P, et al. Coenzyme Q10 improves mitochondrial respiration in patients with mitochondrial cytopathies. An in vivo study on brain and skeletal muscle by phosphorous magnetic resonance spectroscopy. Cell Mol Biol (Noisy-le-grand) 1997 Jul;43(5):741–749. [PubMed] [Google Scholar]
- 10.Barbiroli B, lotti S, Lodi R. Improved brain and muscle mitochondrial respiration with CoQ. An in vivo study by 31P-MR spectroscopy in patients with mitochondrial cytopathies. Biofactors. 1999;9(2–4):253–260. doi: 10.1002/biof.5520090221. [DOI] [PubMed] [Google Scholar]
- 11.Lotti S, Lodi R, Frassineti C, Zaniol P, Barbiroli B. In vivo assessment of mitochondrial functionality in human gastrocnemius muscle by 31P MRS. The role of pH in the evaluation of phosphocreatine and inorganic phosphate recoveries from exercise. NMR Biomed. 1993 Jul-Aug;6(4):248–253. doi: 10.1002/nbm.1940060404. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983 Jul;1(1):77–94. [PubMed] [Google Scholar]
- 13.Kemp GJ, Taylor DJ, Radda GK. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed. 1993 Jan-Feb;6(1):66–72. doi: 10.1002/nbm.1940060111. [DOI] [PubMed] [Google Scholar]
- 14.Thompson CH, Kemp GJ, Sanderson AL, Radda GK. Skeletal muscle mitochondrial function studied by kinetic analysis of postexercise phosphocreatine resynthesis. J Appl Physiol. 1995 Jun;78(6):2131–2139. doi: 10.1152/jappl.1995.78.6.2131. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007 Feb;35(2):235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- 16.Filipek PA, Juranek J, Nguyen MT, Cummings C, Gargus JJ. Relative carnitine deficiency in autism. J Autism Dev Disord. 2004 Dec;34(6):615–623. doi: 10.1007/s10803-004-5283-1. [DOI] [PubMed] [Google Scholar]
- 17.Correia C, Coutinho AM, Diogo L, et al. Brief report: High frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord. 2006 Nov;36(8):1137–1140. doi: 10.1007/s10803-006-0138-6. [DOI] [PubMed] [Google Scholar]
- 18.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994 Oct;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 19.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000 Jun;30(3):205–223. [PubMed] [Google Scholar]
- 20.Erickson LC, Scott-Van Zeeland AA, Hamilton G, Lincoln A, Golomb BA. Brief Report: Approaches to (31)P-MRS in Awake, Non-Sedated Children with and without Autism Spectrum Disorder. J Autism Dev Disord. 2012 Jun;42(6):1120–1126. doi: 10.1007/s10803-011-1359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997 Nov;129(1):35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 22.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001 Jul;31(4):269–286. doi: 10.1016/s0010-4825(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton G, Patel N, Forton DM, Hajnal JV, Taylor-Robinson SD. Prior knowledge for time domain quantification of in vivo brain or liver 31P MR spectra. NMR Biomed. 2003 May;16(3):168–176. doi: 10.1002/nbm.821. [DOI] [PubMed] [Google Scholar]
- 24.Murphy EJ, Rajagopalan B, Brindle KM, Radda GK. Phospholipid bilayer contribution to 31P NMR spectra in vivo. Magn Reson Med. 1989 Nov;12(2):282–289. doi: 10.1002/mrm.1910120218. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton G, Allsop JM, Patel N, et al. Variations due to analysis technique in intracellular pH measurements in simulated and in vivo 31P MR spectra of the human brain. J Magn Reson Imaging. 2006 Apr;23(4):459–464. doi: 10.1002/jmri.20524. [DOI] [PubMed] [Google Scholar]
- 26.Pettegrew JW, Keshavan MS, Panchalingam K, et al. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch Gen Psychiatry. 1991 Jun;48(6):563–568. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- 27.Petroff OA, Prichard JW. Cerebral pH by NMR. Lancet. 1983;2:105–6. doi: 10.1016/s0140-6736(83)90088-0. [DOI] [PubMed] [Google Scholar]
- 28.Sign Test. 2005 http://www.fon.hum.uva.nl/Service/Statistics/Sign_Test.html.
- 29.Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002;59:1406–11. doi: 10.1212/01.wnl.0000033795.17156.00. [DOI] [PubMed] [Google Scholar]
- 30.Taylor DJ, Kemp GJ, Radda GK. Bioenergetics of skeletal muscle in mitochondrial myopathy. J Neurol Sci. 1994 Dec 20;127(2):198–206. doi: 10.1016/0022-510x(94)90073-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.