Abstract
We discuss the rationale for a trial of a novel biologic immunotherapy in schizophrenia (SZ). Available antipsychotic treatments for SZ are often limited by partial effectiveness and significant side effects. Thus, the search for novel medications is of high priority. All current antipsychotics function primarily by blocking D2-type dopamine receptors. An emerging theory of SZ postulates disturbances of cytokines and inflammatory mediators (i.e., the cytokine model), possibly originating in part from infectious exposures. Cytokines are one of the most important components of the immune system that orchestrate the response to infectious and other exogenous insults. Preclinical models of SZ support a convergence between a role for certain cytokines in the pathophysiology of SZ and major neurochemical postulates of the disorder, including the dopamine and glutamate hypotheses. Furthermore, several cytokines are elevated in plasma in SZ, and Positron Emission Tomography (PET) studies have shown active inflammation in the brains of individuals with psychosis. Treatment studies of certain anti-inflammatory agents, such as celecoxib and aspirin, in patients with SZ have provided further support for neuroinflammation in this disorder. The recent development of approved biological therapies for autoimmune diseases provides us with new opportunities to directly target cytokine signaling as a novel treatment strategy in SZ. In addition, advances in imaging, immunology, and psychopharmacology have paved the way for utilizing measures of target engagement of neuroimmune components that would facilitate the identification of patient subgroups who are most likely to benefit from cytokine modulation.
Keywords: schizophrenia, cytokine, inflammation, interleukin, microbial, neuroimmune
Background
All current antipsychotic medications for schizophrenia (SZ) function primarily by blocking D2-type dopamine receptors (1), though many individuals are only partially responsive to these medications (2). In addition, their effects on negative symptoms (3–6) and cognitive deficits (1, 7–14) are limited. Therefore, there is a great need for new psychopharmacologic agents for SZ. An emerging theory of SZ derives from a body of literature (15–17) that postulates disturbances of neuroimmunity in this disorder. Spurred by advances in infectious disease and immunologic research, there has been a renewed interest in microbial pathology, neuroinflammation, and SZ.
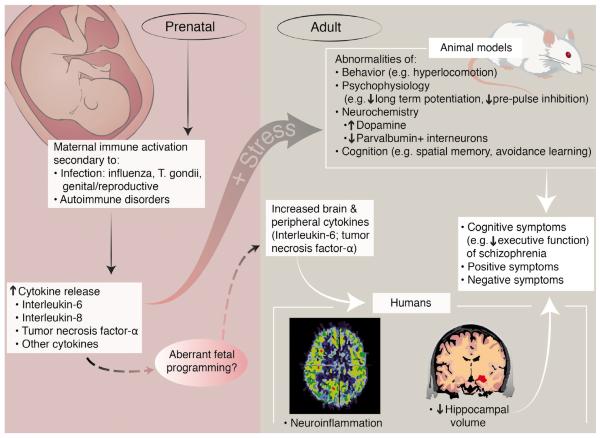
In this manuscript, we review the rationale and treatment strategies for biological immunotherapy for SZ. In particular, we focus on medications aimed at modulating cytokine function and discuss key issues in the development and implementation of these approaches. Throughout this paper we will refer to this rationale and approach as the `cytokine model of SZ' (Figure 1). We first summarize the epidemiological and preclinical evidence for early life infection in the etiology of SZ, links between cytokine dysfunction and the dopamine and glutamate hypotheses, and clinical and imaging studies of inflammation and cytokine disturbances in SZ. Following a review of treatment approaches involving anti-inflammatory medications conducted to date, we discuss how these findings can be translated into novel therapeutic strategies, such as medications that directly target cytokines, including the identification of patients most likely to benefit from these medications and challenges of these approaches.
Figure 1.
The cytokine model of schizophrenia. Artwork by Applied Art, LLC
Infection in Schizophrenia
An emerging literature suggests that prenatal exposure to pathogenic microbes may contribute to the etiopathogenesis of SZ (for review, see 17). While earlier studies, primarily on influenza, were based on ecologic data, more recent investigations have capitalized on birth cohorts with prospectively acquired data from serum bioassays on infectious exposures during the prenatal period. These infections include not only influenza (18), but also Toxoplasma gondii (T. gondii) (19, 20), genital reproductive infections (21), and herpes simplex virus type 2 (22, 23). While not all studies have yielded evidence of associations (24), the majority suggest an increased risk of SZ in offspring of mothers with prenatal infection. Evidence also suggests that exposure to T. gondii during periods other than pregnancy may also be related to SZ (25–28).
Further epidemiologic evidence has supported infections and autoimmune dysfunction as risk factors for SZ. In a recent prospective, nationwide study, hospital contacts for infections and autoimmune diseases prior to onset of SZ were associated with an elevated risk of the disorder (29). These associations increased with the number of infections in a dose-response manner and there was synergy between autoimmune diseases and infections. The risk of SZ was greater if the infection occurred closer to the onset of SZ, although associations were observed as long as 15 years before the diagnosis.
Several models have attempted to explain how prenatal infections increase the risk of SZ in offspring of infected mothers (17). The most parsimonious model suggests that cytokines mediate the effects of infection (17, 30). Cytokines are a family of soluble proteins that play an important role as the systemic mediators of the host response to infection (17), are critically involved in the inflammatory response to non-infectious agents and insults, and contribute to normal development and function of the CNS (17). Cytokines have been categorized into those that initiate pro-inflammatory versus anti-inflammatory processes (Table 1). Pro-inflammatory cytokines, such as interleukin-6 (IL-6) (which in certain circumstances also has anti-inflammatory effects) or tumor necrosis factor-alpha (TNF-alpha), may play roles in cytotoxicity as well as influence dopaminergic and glutamatergic pathways and cognitive processes that are implicated in the pathophysiology of SZ (see “Cytokines and the Dopamine and Glutamate Hypotheses of Schizophrenia”). Cytokine activity can also trigger other biological events, such as activation of the hypothalamo-pituitary adrenal (HPA) axis (31), and is associated with increases in oxidative stress (32). Furthermore, maternally generated cytokines may cross the placenta (33) and blood-brain barrier (34). In this review, we will focus mainly on the pro-inflammatory cytokine IL-6 since there is a relatively robust preclinical and clinical literature on a role of IL-6 in SZ, although other cytokines, such as TNF-alpha and IL-2, may be involved.
Table 1.
Neurotransmitter-Cytokine Relationships*
| Pro-inflammatory |
|---|
| IL-1 |
| IL-2 |
| IL-8 |
| IL-6 |
| TNF-α |
| Anti-Inflammatory |
| IL-10 |
The Maternal Immune Activation Model
The Maternal Immune Activation (MIA) model of SZ has provided a wealth of data on the potential connections between prenatal infection, cytokines, and SZ. We focus here on select studies from this literature. (see Patterson et al. and Meyer et al. (35, 36) for comprehensive reviews). These include studies involving administration of the double stranded RNA poly I:C, and of lipopolysaccharide (LPS), both of which induce strong innate immune responses, to pregnant rodents and, more recently, primates. Offspring of these pregnancies evidenced behavioral, neurochemical, psychophysiologic, and histologic abnormalities found in patients with SZ. Of particular relevance to the dopaminergic hypothesis of SZ, administration of poly I:C to pregnant rodents causes an increased number of mesencephalic dopamine neurons in the fetal brain during mid to late gestation, accompanied by changes in fetal expression of several genes involved in dopamine neuron development (37).
The cytokine IL-6 appears to play an especially important role. Smith et al. investigated the potential contribution of several cytokines to the abnormalities observed in MIA models of SZ (38). The authors found that injection of poly(I:C) into pregnant mice produced offspring with pre-pulse inhibition (PPI) abnormalities and social interaction deficits, analogous to observations in SZ. Co-administration of anti-IL-6 antibodies neutralized these abnormalities. Similarly, offspring of IL-6 knockout mice given poly(I:C) did not exhibit these deficits (38). Samuelsson et al. injected pregnant rats directly with IL-6 and found that adult offspring exhibited increased IL-6 levels, increased hippocampal IL-6 mRNA, hippocampal astrogliosis and neuronal loss, and impaired spatial learning (39). These data suggest that IL-6 is required for detrimental effects of MIA on the fetal brain. Importantly, downstream of the IL-6 receptor, activation of IL-6 response genes was found both in the placenta and in the fetal brain of MIA-exposed offspring, and IL-6 messenger RNA was induced as well (40–42).
Evidence also suggests that MIA is associated with elevations of cytokines in offspring at homologous ages to the usual age of onset of SZ (43, 44). Prenatal exposure to LPS caused an increase in serum proinflammatory cytokine levels, including IL-2, IL-6, and TNF-alpha during this stage of life. Interestingly, these effects were reversed by haloperidol. These findings, and those noted above on hyperdopaminergia in MIA-exposed mice, provide support for aberrant fetal programming of adult immune hyperactivity and its relationship with dopamine dysfunction in SZ and antipsychotic treatment effects coincident with normalization of immune function (see section “Clinical Studies of Neuroinflammation in Schizophrenia”).
Preclinical studies of MIA and SZ have generally examined this risk factor alone. In a recent study, Giovanoli et al. (45) investigated whether MIA increases the vulnerability to peripubertal stress on brain and behavioral phenotypes in SZ. The authors found synergistic effects of MIA and peripubertal stress on sensorimotor gating deficiency, assessed by PPI of the acoustic startle reflex, and in behavioral hypersensitivity to amphetamine and dizocilpine, which alter the function of dopamine and of the NMDA receptor, respectively; such effects were not observed when each putative risk factor was administered alone. These two factors also interacted to cause elevated dopamine levels in the hippocampus. Moreover, MIA markedly increased vulnerability to stress-induced neuroimmunologic disruptions during the peripubertal period, evidenced by an increase in hippocampal and prefrontal markers representing activated microglia, accompanied by elevated levels of the proinflammatory cytokines IL-1-beta and TNF-alpha.
In summary, preclinical models of SZ support the potential involvement of cytokine disturbances, most specifically IL-6, in the etiopathogenesis of SZ. However, internal validity for these models would be greatly enhanced by linking these disturbances with other putative pathophysiological processes in SZ, discussed in the next section.
Cytokines and the Dopamine and Glutamate Hypotheses of Schizophrenia
The dopamine hypothesis of SZ proposes that positive symptoms result from dopamine hyperactivity (46), while the negative symptoms and cognitive deficits of SZ are thought to be related, at least in part, to a cortical dopamine deficit (Weinberger (47); Davis et al. (48)). In addition to the MIA models discussed above, several studies of adult rodents have revealed intriguing interactions between cytokines and dopaminergic signaling.
Zalcman et al. showed that IL-6 administration to rodents causes increased behavioral activation, including ambulatory exploration and digging, and modestly increased locomotion (49), behaviors which model hyperdopaminergic-related psychotic symptoms in SZ. The authors further showed that repeated administration of IL-6 sensitizes rats to the locomotor-stimulating effects of amphetamine, further suggesting a close interaction between IL-6 and the mesolimbic dopaminergic system (50). Furthermore, in an astrocytic cell line, both acute and chronic exposure to methamphetamine increased IL-6 mRNA and protein levels (51).
The cytokine model of SZ is also consistent with the glutamate hypothesis of SZ. This hypothesis originated largely from observations that phencyclidine (PCP) and ketamine, which block neurotransmission at the N-methyl-D-aspartate (NMDA) receptor, induce positive, negative, and cognitive symptoms (52–57), suggesting that reduction in NMDA functioning may be one pathophysiological mechanism in SZ (rev. in (58)). Behrens et al. hypothesized a connection between IL-6 and the psychotomimetic effects of ketamine (59). They found that, in mice, ketamine disrupts parvalbumin containing interneurons (PV+), aberrations of which have been implicated in SZ (60) through activation of NADPH-oxidase (61), an effect that was mimicked by IL-6 administration and reversed by neutralization of NADPH-oxidase (59). Ketamine administration also induces expression of IL-6, and IL-6 antibodies abolish the effects of ketamine on PV+ interneurons (59). The authors also reported that, in IL-6 knockout mice, ketamine did not alter PV+ interneurons nor did it activate NADPH-oxidase. These findings suggest that IL-6 may be a mediator of the deleterious effects of ketamine on PV+ interneurons, which are critical in the regulation of pyramidal cell activity and possibly more directly in cognitive processes such as working memory (60).
Furthermore, overexpression of IL-6 has also been associated with cognitive deficits, such as spatial memory and learning abnormalities, and related deficits in long-term potentiation (LTP) in preclinical models, consistent with observations in SZ. Applying even low levels of IL-6 to hippocampal slices impairs LTP (62). In addition, application of an IL-6 antibody to normal rats increased LTP and hippocampus-dependent spatial alternation learning (63). Braida et al. showed that IL-6 knockout mice showed enhanced learning on the radial maze (64), while Heyser et al demonstrated that mice that overexpress IL-6 have avoidance learning deficits, which were associated with loss of PV+ neurons in the hippocampus, as well as increased gliosis and microglial activation (65). These effects on LTP, spatial memory/learning, and avoidance learning are directly relevant to SZ as they are thought to represent key cognitive deficits (66).
In summary, preclinical models of SZ support a role for IL-6 in pathophysiological processes implicated in SZ, including dopamine and glutamate dysfunction, as well as relevant cognitive abnormalities. One potential implication of these findings is that antagonism of the effects of IL-6 using biological immunotherapy might ameliorate positive, negative, and cognitive deficits in SZ (see “Discussion”).
Clinical Studies of Neuroinflammation in Schizophrenia
There is substantial evidence from adult patients, based on immunologic, neuroimaging, and pharmacologic approaches, of altered cytokine activity in SZ.
Cytokine Levels in Clinical Studies
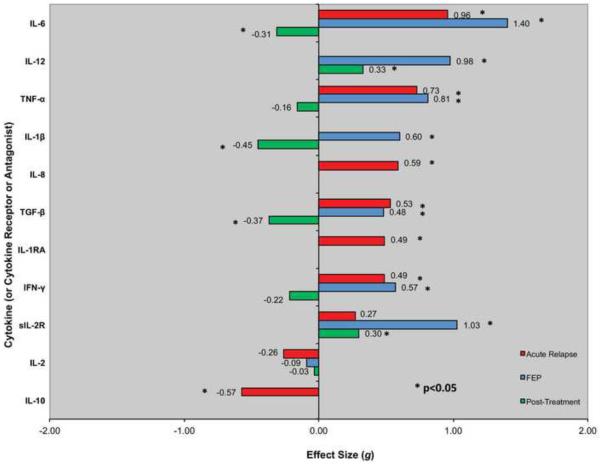
In a recent meta-analysis, Miller et al. reported that numerous cytokines are elevated in peripheral blood of individuals with SZ (Figure 2) (67). IL-6 levels were increased in the plasma of both first-episode (effect size = 1.4) and acutely relapsed (effect size = 0.96) patients, while IL-6 levels significantly decreased after treatment (effect size = −0.31) (67). Although these studies are observational, they suggest that IL-6 is a state marker of SZ that normalizes with treatment. Similarly, TNF-alpha levels are elevated in the plasma of both acutely relapsed and first-episode patients with SZ. However, it is important to emphasize that most studies did not assess or control for potential confounding factors that can influence blood cytokine levels (e.g., age, race, sex, body mass index). Genetic factors, including polymorphisms in cytokine genes that impact on SZ risk and/or cytokine levels, have also not been adequately considered. Thus, additional well-controlled studies are needed to identify the most robust potential therapeutic target(s). There are also reports of associations between IL-6 levels and total duration of illness, positive symptoms, total psychopathology (67), and cognition (68). Further studies should explore relationships between cytokine levels and symptoms.
Figure 2.
Blood cytokine levels in patients with schizophrenia. Reprinted from Biological Psychiatry, 70 (7), Miller BJ, Buckley P, Seabolt W, Mellor A, and Kirkpatrick B, Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects, Pages 663–671, Copyright 2011, with permission from Elsevier.
The role of antipsychotic medications on these effects is less well understood. Although IL-6 levels decrease with treatment as shown in the meta-analysis of Miller et al. (67), it is not clear whether these effects are directly related to treatment or to the phase of the illness. Himmerich et al. examined this question by adding antipsychotic medications to plasma drawn from healthy control subjects that was stimulated by toxic shock syndrome toxin, producing elevated cytokine levels (69). The antipsychotic medications had no effects on plasma IL-6 levels. These data, taken together with findings from clinical studies on the effects of antipsychotic medications on cytokine levels (analyzed and reviewed in detail by Miller et al. and Potvin et al.) (67, 70), suggest that IL-6 levels decrease during treatment with antipsychotic medications, but this effect may be related to the state of the illness rather than to the medications themselves. Further investigation is warranted. However, findings of abnormal cytokine abnormalities in first-episode psychosis support an association that is independent of antipsychotic medications.
Imaging Studies
Imaging studies, primarily positron emission tomography (PET) of a peripheral benzodiazepine receptor, have also supported persistent inflammation in the brains of individuals with SZ. This receptor is widely distributed in the body, though has low levels in brain (71). Active neuroinflammation increases levels of this receptor, primarily the result of a microglial response as demonstrated by ligand binding studies in both rodent models of inflammation and human disease (72, 73). Doorduin et al., using the benzodiazepine receptor PET ligand 11C-(R)-PK11195, found increased binding potential in hippocampus in patients versus comparison subjects (74). Van Berckel et al., using the same ligand, found increased binding potential in total gray matter in patients (75), while Takano et al. found no differences in regional binding potential between patients and comparison subjects using the peripheral benzodiazepine receptor ligand [11C]DAA1106 but did observe correlations between positive symptoms and binding potential of this ligand in medial frontal, medial temporal, and occipital cortical regions (76).
While no studies have investigated the relationship between neuroinflammation in SZ and peripheral cytokine levels, several studies have investigated brain morphology in relation to cytokine levels. In an MRI study, increased IL-6 gene expression in blood leukocytes was associated with diminished left hippocampal volume in SZ (77). The authors suggested a synergistic effect of IL-6 with brain derived neurotrophic factor (BDNF) and cortisol on hippocampal volume. Elevated peripheral blood levels of IL-6 were also related to diminished hippocampal grey matter volume in healthy adults at mid-life (78).
In spite of this extensive literature, these findings have yet to be reconciled with the lack of evidence of gliosis in SZ. Schneider and Dwork (79) have extensively reviewed the postmortem literature on gliosis and SZ. The review included positive studies (indicating increased immunoreactive microglia in SZ) including those by Bayer et al. (80), Radewicz et al. (81), and Steiner et al. (82), though some negative findings were also reported. The authors concluded that all studies reviewed were too small, had methodological flaws, and possibly biased cell counting methods. However, they further concluded that “some of the positive findings in the literature cannot easily be dismissed,” and called for further research. Furthermore, as noted above, cytokines can alter brain function through mechanisms that are not believed to involve gliosis, including dopaminergic and glutamatergic effects.
In summary, these results support neuroinflammation in SZ, as well as relationships between IL-6 and diminished hippocampal volumes, further suggesting a potential role for biological immunotherapy, particularly medications that antagonize IL-6, in SZ.
Treatment Studies
Treatment studies of anti-inflammatory agents also support a neuroimmune component in SZ and provide evidence that biological immunotherapy can improve symptoms. The cyclo-oxygenase 2 inhibitor celecoxib has been administered as an add-on agent to antipsychotic medications in four randomized, placebo-controlled clinical trials (83–86), and aspirin in one (87). Four of these studies showed at least partial benefit on the PANSS (83–85, 87) or CGI scales (84), and one showed no benefit (86). Importantly, both Muller et al. (83, 88) and Laan et al. (87) reported associations between response to cyclo-oxygenase inhibition and an impaired type 1 to type 2 immune balance, which is normalized by treatment with cyclo-oxygenase inhibitors. This finding suggests that enriching samples with patients who possess abnormal levels of inflammatory markers might increase the potential benefit of future treatments. The anti-inflammatory agent minocycline has also been tested as an add-on agent in two randomized, blinded clinical trials in SZ, with improvements observed on negative symptoms and executive functioning (89, 90).
Although these findings are promising, overall symptom improvement in these studies and others that investigated putative anti-inflammatory agents (e.g., N-acetylcysteine, estrogen, minocycline, davunetide, fatty acids, etc.) was modest, as reviewed in a meta-analysis by Nitta et al. (91) as well as in a review by Sommer et al. (92). Moreover, many of these anti-inflammatory medications have relatively nonspecific actions on neuroimmune mechanisms or cytokines. The literature reviewed in the preceding sections suggests that a biological immunotherapy that directly targets cytokines is also worthy of study.
Discussion
The cytokine model of SZ suggests increased inflammation in the brains of individuals with SZ, possibly related to infection during the prenatal period or early life and to persistent inflammation into adulthood. This hypothesis is supported by epidemiological data indicating an elevated risk of SZ in individuals who were exposed to infectious pathogens in utero and to autoimmune diseases and serious infection. The findings summarized in this paper suggest a pathophysiological model in which IL-6 plays a key role, and this may generalize to other pro-inflammatory cytokines such as TNF-alpha or IL-2. Elevated IL-6 levels have a wide range of adverse effects on brain structure and function relevant to SZ. These consequences include facilitation of dopaminergic sensitization, increased vulnerability of PV+ interneurons to toxic stimuli, diminished hippocampal volumes, and impaired glutamatergic functioning. It is postulated that aberrant fetal programming results in elevation of IL-6 levels, particularly around the time of puberty, and when reinforced by peri-pubertal stress, the elevated IL-6 levels and structural and functional deficits are proposed to interact with one another producing positive and negative symptoms, and cognitive deficits. This model is elaborated in Figure 1.
Although treatment studies aimed at modulating aspects of immune function have been innovative and promising, the effects have been generally modest. The findings reviewed herein suggest a new strategy that is consistent with the array of evidence reviewed above of overactivation of particular cytokines in SZ. Specifically, we propose that a biological immunotherapy that targets specific cytokines, such as opposing the effects of IL-6, may represent a useful and novel strategy for drug development, including clinical trials, in SZ. To date, however, no such treatment trial has been published. The development, approval, and use of these innovative therapies for autoimmune diseases in recent years has provided candidate agents that target specific cytokines in SZ. These therapeutic agents include antibodies or antibody components that function by neutralizing the target cytokine, either by binding the receptor or the cytokine (93, 94). Potential mechanisms of actions of these agents include the prevention of peripheral receptors from crossing the blood brain barrier (i.e., by binding to them) and preventing induction of inflammatory cascades in the periphery that could have downstream effects on the central nervous system. Tocilizumab, for example, is an anti-IL-6 receptor antibody that is FDA-approved for rheumatoid arthritis in individuals who have not responded to at least one TNF-alpha therapy and for juvenile idiopathic arthritis. Testing agents that modulate IL-6 and other cytokines in randomized clinical trials would not only provide evidence of their efficacy but may also represent a first step toward addressing whether cytokines, such as IL-6, play a causal role in SZ or are merely correlated with the disorder and its manifestations.
In the only known study to date of a cytokine antagonist in a psychiatric disorder, infliximab, a TNF-alpha antibody, was associated with significant improvement in HAM-D scores at 12 weeks in patients with treatment resistant depression and elevated baseline TNF-alpha levels (95). Further, while there were no significant group differences in the change in HAM-D scores, there was a significant time by treatment interaction favoring infliximab-treated subjects with greater levels of C-reactive protein at baseline. Although infliximab is not known to cross the blood brain barrier, this study indicates that direct action on the CNS is not necessary for a behavioral response to the medication and provides “proof-of-concept” for analogous treatment studies in SZ. Similarly, Abbasi et al. recently reported that adjunctive celecoxib significantly reduced serum IL-6, and that change in IL-6 levels and change in HAM-D scores were significantly associated in subjects receiving either celecoxib or placebo (96). As with all pharmacological approaches, the potential benefits of biological therapies would need to be considered in light of potential risks, including infection, immunosuppression, and cytopenia (e.g., platelets, neutrophils).
While many challenges need to be addressed in the development and use of immune modulators in SZ, we focus on several questions that appear most relevant. First, would a biological therapy be useful in an illness in which an infectious or inflammatory insult may have contributed in only a subgroup of individuals? To address this, we suggest that treatment with these agents may need to be predicated on the presence of measurable neuroimmune abnormalities that assess the degree to which an agent engages its target, a critical component of drug development (97). However, there remains no clear biomarker to assess either structural or functional target engagement of biological immunotherapies. For example, the response of peripheral blood markers (e.g., cytokines or cytokine receptors) to treatments that target the neuroimmune system are largely unknown. Furthermore, while biomarkers of inflammation in PET studies exist, the relationship to treatment effects, or longitudinal changes in SZ in general, are also unknown. These caveats aside, one potential strategy may involve quantifying serum or CSF levels of the cytokine being targeted (and potentially other cytokines) before and after treatment. In addition, based on evidence reviewed above, morphometric MRI, neuroinflammation-based PET, or high resolution fMRI (98) may be used to measure target engagement. One may also enrich the sample by selecting individuals with elevated baseline levels of inflammatory markers, such as IL-6 or C-reactive protein, as supported by the study by Raison et al. (95).
Second, it is not clear whether the mechanism of action of immune modulators overlap with D2-type dopamine receptor-based drugs leading to synergistic effects or whether this overlap would result in a negation of effects. This would have clear implications for the specific therapeutic strategy, in particular whether these medications should be used adjunctively with existing antipsychotics. A third key question concerns the patient population(s) (e.g. chronic, acute, prodromal) that would benefit most from these treatments. The vast majority of clinical studies that we have reviewed above concern chronically ill patients; however, it is conceivable that detrimental effects of cytokine overactivity on brain structure and functions relevant to SZ might occur during the prodromal phase of the illness or earlier. Similarly, clozapine may have differential effects on cytokine levels, compared to other agents (99). Therefore, treatment resistance would be another important factor to consider. Additional considerations for the potential clinical use of these immune modulators include their high cost and parenteral administration, which may affect treatment compliance.
In conclusion, we propose a cytokine model of SZ in which integrating technological advances in imaging, immunology, and psychopharmacology would facilitate the development of new and more effective biological immunotherapies targeted at modulating cytokine activity and other components of the neuroimmune system. A multidisciplinary program of research offers the promise of tailored psychopharmacological interventions based, for example, on individual peripheral and central neuroimmune biomarker profiles, one step toward the ultimate goal of personalized medicine.
Acknowledgments
Dr. Girgis is supported by NCRR grant 2KL2RR024157-06 (Henry Ginsberg). Dr. Brown's work was supported by NIH grant 5K02 MH065422.
Financial Disclosures: Dr. Girgis receives research support from Otsuka Pharmaceuticals. Dr. Brown and Ms. Kumar report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 2.Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: an update and critical review of the pharmacology and clinical profiles of current and future therapeutic agents. Molecular Psychiatry PMCID: In Process. doi: 10.1038/mp.2012.47. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. J Clin Psychiat. 1997;58:538–546. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- 4.Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, et al. Effects of Clozapine on Positive and Negative Symptoms in Outpatients with Schizophrenia. Am J Psychiat. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- 5.Kasper S, Lerman MN, McQuade RD, Saha A, Carson WH, Ali M, et al. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol. 2003;6:325–337. doi: 10.1017/S1461145703003651. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer HY, Casey DE, Garver DL, Marder SR, Masand PS, Miller D, et al. Assessing the effects of atypical antipsychotics on negative symptoms. J Clin Psychiat. 1998;59:28–34. [PubMed] [Google Scholar]
- 7.Green MF, Marshall BD, Wirshing WC, Ames D, Marder SR, McGurk S, et al. Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am J Psychiat. 1997;154:799–804. doi: 10.1176/ajp.154.6.799. [DOI] [PubMed] [Google Scholar]
- 8.Kern RS, Green MF, Marshall BD, Wirshing WC, Wirshing D, McGurk S, et al. Risperidone vs. haloperidol on reaction time, manual dexterity, and motor learning in treatment-resistant schizophrenia patients. Biol Psychiat. 1998;44:726–732. doi: 10.1016/s0006-3223(98)00088-2. [DOI] [PubMed] [Google Scholar]
- 9.Green MF, Marder SR, Glynn SM, McGurk SR, Wirshing WC, Wirshing DA, et al. The neurocognitive effects of low-dose haloperidol: A two-year comparison with risperidone. Biol Psychiat. 2002;51:972–978. doi: 10.1016/s0006-3223(02)01370-7. [DOI] [PubMed] [Google Scholar]
- 10.Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;161:985–995. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- 11.Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, et al. Long-term neurocognitive effects of olanzapine or low-dose haloperidol in first-episode psychosis. Biol Psychiatry. 2006;59:97–105. doi: 10.1016/j.biopsych.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- 13.Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 14.Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker WW, Keefe RS, et al. Cognitive Effects of Antipsychotic Drugs in First-Episode Schizophrenia and Schizophreniform Disorder: A Randomized, Open-Label Clinical Trial (EUFEST) Am J Psychiat. 2009;166:675–682. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- 15.Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry. 2008;13:470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- 17.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 19.Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Torrey EF, et al. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry. 2007;61:688–693. doi: 10.1016/j.biopsych.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Brown AS, Schaefer CA, Quesenberry CP, Jr., Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 21.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163:927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- 22.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequentpsychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 23.Buka SL, Cannon TD, Torrey EF, Yolken RH. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry. 2008;63:809–815. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Brown AS, Schaefer CA, Quesenberry CP, Jr., Shen L, Susser ES. No evidence of relation between maternal exposure to herpes simplex virus type 2 and risk of schizophrenia? Am J Psychiatry. 2006;163:2178–2180. doi: 10.1176/ajp.2006.163.12.2178. [DOI] [PubMed] [Google Scholar]
- 25.Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amminger GP, McGorry PD, Berger GE, Wade D, Yung AR, Phillips LJ, et al. Antibodies to infectious agents in individuals at ultra-high risk for psychosis. Biol Psychiatry. 2007;61:1215–1217. doi: 10.1016/j.biopsych.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Niebuhr DW, Millikan AM, Cowan DN, Yolken R, Li Y, Weber NS. Selected infectious agents and risk of schizophrenia among U.S. military personnel. Am J Psychiatry. 2008;165:99–106. doi: 10.1176/appi.ajp.2007.06081254. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen MG, Stevens H, Pedersen CB, Norgaard-Pedersen B, Mortensen PB. Toxoplasma infection and later development of schizophrenia in mothers. Am J Psychiatry. 2011;168:814–821. doi: 10.1176/appi.ajp.2011.10091351. [DOI] [PubMed] [Google Scholar]
- 29.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–1310. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore JH, Jarskog LF. Exposure to infection and brain development: cytokines in the pathogenesis of schizophrenia. Schizophr Res. 1997;24:365–367. doi: 10.1016/s0920-9964(96)00123-5. [DOI] [PubMed] [Google Scholar]
- 31.Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain, behavior, and immunity. 1995;9:253–275. doi: 10.1006/brbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- 32.Chen CY, Huang YL, Lin TH. Association between oxidative stress and cytokine production in nickel-treated rats. Arch Biochem Biophys. 1998;356:127–132. doi: 10.1006/abbi.1998.0761. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Ohls RK, Rosa C, Shah M, Richards DS, Christensen RD. Maternal and umbilical serum concentrations of granulocyte colony-stimulating factor and its messenger RNA during clinical chorioamnionitis. Obstet Gynecol. 1995;86:428–432. doi: 10.1016/0029-7844(95)00189-x. [DOI] [PubMed] [Google Scholar]
- 34.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 35.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer U, Engler A, Weber L, Schedlowski M, Feldon J. Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience. 2008;154:701–709. doi: 10.1016/j.neuroscience.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 38.Smith SE, Li J, Garbett K, Mimics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuelsson AM, Alexanderson C, Molne J, Haraldsson B, Hansell P, Holmang A. Prenatal exposure to interleukin-6 results in hypertension and alterations in the renin-angiotensin system of the rat. J Physiol. 2006;575:855–867. doi: 10.1113/jphysiol.2006.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsiao E, Patterson PH. Maternal immune activation evokes IL-6-dependent downstream signaling in the placenta and fetal brain. Paper presented at: Program No. 436.19, Neuroscience Meeting Planner; Chicago, IL. 2009. [Google Scholar]
- 41.Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain, behavior, and immunity. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero E, Ali C, Molina-Holgado E, Castellano B, Guaza C, Borrell J. Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology. 2007;32:1791–1804. doi: 10.1038/sj.npp.1301292. [DOI] [PubMed] [Google Scholar]
- 44.Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2010;15:372–383. doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- 45.Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 46.Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- 47.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 48.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 49.Zalcman S, Murray L, Dyck DG, Greenberg AH, Nance DM. Interleukin-2 and -6 induce behavioral-activating effects in mice. Brain Res. 1998;811:111–121. doi: 10.1016/s0006-8993(98)00904-4. [DOI] [PubMed] [Google Scholar]
- 50.Zalcman S, Savina I, Wise RA. Interleukin-6 increases sensitivity to the locomotor-stimulating effects of amphetamine in rats. Brain Res. 1999;847:276–283. doi: 10.1016/s0006-8993(99)02063-6. [DOI] [PubMed] [Google Scholar]
- 51.Shah A, Silverstein PS, Singh DP, Kumar A. Involvement of metabotropic glutamate receptor 5, AKT/PI3K signaling and NF-kappaB pathway in methamphetamine-mediated increase in IL-6 and IL-8 expression in astrocytes. J Neuroinflammation. 2012;9:52. doi: 10.1186/1742-2094-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luby ED, Gottlieb JS, Cohen BD, Rosenbaum G, Domino EF. Model psychoses and schizophrenia. Am J Psychiatry. 1962;119:61–67. doi: 10.1176/ajp.119.1.61. [DOI] [PubMed] [Google Scholar]
- 53.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 54.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 55.Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, et al. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–1649. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- 56.Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- 57.Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 58.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 61.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 62.Tancredi V, D'Antuono M, Cafe C, Giovedi S, Bue MC, D'Arcangelo G, et al. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- 63.Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, et al. Interleukin-6: a cytokine to forget. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 64.Braida D, Sacerdote P, Panerai AE, Bianchi M, Aloisi AM, Iosue S, et al. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav Brain Res. 2004;153:423–429. doi: 10.1016/j.bbr.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 65.Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review ofthe evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 67.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller BE, Mellor A, Buckley PF. Interleukin-6 and Cognition in Non-Affective Psychosis. Schizophr Bull. 2013;39:S242. [Google Scholar]
- 69.Himmerich H, Schonherr J, Fulda S, Sheldrick AJ, Bauer K, Sack U. Impact of antipsychotics on cytokine production in-vitro. J Psychiatr Res. 2011;45:1358–1365. doi: 10.1016/j.jpsychires.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 71.Anholt RR, De Souza EB, Oster-Granite ML, Snyder SH. Peripheral-type benzodiazepine receptors: autoradiographic localization in whole-body sections of neonatal rats. J Pharmacol Exp Ther. 1985;233:517–526. [PubMed] [Google Scholar]
- 72.Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE. Macrophage and astrocyte populations in relation to [3H]PK 11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab. 1991;11:314–322. doi: 10.1038/jcbfm.1991.64. [DOI] [PubMed] [Google Scholar]
- 73.Benavides J, Cornu P, Dennis T, Dubois A, Hauw JJ, MacKenzie ET, et al. Imaging of human brain lesions with an omega 3 site radioligand. Ann Neurol. 1988;24:708–712. doi: 10.1002/ana.410240603. [DOI] [PubMed] [Google Scholar]
- 74.Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- 75.van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 76.Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET studywith [11C]DAA1106. Int J Neuropsychopharmacol. 2010;13:943–950. doi: 10.1017/S1461145710000313. [DOI] [PubMed] [Google Scholar]
- 77.Mondelli V, Cattaneo A, Belvederi Murri M, Di Forti M, Handley R, Hepgul N, et al. Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. J Clin Psychiatry. 2011;72:1677–1684. doi: 10.4088/JCP.10m06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schnieder TP, Dwork AJ. Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry. 2011;69:134–139. doi: 10.1016/j.biopsych.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett. 1999;271:126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- 81.Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. Journal of neuropathology and experimental neurology. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- 82.Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG, et al. Distribution of HLADR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112:305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- 83.Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 84.Muller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 85.Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry. 2005;57:1594–1596. doi: 10.1016/j.biopsych.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 87.Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlledtrial. J Clin Psychiatry. 2010;71:520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 88.Muller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Moller HJ, et al. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 2004;254:14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- 89.Chaudhry IB, Hallak J, Husain N, Minhas F, Stirling J, Richardson P, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol. 2012;26:1185–1193. doi: 10.1177/0269881112444941. [DOI] [PubMed] [Google Scholar]
- 90.Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, et al. A doubleblind, randomized study of minocycline for the treatment of negative and cognitive symptoms in earlyphase schizophrenia. J Clin Psychiatry. 2010;71:138–149. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- 91.Nitta M, Kishimoto T, Muller N, Weiser M, Davidson M, Kane JM, et al. Adjunctive Use of Nonsteroidal Anti-inflammatory Drugs for Schizophrenia: A Meta-analytic Investigation of Randomized Controlled Trials. Schizophr Bull. 2013;39:1230–1241. doi: 10.1093/schbul/sbt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. Efficacy of Anti-inflammatory Agents to Improve Symptoms in Patients With Schizophrenia: An Update. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 94.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 95.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. Arch Gen Psychiatry. 2012:1–11. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abbasi SH, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S. Effect of celecoxib addon treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J Affect Disord. 2012;141:308–314. doi: 10.1016/j.jad.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 97.Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, et al. Translating glutamate: from pathophysiology to treatment. Science translational medicine. 2011;3:102mr102. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roge R, Moller BK, Andersen CR, Correll CU, Nielsen J. Immunomodulatory effects of clozapine and their clinical implications: what have we learned so far? Schizophr Res. 2012;140:204–213. doi: 10.1016/j.schres.2012.06.020. [DOI] [PubMed] [Google Scholar]