Abstract
Point-of-care platforms can provide fast responses, decrease the overall cost of the treatment, allow for in-home determinations with or without a trained specialist, and improve the success of the treatment. This is especially true for microfluidic paper-based analytical devices (μPAD), which can enable the development of highly efficient and versatile analytical tools with applications in a variety of biomedical fields. The objective of this work was the development of μPADs to identify and quantify levels of nitrite in saliva, which has been proposed as a potential marker of periodontitis. The devices were fabricated by wax printing and allowed the detection of nitrite by a colorimetric reaction based on a modified version of the Griess reaction. The presented modifications, along with the implementation of a paper-based platform, address many of the common drawbacks (color development, stability, etc.) associated with the Griess reaction and are supported by results related to the design, characterization, and application of the proposed devices. Under the optimized conditions, the proposed devices enable the determination of nitrite in the 10 to 1000 μmol L−1 range with a limit of detection of 10 μmol L−1 and a sensitivity of 47.5 AU [log (μmol L−1)]−1. In order to demonstrate the potential impact of this technology in the healthcare industry, the devices were applied to the analysis of a series of real samples, covering the relevant clinical range.
Keywords: paper-based microfluidic analytical device, nitrite, saliva, Griess Reagent
1. Introduction
Paper-based microfluidic analytical devices (μPAD) are emerging platforms that are simple, easy to use, portable, inexpensive, and disposable. In addition, they require small volumes of sample, minimal use of supporting equipment, and no external power source since fluidic movement is controlled by capillarity and evaporation [1]. Compared to traditional microfluidic devices, paper-based devices offer lower fabrication cost (as low as $0.01 per device) and do not require large microfabrication facilities, making them practical for point-of-care diagnostics in limited-resources environments [1–3]. Among other potential applications [4], μPADs can be used to aid in the diagnosis of diseases such as lower respiratory infections, acquired immunodeficiency syndrome, malaria, and oral diseases [2].
Oral diseases are a major public health concern of both developed and developing countries, having a larger incidence in communities with limited resources [5]. According to a recent national survey held between 2009–2010 [6], approximately 50% of North American adults are affected by periodontitis. This disease can lead to the formation of tooth abscess, tooth loss, or infection of the surrounding tissue and bones [7]. In addition, periodontal disease can increase the risk factors for systemic diseases such as coronary heart disease, atherosclerosis, and stroke [8]. The initiation and the progression of periodontitis are dependent on the presence of virulent microorganisms. However, the host response to the pathogenic infection is critical to disease progression [9]. Traditional methods of diagnosis of periodontitis include probing the gingival tissues for bleeding and suppuration, inspecting for biofilm deposits, and taking X-rays to evaluate the teeth and bone [10, 11]. Because these techniques require the patient to be evaluated by a specialist at an off-site location, their use in developing countries and remote areas is very limited. Alternatively, periodontitis can be traced in saliva (non-invasively and easily-accessible [12–17]) using inflammatory biomarkers produced by either the host or the pathogenic Gram-negative bacteria [9, 13]. Although research in this area is still in the early stages, the concentration of nitrite (an antimicrobial agent [12] produced from nitric oxide released by endothelial NO-synthases [14]) in saliva has been associated with the clinical symptoms of periodontitis (gingival redness, swelling, and bleeding). More importantly, and although the relevant clinical range can be affected by several variables, most reports indicate that the concentration of nitrite can be used as an indicator of the progression of the disease.
A variety of analytical methods have been developed for the quantification of nitrite [18]. Among them, spectrophotometric methods (mostly based on the Griess reaction [19]) offer an adequate balance between simplicity, instrumental needs, cost, and sensitivity. Although it has been extensively used, common drawbacks of the method include the need of a spectrophotometer as well as large reagent and sample volumes. Even though attempts to integrate this method with μPADs have been presented [20–22], the technology still suffers from many drawbacks including adverse color development and limited stability [23].
Aiming to develop a suitable tool for the detection of nitrite that could lead to further understanding of the progression and treatment of periodontitis and to guide professionals to effectively plan for on-site campaigns to control periodontal diseases [24], this paper describes the development of a user-friendly μPAD for the quantification of nitrite. Results related to the optimization of the analytical platform and the analyses of real samples are herein presented.
2. Materials and Methods
2.1 Reagents
The Griess reagent was prepared by mixing 1:1 ratio of 1% (w/v) sulfanilamide (Sigma-Aldrich; St. Louis, MO) in 5% (v/v) phosphoric acid solution (Fluka; Buchs, SG Switzerland) and 0.1% (w/v) N-naphthylethylenediamine dihydrochloride (NED; Sigma-Aldrich; St. Louis, MO) in water. Artificial saliva was prepared in accordance to Yildirmaz et al. [10], and contained 0.4 mg mL−1 NaCl (Sigma-Aldrich; St. Louis, MO), 0.4 mg mL−1 KCl (EM Science; Gibbstown, NJ), 0.8 mg mL−1 CaCl2 (Alfa Aesar; Ward Hill, MA), 0.69 mg mL−1 NaH2PO4 (Fisher Scientific; Waltham, MA), and 0.0163 mg mL−1 Na2S (Sigma-Aldrich; St. Louis, MO). A stock solution of 10 mmol L−1 sodium nitrite (Sigma, St. Louis, MO) was prepared in artificial saliva and subsequent dilutions were prepared for each of the calibration standards. Commercially available Griess reagent in acetic acid was obtained from Fluka (Buchs, SG Switzerland), hydrochloric acid from Synth (Diadema, SP Brazil), glucose from Mallinckrodt (Hazelwood, MO), ascorbic acid from Fisher Scientific Company (Hampton, NH), butylated hydroxytoluene from Sigma-Aldrich (St. Louis, MO), uric acid from Alfa Aesar (Ward Hill, MA), and sodium dodecyl sulfate from Sigma-Aldrich (St. Louis, MO). All chemicals were used as received and all solutions were prepared in 18 MΩ-cm water (NANOpure Diamond, Barnstead; Dubuque, Iowa).
2.2 Fabrication of μPADs
The selected prototype for the μPADs used in this manuscript contains a main channel with four identical arms and four round “testing zones”, which are designed using black lines and shapes on a white background using CorelDraw® Graphics Suite X5 (shown in the Supplementary Information). Although all four testing zones were dedicated for the analysis of nitrite, the chip was conceived with the future goal of including controls for standard additions or measuring multiple analytes including proteins, carbohydrates [25, 26], and metabolites [3]. The designs were printed with wax toner (Genuine Xerox Solid Ink Black; Xerox; Norwalk, CT) onto Grade No. 1 Thin Chromatography Paper from Whatman® (VWR; Radnor, PA) using a laser printer (Xerox Phaser 8560; Norwalk, CT). The paper is considered an ideal substrate for the proposed μPAD because it is made from cellulose and provides a network of hydrophilic micro-channels (scanning electron microscopy image shown in Supplemental Information) for sample uptake through capillary action, avoiding the application of driving potential or pressure. After printing, the μPADs were heated for 2 min at 150 °C with a hot iron press to melt the wax toner through the cellulose, creating the hydrophobic barriers to guide fluid movement. The dimensions of the finished μPADs are 24 mm by 24 mm, with a 2 mm width for the main channel and 3 mm diameter for the testing zone.
2.3 Analysis technique
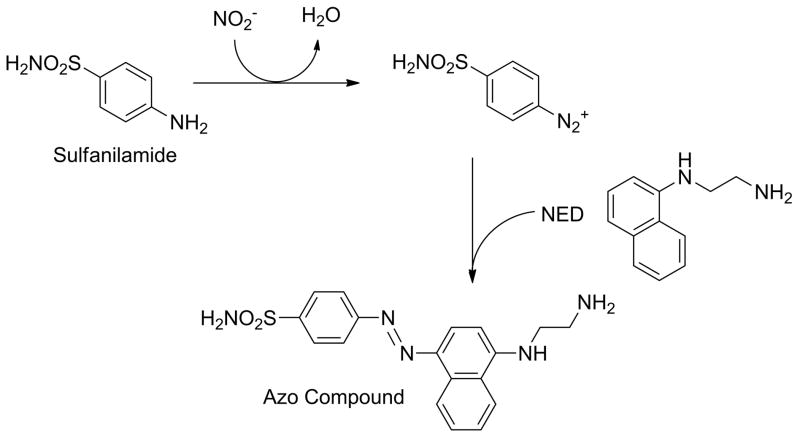
After fabrication of the μPADs, 0.5 μL of Griess reagent were spotted on the testing zones (detection areas) and allowed to dry for 15 min. For the analysis procedure, a 12-μL droplet of either sample or standard solution was dispensed onto a hydrophobic material, such as Parafilm (Pechiney Plastic Packaging Company; Chicago, IL). The main channel of the μPAD was then brought into vertical contact with the droplet to allow sample uptake by capillarity and drive the solution into the branched channels and testing zones. In the presence of nitrite, the two components of the Griess reagent lead to the formation of a magenta azo compound, as described by the reaction scheme in Figure 1. Under optimized conditions, the color intensity can be related to the concentration of nitrite present in the sample, which in turn can be used as an indication of the progression of periodontitis.
Figure 1.
Reaction scheme of the production of the azo dye by the interaction of the Griess reagent and nitrite.
After sample uptake, the μPAD was allowed to dry for 15 min. Finally, the μPAD was scanned using a basic flatbed scanner under optimized brightness and contrast settings (Canon CanoScan LiDE 700F; Lake Success, NY) and the image was analyzed using Adobe® Photoshop® CS6. The color intensity was calculated by measuring the magenta channel in Photoshop® (when set to CMYK format). Additionally, the image can be converted to grayscale and analyzed using the gray channel as the primary histogram color, if required. The average intensity of the selected color channel yields a numerical value, which can be used to quantify the concentration of nitrite in the sample.
3. Results and Discussion
Multiple generations of the μPADs were sequentially designed to address the practicality and applicability of an analytical platform for the detection of nitrite. Initial experiments, performed with the device described in section 2.2, revealed two critical flaws in the traditional protocol. First, significant degradation of the Griess reagent was noticed once spotted on the μPAD, causing the testing zone to blush a noticeable faint pink within min and regardless of the presence of nitrite. This effect significantly affected the limit of detection of the assay. Second, the sample uptake by capillarity resulted in an uneven distribution of the azo dye in the testing zones of the μPAD, yielding to the formation of a color gradient that impaired the quantification step. In order to minimize the aforementioned effects, the stability of the Griess reagent spotted on the μPAD and subsequent color development were investigated as a function of solvent and stabilizer type, temperature, and storage environment.
3.1 Color development and stability
Considering that significant differences in color development and stability can be observed for the type and ratio of reagents used to prepare the Griess solution [27], the acetic acid present in the commercial Griess reagent was replaced by either 10% HCl or 5% H3PO4. While it was observed that both solutions provided a more acidic environment for the reaction and significantly increased the development of the color [23, 28], the use of HCl adversely affected the sensitivity of the analysis at low concentrations (< 50 μmol L−1), limiting the applicability of the method to clinical samples. As described by Fox [29], the color development in the selected reaction results as a balance of the formation of HNO2, protonation of the naphthalene derivative, and the potential decomposition of the azo dye at lower pH values (such as 10% HCl). According to our results, and although the pink blushing resulting from the degradation of the Griess reagent was still evident, it was concluded that 5% H3PO4 provided the best analytical performance of the device and was implemented in all subsequent experiments.
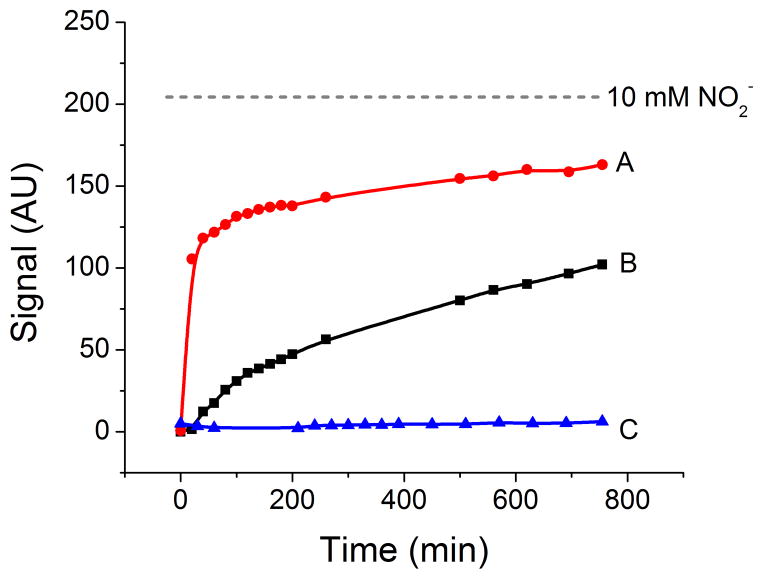
Because a gradual degradation of the reagent was observed in all devices (vide infra, Figure 3), the efficiency of a series of stabilization methods was also investigated. Considering that aniline-like compounds are known to readily oxidize in air, it was hypothesized that the pink blushing was linked to an oxidation during the drying, storage, and/or usage steps. For the specific case of sulfanilamide, Seikel [30] and Ermakova et al. [31] reported that its decomposition proceeds mostly to the formation of azobenzene-4,4′-disulphonamide and azoxybenzene-4,4′-disulphonamide. Therefore, the objective was to implement the simplest and least expensive strategy that can effectively limit the exposure of the reagents to the environment without interfering with the analysis. The first series of experiments aimed at the inclusion of encapsulating agents, prepared in solution with the Griess reagent. These included glucose (for entrapment within its crystalline structure) and sodium dodecyl sulfate (for entrapment in micelles) [32]. Although potentially useful, the addition of glucose yielded to the formation of a brown color (Maillard reaction) and the addition of SDS produced a dramatic loss of color uniformity after sample uptake. In increasing order of complexity, the second strategy was to include an antioxidant in the sample spot. However, neither ascorbic acid, butylated hydroxytoluene, nor uric acid [33] showed significant effects at preventing the degradation of the reagent. The third strategy to increase the stability of the reagent included physical alterations to the μPAD by the addition of a layer of clear adhesive tape, lamination, or by placing the chip inside vacuum-sealed zip-lock bags until use. Although slight improvements in stability were observed, the devices were deemed as ineffective within few hours. Based on these experiments and considering the cost/benefit of the potential solutions to the color development, a new design of the μPADs was pursued.
Figure 3.
Development of the background signal (in arbitrary units) of the Griess reagent when dried and stored in air (A), when dried under nitrogen and stored in air (B) and when dried and stored under nitrogen (C). For comparison purposes, the figure also includes the signal obtained when 10 mmol L−1 nitrite was added. All experiments were conducted at room temperature.
3.2 Redesign of the μPAD
One of the most important limitations of the Griess reagent is its poor stability upon mixture of the components. Therefore, and following the strategy presented by Abe and Schilling [34, 35], the μPAD was redesigned to separate the components of the Griess reagent and to minimize the degradation during storage (Figure 2). It is worth mentioning that because sulfanilamide and NED both compete for the reaction with nitrite, 0.5 μL of sulfanilamide were spotted in the uptake zones and 0.5 μL of NED were spotted the testing zone, ensuring the sequential reaction with sulfanilamide and the formation of the azo dye in the testing zones during sample uptake [27]. The dimensions of the final design of the μPAD are 24 mm by 24 mm, with a 3 mm width in the main channel and 3 mm diameter for the uptake and testing zones.
Figure 2.
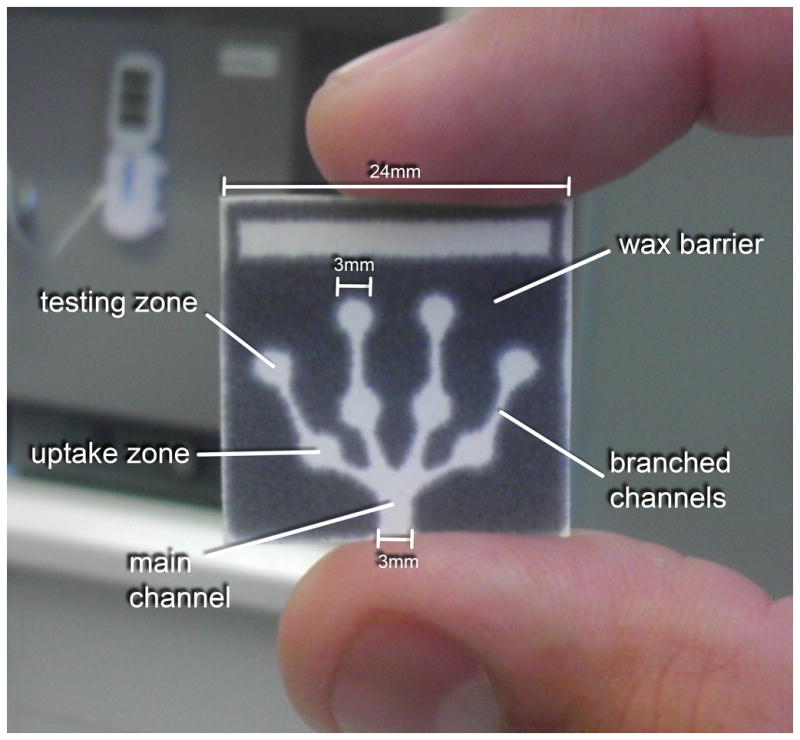
The redesigned μPAD for nitrite detection in saliva showing the hydrophilic main channel, branched channels, uptake zones, and testing zones surrounded by the hydrophobic wax barrier.
This design for the μPAD showed significant improvements in terms of color gradient and color intensity. Although significant reductions of the pink blushing were observed, the phenomenon was still evident in the testing zones when blank solutions (0 μmol L−1 nitrite) were allowed to dry in air for 15 min. As a representative example of the results obtained, Figure 3 (trace A) shows the color development as a function of time observed when the Griess reagent was spotted on the μPAD, dried and stored at room temperature, under normal atmospheric conditions. The severity of this process is evident when the signal observed for the blank is compared to the signal obtained upon the addition of a solution containing 10 mmol L−1 nitrite (Figure 3, dotted line). This observation, that was consistent with the behavior of the traditional Griess reagent, was considered detrimental to the detection of low levels of nitrite (< 50 μmol L−1). In order to identify the most critical step affecting the color development and propose an adequate strategy to increase shelf life, the devices were dried under a stream of nitrogen and then stored in air. As it can be observed in Figure 3 (trace B), although a significant reduction in the blushing process was obtained, these results still show noticeable pink blushing, limiting the possibility of preparing and shipping the chips to remote locations. Alternatively, and confirming that the described pink blushing could be attributed to the oxidation of the Griess reagents, significant improvements in stability were observed when the μPADs were dried and stored under nitrogen (Figure 3, trace C). Advantageously, once the reaction with nitrite was completed and the chip was dried, the color was stable for several hours; giving ample time to scan the devices and perform the data analysis.
In summary, and although the color development was only followed for 12 hours, the results indicate that the contributions of non-oxidative processes to the pink blushing are negligible and should not affect the signal-to-noise ratio. More importantly, these experiments indicate that the degradation of the Griess reagent during the manufacturing and storage process could be minimized by simply spotting the sulfanilamide and NED in separate wells and limiting their exposure to oxygen. This could be efficiently achieved by placing the devices under nitrogen in a disposable plastic bag. Although the requirement of nitrogen to dry the μPADs (after introducing the sample) could hinder the development of some point-of-care applications involving the analysis of low concentrations of nitrite, it is envisioned that professionals can still use the μPADs at on-site diagnosis centers. For testing locations with limited resources, the μPADs can be dried using a can of compressed nitrogen, which is readily available. In addition, semi-quantitative analysis of nitrite on wet μPADs can be performed by comparing the color developed from the azo dye to a color scale (also printed on the paper), making the overall analysis time even shorter.
3.3 Effect of temperature
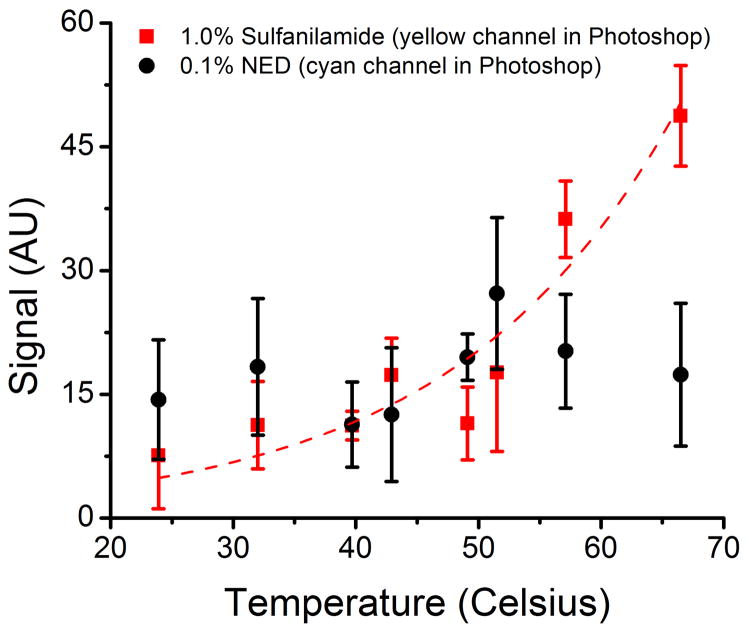
In order to evaluate the effect of temperature on the color development of the proposed design, μPADs spotted with sulfanilamide and NED (in separated zones) were exposed to temperatures ranging from 24 °C to 67 °C under nitrogen, over a period of two hours for each temperature level. The developed color in the zone spotted with sulfanilamide (faint yellow color) and in the zone spotted with NED (faint blue color) was evaluated using either the yellow or cyan color channel in Photoshop, respectively. The results are summarized in Figure 4. Although all μPADs developed some blushing, the temperature had a more significant effect on sulfanilamide, which developed considerable color intensity above 49 °C. These results indicate that while refrigeration would be required for shipping and storage in high-temperature locations, the proposed μPADs can be stored and shipped under ambient temperature conditions to most areas with mild temperatures without significant effects to their analytical performance.
Figure 4.
Effect of temperature on the development of background signal (in arbitrary units) in the zones spotted with each component of the Griess reagent (under nitrogen). Lines added to guide the eyes.
3.4 Analytical performance
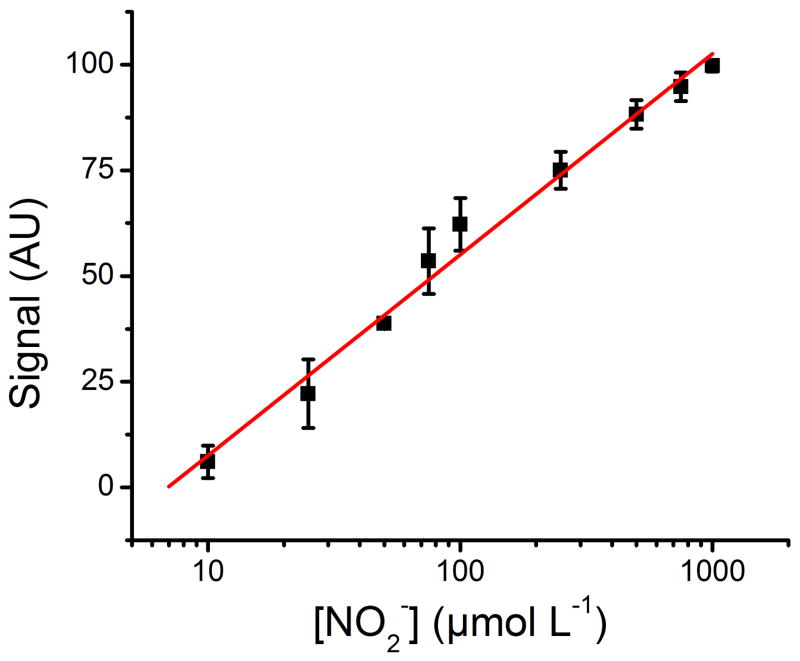
In order to quantify nitrite in pertinent samples, calibration standards were prepared using artificial saliva and covered a 10 – 1000 μmol L−1 range of nitrite (including a blank solution to evaluate blushing during the drying, analysis, and storage process). For this calibration curve, the μPADs shown in Figure 2 were printed, spotted with sulfanilamide and NED (in separated zones), and dried/stored under nitrogen at room temperature until use. Once exposed to the standard solution, the devices were dried under a stream of nitrogen, scanned, and analyzed using the gray channel in Photoshop®. As shown in Figure 5, a linear relationship was obtained between the color intensity (I) and the logarithm of the concentration of nitrite (I = 47.522 * log [NO2−] − 39.973, R2= 0.988)
Figure 5.
Analytical calibration curve exhibiting logarithmic behavior from 10 – 1000 μmol L−1 nitrite. The standard solutions were prepared in artificial saliva and analyzed using the newly designed μPAD, a scanner, and the gray channel in Photoshop®. Signal was normalized against a selected blank location on the μPAD.
This calibration curve allowed determining an experimental limit of detection of 10 μmol L−1. It is worth noting that the calibration curve shows high correlation under the logarithmic scale, allowing the μPADs to be applied in a clinically relevant range without dilutions. This logarithmic correlation can be attributed to a combination of the sensitivity of the flatbed scanner to distinguish between darker shades of the azo dye, saturation of the azo dye on the analyzable surface of the μPAD, and the consumption of sulfanilamide and NED by the standard solutions.
3.5 Analysis of nitrite in saliva
Experiments involving saliva from human volunteers were conducted in accordance with and upon approval of the Institutional Review Board at the University of Texas at San Antonio. All subjects received informed consent forms before inclusion in the study and voluntarily agreed to participate. Samples of saliva were collected from six subjects 2.5 hours after consumption of food. The samples were filtered using a 0.2 μm PTFE filter (VWR; Radnor, PA) and analyzed for nitrite using the μPAD. Table 1 summarizes the results from six subjects using the μPAD as a platform for nitrite detection.
Table 1.
Concentration of salivary nitrite in samples collected from the three males and three females volunteers.
| Sample # | μPAD Signal (AU) | μPAD [NO2−] (μmol L−1) | UV [NO2−] (μmol L−1) |
|---|---|---|---|
| 1 | 26 ± 12 | 25 ± 14 | 26.1 ± 0.2 |
| 2 | 70 ± 6 | 209 ± 59 | 161.7 ± 0.2 |
| 3 | 89 ± 3 | 521 ± 79 | 434.2 ± 0.2 |
| 4 | 97 ± 3 | 766 ± 99 | 765.6 ± 2.8 |
| 5 | 26 ± 13 | 25 ± 15 | 25.7 ± 0.0 |
| 6 | 33 ± 9 | 35 ± 15 | 37.3 ± 0.2 |
As it can be observed, the samples of saliva (obtained from presumably healthy subjects with no periodontal disease, as identified by the subjects themselves) yielded concentrations in a range spanning from 25 to 766 μmol L−1. In order to validate these results, the saliva samples were also analyzed by spectrophotometry (measuring absorbance at 548 nm; Thermo Genesys 10UV, Waltham, MA) using the Griess method [36]. As it can be also observed in Table 1, a very good correlation was obtained between the two methods, demonstrating the suitability of the μPAD platform to quantify nitrite concentration. It is important to note that although the clinical relevance of these results is still relative [15] and could be affected by a series of specific variables (including sample preparation [37], diet [38], general health, genetics, and gender), the proposed devices were able to assess the nitrite concentration in a range that spans normal healthy conditions. It is also interesting to note that, although identified as healthy, the two individuals with the highest nitrite concentration (#3 and #4) disclosed recent lip-biting events, which could yield an inflammatory response in the oral tissue and the subsequent increase in nitrite concentration in saliva. Additional experiments are currently underway to further understand this effect and validate the clinical results with a larger pool of human samples.
It is also important to note that the analysis of the saliva samples using the μPADs were analyzed using an office-grade scanner, yielding significantly larger errors than the traditional methodology. Although this presents a limitation, we believe the advantages of the μPADs in terms of portability, stability, cost, sample handling, and analysis time clearly surpass the disadvantages. In addition, the test could also become an additional tool to support the diagnostic expertise of the dentists, not only relying on the number (and the large error) obtained in the device.
4. Conclusions
The capability of the μPADs to yield quantitative results for non-invasive monitoring of nitrite in saliva was examined. In order to maximize the color development, the acetic acid of the Griess reagent was replaced by 5% H3PO4. Although early experiments demonstrated that the oxidation of the components of the Griess reagent can yield to the development of a pink blushing on the detection zones, the possibility of separating the components in the μPAD and the use of nitrogen to dry and store the devices significantly enhanced their shelf life. The optimized configuration was used to provide a quick, easy, and inexpensive analytical method of quantifying nitrite levels in human saliva. This methodology could aid research leading to establish a definitive relationship between salivary nitrite levels and periodontitis. As the proposed μPADs may also be used to quantify nitrite in food [39] and water samples [40], as well as other biological samples (such as urine [41]), the approach may become vital to environments where semi-quantitative results are required or when the gains in analysis time, cost, and portability justify the slight loss in analytical performance. In addition, with the concomitant use of simple devices, such as phones with cameras, one can transfer data to an analyst at an off-site location for immediate analysis [24].
Supplementary Material
Implemented a modified version of the Griess reaction on microfluidic paper-based analytical devices (μPAD) to quantify levels of nitrite in saliva
Detection performed in few minutes covering the 10 – 1000 μmol L−1 nitrite range
Reliable alternative to benchtop instrumentation for nitrite determination
Acknowledgments
The authors thank the financial support provided by the National Science Foundation through the International Research Experience for Students (OISE-0965814) and the Partnership for Education and Research in Materials (DMR-0934218). The support from the National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health, the University of Sao Paulo and the National Council for Scientific and Technological Development (CNPq, Brazil) are also acknowledged. The authors would also like to thank Matthew T. Gordon for his contributions to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Anal Chem. 2010;82:3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 2.Mao XL, Huang TJ. Lab Chip. 2012;12:1412–1416. doi: 10.1039/c2lc90022j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrilho E, Martinez AW, Whitesides GM. Anal Chem. 2009;81:7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 4.Coltro WKT, de Jesus DP, da Silva JAF, do Lago CL, Carrilho E. Electrophoresis. 2010;31:2487–2498. doi: 10.1002/elps.201000063. [DOI] [PubMed] [Google Scholar]
- 5.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. B World Health Organ. 2005;83:661–669. [PMC free article] [PubMed] [Google Scholar]
- 6.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 7.W.H. Organization. Oral health - Fact Sheet N°318. 2012 http://www.who.int/mediacentre/factsheets/fs318/en/index.html.
- 8.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Periodontol 2000. 1997;14:216–248. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 9.Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Dent Clin North Am. 2005;49:551–571. vi. doi: 10.1016/j.cden.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. J Clin Periodontol. 1986;13:590–596. doi: 10.1111/j.1600-051x.1986.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 11.Cutler CW, Stanford TW, Abraham C, Cederberg RA, Boardman TJ, Ross C. J Clin Periodontol. 2000;27:134–143. doi: 10.1034/j.1600-051x.2000.027002134.x. [DOI] [PubMed] [Google Scholar]
- 12.Allaker RP, Mendez LSS, Hardie JM, Benjamin N. Oral Microbiol Immunol. 2001;16:253–256. doi: 10.1034/j.1399-302x.2001.160410.x. [DOI] [PubMed] [Google Scholar]
- 13.Menaka KB, Ramesh A, Thomas B, Kumari NS. J Indian Soc Periodontol. 2009;13:75–78. doi: 10.4103/0972-124X.55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lappin DF, Kjeldsen M, Sander L, Kinane DF. J Periodontal Res. 2000;35:369–373. doi: 10.1034/j.1600-0765.2000.035006369.x. [DOI] [PubMed] [Google Scholar]
- 15.Reher VG, Zenobio EG, Costa FO, Reher P, Soares RV. J Oral Sci. 2007;49:271–276. doi: 10.2334/josnusd.49.271. [DOI] [PubMed] [Google Scholar]
- 16.Batista AC, Silva TA, Chun JH, Lara VS. Oral Dis. 2002;8:254–260. doi: 10.1034/j.1601-0825.2002.02852.x. [DOI] [PubMed] [Google Scholar]
- 17.Aurer A, Aleksic J, Ivic-Kardum M, Aurer J, Culo F. J Clin Periodontol. 2001;28:565–568. doi: 10.1034/j.1600-051x.2001.028006565.x. [DOI] [PubMed] [Google Scholar]
- 18.Moorcroft MJ, Davis J, Compton RG. Talanta. 2001;54:785–803. doi: 10.1016/s0039-9140(01)00323-x. [DOI] [PubMed] [Google Scholar]
- 19.Weselsky P, Benedikt R. Ber Dtsch Chem Ges. 1879;12:226–230. [Google Scholar]
- 20.Klasner SA, Price AK, Hoeman KW, Wilson RS, Bell KJ, Culbertson CT. Anal Bioanal Chem. 2010;397:1821–1829. doi: 10.1007/s00216-010-3718-4. [DOI] [PubMed] [Google Scholar]
- 21.He Q, Ma C, Hu X, Chen H. Anal Chem. 2012;85:1327–1331. doi: 10.1021/ac303138x. [DOI] [PubMed] [Google Scholar]
- 22.Martinez AW, Phillips ST, Nie ZH, Cheng CM, Carrilho E, Wiley BJ, Whitesides GM. Lab Chip. 2010;10:3428–3428. doi: 10.1039/c0lc00021c. [DOI] [PubMed] [Google Scholar]
- 23.Senra-Ferreiro S, Pena-Pereira F, Lavilla I, Bendicho C. Anal Chim Acta. 2010;668:195–200. doi: 10.1016/j.aca.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 24.Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM. Anal Chem. 2008;80:3699–3707. doi: 10.1021/ac800112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz J. J Clin Periodontol. 2001;28:710–712. doi: 10.1034/j.1600-051x.2001.028007710.x. [DOI] [PubMed] [Google Scholar]
- 26.Oates TW. J Evid Based Dent Pract. 2012;12:154–155. doi: 10.1016/j.jebdp.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Promega. Griess Reagent System Protocol. 2012 http://www.promega.com/tbs/tb229/tb229.pdf.
- 28.Giustarini D, Rossi R, Milzani A, Dalle-Donne I. In: Method Enzymol. Enrique C, Lester P, editors. Academic Press; 2008. pp. 361–380. [DOI] [PubMed] [Google Scholar]
- 29.Fox JB. Anal Chem. 1979;51:1493–1502. [Google Scholar]
- 30.Seikel MK. J Am Chem Soc. 1940;62:1214–1216. [Google Scholar]
- 31.Ermakova GA, Skachilova SY, Voronin VG, Shramova ZI. Pharm Chem J. 1983;17:582–589. [Google Scholar]
- 32.Hagopian DS, Riley JG. Aquacult Eng. 1998;18:223–244. [Google Scholar]
- 33.Denisov ET, Afanas’ev IB. Oxidation and Antioxidants in Organic Chemistry and Biology. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- 34.Abe K, Kotera K, Suzuki K, Citterio D. Anal Bioanal Chem. 2010;398:885–893. doi: 10.1007/s00216-010-4011-2. [DOI] [PubMed] [Google Scholar]
- 35.Schilling KM, Lepore AL, Kurian JA, Martinez AW. Anal Chem. 2012;84:1579–1585. doi: 10.1021/ac202837s. [DOI] [PubMed] [Google Scholar]
- 36.Molecular Probes, Griess Reagent Kit for Nitrite Determination. 2008 http://www.mobitec.de/probes/docs/media/pis/mp07921.pdf.
- 37.Morelatto RA, Benavidez TE, Baruzzi AM, Solís VM, LdBlanc SA. AJAC. 2011;2:683–688. [Google Scholar]
- 38.Tsikas D. Free Radical Res. 2005;39:797–815. doi: 10.1080/10715760500053651. [DOI] [PubMed] [Google Scholar]
- 39.Walker R. Food Addit Contam. 1990;7:717–768. doi: 10.1080/02652039009373938. [DOI] [PubMed] [Google Scholar]
- 40.Fan AM, Steinberg VE. Regul Toxicol Pharm. 1996;23:35–43. doi: 10.1006/rtph.1996.0006. [DOI] [PubMed] [Google Scholar]
- 41.Lundberg JON, Carlsson S, Engstrand L, Morcos E, Wiklund NP, Weitzberg E. Urology. 1997;50:189–191. doi: 10.1016/S0090-4295(97)00257-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.