Abstract
Mucopolysaccharidosis type IIIA (MPS IIIA) is a neurodegenerative disease with behavioral symptoms unique among the mucopolysaccharidoses. Children with MPS IIIA reportedly mouth things, explore novel environments almost continuously, disregard danger, and empathize/socialize and comply less with parents. These characteristics resemble Klüver–Bucy syndrome (K-Bs). To test the K-Bs hypothesis, 30 children with MPS IIIA were compared to 8 ‘post-transplant’ Mucopolysaccharidosis type IH patients in an experimental “Risk Room”. The room contained attractive and mildly frightening objects, exposure to a 92 dB startle noise triggered by contact with an attractive toy, mother’s return after a brief absence, and compliance with her clean-up directive. Children with MPS IIIA: 1) left mother sooner, 2) wandered more, 3) were more likely to approach frightening objects, 4) were less likely to respond to loud noise with whole body startle, 5) were less likely to avoid the toy associated with the startle noise, 6) interacted less with mother upon her return, and 7) complied less with her clean-up command. K-Bs is associated with loss of amygdala function. Brain MRIs of a subset of the children with MPS IIIA showed volume loss that was greater in the amygdala than the hippocampus; only amygdala loss correlated with reduced fearfulness. MPS IIIA may be the first identified pediatric disease presenting systematically as a K-Bs variant. If validated by further studies, the K-Bs hypothesis of MPS IIIA would provide important clinical and theoretical information for the guidance of families as well as markers for natural disease progression and treatment effects.
Keywords: amygdala, fear conditioning, orality, Sanfilippo syndrome, startle
Mucopolysaccharidosis type IIIA (MPS IIIA) is a lysosomal storage disorder caused by inherited reduction in the activity of sulfamidase, which metabolizes heparan sulfate. Its incidence is about one in 100,000 births (Baehner et al., 2005; Meikle, Hopwood, Clague, & Carey, 1999; Poorthuis et al., 1999). MPS IIIA involves progressive central nervous system dysfunction, neurocognitive decline and notable behavioral abnormalities. Children with MPS IIIA die in their teens or early twenties, primarily from neurologic causes. Cleary and Wraith (1993) proposed a three stage model of disease progression: 1) developmental delays, especially in language; 2) behavioral and sleep abnormalities; 3) loss of mobility, feeding difficulties, and seizures. Anecdotally, parents report that children with MPS IIIA exhibit many odd and troubling behaviors during Stage 2 which differ markedly from those of children with other MPS diseases, such as MPS IH (Hurler syndrome), who are equally cognitively impaired (e.g., Colville & Bax, 1996; Robertson, Klug, & Rogers, 1998). Little is known scientifically about the Stage 2 behavioral phenotype of MPS IIIA. Characterizing this phenotype can provide guidance and support to these families, markers for natural disease progression, and perhaps some insight into underlying brain pathology.
To develop hypotheses about the behavioral phenotype, telephone interviews were conducted with parents of children with MPS IIIA, clinical reports were gathered and observations made by the authors. One salient parent-reported behavior is “orality”, the tendency to put things in the mouth. The children’s heedlessness of danger is of greater concern, often requiring extensive “child-proofing” of the home and unremitting parental attention outside it. Empathic capacity and social affiliation are diminished, sometimes severely so, compared to same age peers. These children are described as frequently oppositional and aggressive, thwarting attempts at discipline. We observed the children’s almost non-stop exploratory locomotion in novel situations interspersed with brief and repeated object handling.
Many of these characteristics resemble those of Klüver–Bucy syndrome (K-Bs) in monkeys following bilateral temporal lobectomy. The original observations included exploring items by mouth, “hyper-metamorphosis” (repeated exploration of objects), and dampening of emotional expression, including diminished fear (for review see Neylan, 1997). Subsequent studies on rhesus and other species associated most of these effects with amygdala dysfunction. These studies also highlighted reduced and inappropriate social interactions (e.g., Meunier, Bachevalier, Murray, Málková, & Mishkin, 1999.) Reported cases of K-Bs in human adults result from diverse etiologies but show similar behavioral features (e.g., Aygun, Guven, Altintop, & Doganay, 2003; Janszky, Fogarasi, Magalova, Tuxhorn, & Ebner, 2005). K-Bs secondary to herpes encephalitis, anoxic-ischemic encephalopathy and other conditions is reported in children (e.g., Jha, Kumar, Kumar, & Kumar, 2005; Pradhan, Singh, & Pandey, 1998). These individuals show marked indifference and lack of emotional attachment to caretakers; they also show hyperorality and increased masturbation. MRIs confirm anterior temporal lobe damage.
If validated, the hypothesis that MPS IIIA presents as K-Bs would provide important clinical guidance and theoretical insight. However, the K-Bs hypothesis rested on anecdotal reports. More convincing evidence must be developed through experimental observation. Given the constraints of low cognitive function and non-cooperation expected from these children, we developed an experimental “Risk Room” design capitalizing on their spontaneous behavior and reactions to highly salient stimuli. Our specific hypotheses were that, compared to controls, children with MPS IIIA will: 1) Leave mother sooner, 2) wander more, 3) be more likely to approach frightening objects, 4) show reduced or absent whole body startle in response to noise, 5) be less likely to avoid the object associated with the startle noise, 6) interact less with mother in a Strange Situation challenge, and 7) be less compliant with a clean-up command. Because orality is a key feature of KB-S, based on maternal report, we also hypothesized that their frequency of oral behavior is high. To control for the effects of cognitive decline associated with MPS, children with MPS IIIA were compared to those with MPS 1H.
We also hypothesized that reduced amygdala volumes, reflecting the neurodegenerative process, would be associated with Risk Room behaviors. Such associations might occur with amygdala volumes as measured at the time of testing, reflecting prior impaired growth and/or degeneration. Alternatively, behaviors might be associated with volume change over time, reflecting the rate of degeneration. Because a generalized atrophy associated with cognitive decline is also expected, the neuroanatomical specificity of any amygdala-associated effects was determined by comparison to the neighboring hippocampus.
METHOD
Participants
MPS IIIA patients
25 children with MPS IIIA, age 2 to 18 years, were recruited from a Natural History (NH) study sponsored by ShireHGT at the University of Minnesota (Clinicaltrials.gov). This longitudinal study detailed the natural course of MPS IIIA identifying potential endpoints for future treatment trials. Patients in the NH study met the following criteria: 1) MPS IIIA diagnosis confirmed by enzyme or genetic assay, 2) minimum chronological age of one year, 3) developmental age > 12 months on the Vineland Adaptive Behavior Scales. Exclusion criteria were: 1) known hypersensitivity to anesthesia, 2) history of ameliorative treatment with any investigational drug or with 3) hematopoietic cell transplant, 4) significant non-MPS IIIA-related CNS impairment, 5) medications that would significantly interfere with performance, 6) any condition or device precluding MRI, and 7) blindness and/or deafness.
Additional inclusion criteria for this current study were being more than two years old and independently ambulatory. NH study participants came for three visits at 6 month intervals; our neurobehavioral testing was done at the first visit, (one patient, not yet two years old at the first visit, was tested at the second visit.) At the testing visit patients had cognitive assessments, the Vineland Adaptive Behavior Scale and a sedated MRI. The sedated MRI was repeated when the NH patients came for their next visit. After 25 patients had been tested and the study closed, five additional patients who met criteria for entry, and had contacted us to be in this study were seen as clinical patients and given exactly the same measures. They were not different than the 25 previous patients in any way except that the second MRI was not obtained.
MPS IH patients
MPS IH patients were chosen for comparison because 1) they have a similar cognitive course if untreated and 2) they are described as behaviorally normal. MPS IH is similarly rare, but involves more physical abnormalities (e.g. orthopedic, cardiorespiratory, short stature) than MPS IIIA. Both groups have hearing difficulties. Eight post-hematopoietic cell transplant MPS IH patients were recruited from a NIH supported longitudinal study of brain structure and function in MPS disorders Types I, II, and VI (NIH U54NS065768) to form the comparison group.
The Institutional Review Board of The University of Minnesota’s Medical School approved the main NH project and this ancillary Risk Room study. When obtaining written consent for the NH study, parents were separately consented for this study. The five MPS IIIA clinical patients were consented only for this study.
Twenty-four children were initially diagnosed before 6 years of age, consistent with the typical early form of MPS IIIA. Children diagnosed after age 6 were classified as having an atypical form with a slower decline (Heron et al., 2011; Hopwood, 2007; see Table 1). The 8 MPS IH patients were most similar in age and disease course to the children diagnosed with MPS IIIA before age 6.
Table 1.
Dx < 6 yrs are children diagnosed before age 6, Dx > 6 yrs are children diagnosed after age 6
| MPS IH | MPS IIIA Dx < 6 yrs |
MPS IIIA Dx > 6 yrs |
|
|---|---|---|---|
| N, male:female | 3:5 | 17:7 | 3:3 |
| Mean age (SD) at test (months) | 52 (17) | 55 (22) | 149 (54) |
| Cognitive function – DQ | 85 (14.7) | 51 (26.5) | 25 (19.9) |
| Cognitive function – Age Equivalent in months |
41 (14.4) | 24 (12.1) | 33 (21.8) |
Procedures
Experimental Rationale
The Risk Room of the Laboratory-Temperament Assessment Battery (Buss & Goldsmith, 2000) permits evaluation of social/emotional behaviors in lower functioning children. Capitalizing upon children’s tendencies to explore their environment, it contains a mix of attractive and mildly frightening objects. The Risk Room has been extensively used to study children’s temperament including fearfulness (e.g., Hayden et al., 2007.)
Fear has measurable effects at other levels, too. At the reflex level, experimentally induced fear in rats, and clinical anxiety in humans, potentiates auditory startle. These effects are mediated by the amygdala (Davis, 2006). Conversely, amygdala lesions reduce fear-potentiated startle in rats while amygdala degeneration has been associated with loss of startle in a few clinical cases (Angrilli et al., 1996.) The dependence of startle on amygdala function allows a test of the K-Bs hypothesis at the reflex level, tapping amygdala-brainstem connections independent of the complexities of learned behavior.
Many studies demonstrate that the amygdala mediates fear conditioning (e.g., Wilensky, Schafe, Kristensen, & LeDoux, 2006.) That is, when exposed to fear-inducing stimuli, animals with amygdala damage may show immediate signs of fear, but do not subsequently avoid the stimuli associated with fear induction. The same is true for humans (LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998). Electric shock is the typical fear-inducing stimulus, but noise loud enough to induce a startle response is also effective for fear conditioning (Bechara et al., 1995.) To test fear learning, our startle sound was triggered by the child’s first contact with a toy; the child’s subsequent interaction with that toy was recorded.
To quantify social affiliation we used a version of the Strange Situation in which the mother leaves briefly and the child’s response to her return is recorded. A child’s immediate post-separation “reunion behavior” in the Strange Situation is a measure of the depth of “attachment” (affectional bond) with the parent. Finally, to quantify (non)-compliance, we used a one item clean-up task. These naturalistic tasks yield reliable and clinically useful results (Roberts, 2001).
Risk Room Protocol
The Risk Room protocol had 4 phases. Exploration began when mother and child entered the Risk Room. The mother sat in the chair at one end of the room near the door (Fig 1). The child could stay by mother and/or play with the little toy nearby, but to get to the large, attractive truck or doll at the other end of the room, s/he had to pass between the skeleton mask on the left and the gorilla mask and a seated male stranger (observer) wearing sunglasses and ball cap on the right. A pair of 0.61m (2 ft) high shelves formed a partial barrier between child and stranger, but the child could approach the stranger from behind or between the shelves. Exploration measures included: 1) Latency to leave mother, proportion of 10 second intervals during which the child 2) remained next to mother or 3) walked around, 4) latency to first contact with a mask, and 5) interaction with the stranger (scored as 0 or 1). Exploration lasted until the child touched either truck or doll or 10 min elapsed. If 10 min elapsed without contact with truck or doll, the mother was instructed to lead the child by the hand to one of these toys.
Fig. 1.
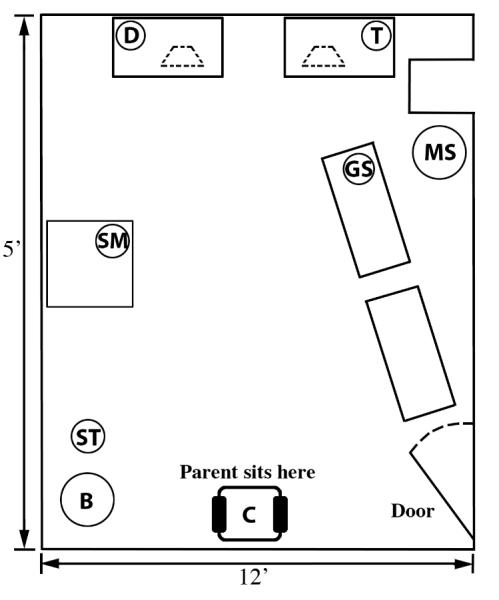
Risk Room layout
B = basket, ST = small toy, SM = skeleton mask, GS = gorilla mask, MS = male stranger, D = large doll, T = large truck
The two toys were on adjacent small tables, each with a loudspeaker beneath. The Startle phase began when the child touched either toy; three brief, 92 dB white noise bursts were delivered at 1 sec intervals. Whole body startle to at least one of the bursts was detected from the videorecording and/or by the observer, as was the child’s subsequent interaction with the toy s/he had touched.
Three minutes after the Startle test, the Strange Situation was initiated by the mother saying “Goodbye, I will be back” and leaving the room for 30 sec. The child’s maximum “reunion response” to mother’s return was scored as follows: 0 = ignore mother, 1= Look at mother, 2= talk to mother without approaching, 3= approach mother, 4= make physical contact/hug1.
For Cleanup-Compliance, the mother gave up to 5 verbal commands, at 30 sec intervals, to put the small toy in the basket. If the child did not comply by the fifth command, she physically showed the child how to put the toy in the basket and asked him/her to do it once more.
Statistical analysis
Interrater reliability for coding the videorecorded behaviors ranged from 0.8 to 1.0; most reliability ratings were >0.9. Outcomes were evaluated for each group and summarized by means with 95% confidence intervals. Groups were compared by t-tests (with unequal variance and Welch degrees of freedom) and chi-squared tests for continuous and categorical variables, respectively. Logrank statistics and score tests from Cox proportional hazards models were used for time-to-event analyses of latencies to leave mother and to touch masks. The Ns in the statistical tests below vary slightly because only partial data were available for two MPS IIIA and two MPS 1H patients.
Other measures from the Natural History study
Orality ratings
Orality is a key feature of K-Bs and is commonly reported by parents of children with MPS IIIA. However, pilot observations indicated that during the brief (5-20 min) Risk Room exposure to arousing objects in a novel situation, oral behaviors were infrequent. We therefore used parent ratings on the 6-item Orality sub-scale of the Sanfilippo Behavior Rating Scale (SBRS, Table 2), a disease-specific rating scale (Potegal & Shapiro, 2009).
Table 2.
Sanfilippo Behavioral Rating Scale: Orality subscale items
| SBRS Orality subscale items |
| Puts everything in his/her mouth |
| Chews on objects |
| Mouths, bites or chews on clothing |
| Attempts to eat any food s/he sees |
| Holds food or drink in mouth before swallowing |
| Stuffs mouth full while eating |
Auditory brainstem responses
MPS IIIA almost always affects hearing. Because behavioral audiology is very difficult with these children, the NH study collected click-evoked, auditory brainstem responses (ABRs, Stone et al., 2009) under midazolam and propofol anesthesia one day after the Risk Room study. Two trials/ear were conducted; each with 1000 rarefaction clicks [rate 33.1/s, intensity 80 dBnHL, per American Clinical Neurophysiology Society guidelines (1994)].
Magnetic Resonance Imaging
While the child was under anesthesia during the baseline visit and again six months later, MRI was acquired on a 3 Tesla Siemens Trio scanner with sequences that included MPRAGE (Magnetization-Prepared Rapid Acquisition with Gradient Echo). Volumetric analyses included manual tracing of the amygdala and hippocampus using Brains2, which allows three-dimensional representation for accurate measurement (Magnotta et al., 2002). Because we found automated parcellation of these structures in young children to be unreliable, manual tracing was done by co-author AA; interrater reliability was assessed by coauthor IN. As previously reported, intrarater reliability was 0.99 and interrater reliability was 0.87 (Ahmed, Nestrasil, Rudser, & Shapiro, 2010).
RESULTS
Demographics
All the children tested were Caucasian; two were Hispanic. See Table 1 for age and gender. On cognitive tests and parent report of adaptive function, almost all children were functioning below age level. Mean (SD) age equivalent cognitive function across all children was 29(14.1) months.
Risk Room
Overall, children with MPS IIIA differed from those with MPS IH in the expected direction on all measures; all but one of these differences showed statistical trends or were statistically significant (Table 3). Most effects were more pronounced for children diagnosed with MPS IIIA before age 6.
Table 3.
Risk Room responses of children with MPS IH (Column 1) compared to all children with MPS IIIA (Column 2) and separately to MPS IIIA subgroups diagnosed before 6 yrs (Column 3) and after 6 yrs (Column 4)
| MPS IH |
MPS IIIA |
MPS IIIA (Diagnosed < 6 yrs) |
MPS IIIA (Diagnosed >= 6 yrs) |
||||
|---|---|---|---|---|---|---|---|
| (N=8) | (N=30) | (N=23) | (N=7) | ||||
| Covariate | Estimate (95% CI) | Estimate (95% CI) | P- value* |
Estimate (95% CI) | P- value* |
Estimate (95% CI) | P- value* |
| Locomotion: | |||||||
| Move-away Latency (sec) | 161.7 (0.0, 399.5) | 40.1 (10.8, 69.4) | 0.07 | 42.3 (4.0, 80.6) | 0.07 | 32.9 (5.3, 60.5) | 0.25 |
| Locomotion: Percent Time | 10.0 (0.0, 22.3) | 51.8 (39.9, 63.8) | <0.001 | 56.7 (42.0, 71.4) | <0.001 | 35.7 (19.8, 51.6) | 0.01 |
| Next to Mother: Percent Time | 71.5 (41.7, 101.3) | 39.6 (26.8, 52.3) | 0.04 | 35.2 (21.6, 48.8) | 0.03 | 53.1 (15.3, 91.0) | 0.36 |
| Touch Mask (%) | 0.0 (0.0, 48.3) | 56.7 (37.7, 74.0) | 0.04 | 65.2 (42.8, 82.8) | 0.02 | 28.6 (5.1, 69.7) | 0.51 |
| Approach Stranger (%) | 16.7 (0.09, 63.5) | 53.3 (34.6, 71.2) | 0.23 | 60.9 (38.8,79.5) | 0.14 | 28.6 (5.1, 69.7) | 1.00 |
| Startle (%) | 75.0 (35.6, 95.5) | 26.7 (13.0, 46.2) | 0.04 | 21.7 (8.3, 44.2) | 0.02 | 42.9 (11.8, 79.8) | 0.46 |
| Hold/return to Trigger Toy (%) | 16.7 (0.9, 63.5) | 75.0 (54.8, 88.6) | 0.03 | 81.0 (57.4, 93.7) | 0.01 | 57.1 (20.2, 88.2) | 0.36 |
| Reunion Score | 3.7 (3.1, 4.0) | 2.3 (1.8, 2.8) | <0.001 | 2.3 (1.8, 2.8) | <0.001 | 2.3 (1.0, 3.6) | 0.04 |
| Clean-up Compliance (%) | 87.5 (46.7, 99.3) | 46.7 (28.8, 65.4) | 0.096 | 39.1 (20.5, 61.2) | 0.05 | 71.4 (30.3, 94.9) | 0.90 |
Comparison to MPS I group
Exploration
Survival analysis indicated a trend for the MPS IIIA group to move away from mother more quickly, logrank (1, N=36) = 3.22, p = .073. On average, they spent about half their time walking around, which was significantly greater than the mean of about 10% for children with MPS 1H, t(22) = 5.55, p <0.001. On average, children with MPS IIIA spent little more than a third of their time in proximity to mother whereas children with MPS IH spent almost three quarters of their time next to her, t(8) = 2.43, p = .04.
Almost 60% of the MPS IIIA group touched at least one scary mask but none of the MPS IH group did so, c2(1,N=36) = 4.37, p = .04. When looking at the masks and/or stranger, three children with MPS IIIA said “Uh-oh”, or “Scary” or otherwise indicated that they perceived that something was “wrong”, but touched and manipulated one or both masks, nonetheless. Fewer children with MPS IH interacted with the stranger (17 % vs. 53%), but the difference between groups was not statistically significant, c2(1,N=36) = 1.43, p=.23.
Response to startle sound and trigger toy
Three quarters of the MPS IH group showed full body startle in response to one of the sound stimuli; less than a third of the MPS IIIA group startled, c2(1,N = 38) = 4.43, p = .04. Conversely, almost three quarters of the MPS IIIA group held onto or returned to the trigger-toy within 2 min after the startle sound; only one of six in the MPS IH group did so c2(1,N = 34) = 5.03, p = .03. Absence of a startle response was not a pre-condition for returning to the toy; 5 of the 8 (63%) children with MPS IIIA who startled held onto/returned to the toy compared to 1 of 6 (17%) of the MPS IH group who startled.
Strange Situation
As Fig 2 shows, the modal response of children with MPS IH to mother’s return was to make physical contact with her. The responses of children with MPS IIIA appeared bimodal; 11 of them ignored their mother or just looked at her; 16 approached or made physical contact with her. The difference between the MPS IIIA and MPS IH groups was significant t(19) = 4.43, p <0.001.
Fig. 2.
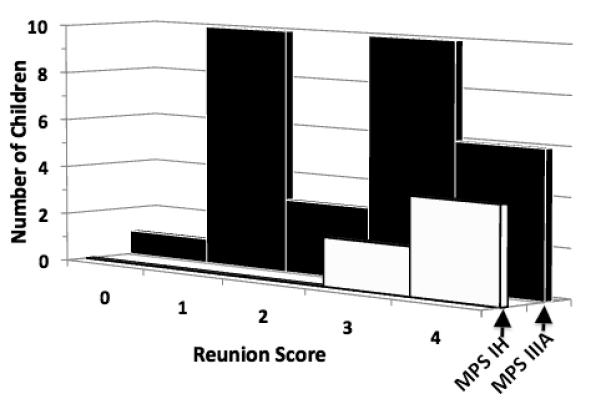
Distribution of reunion responses in children with MPS IH (white bars) and MPS IIIA (black bars)
0 = ignore mother, 1= look at mother, 2= talk to mother without approaching, 3= approach mother, 4= make physical contact/hug
Compliance
Seven of 8 children in the MPS IH group complied with the pick-up command within three verbal repetitions; less than half of the MPSIII group complied even after 5 verbal repetitions and physical direction c2(1,N = 38) = 2.77, p = .096.
Late-onset MPS IIIA subgroup
Differences from the MPS IH group on 7 of the 9 behavioral variables were less pronounced for the MPS IIIA subgroup of 7 children who were diagnosed later than 6 years of age compared to those who were diagnosed earlier (Table 3). The reduced significance of statistical differences between the older MPS IIIA subgroup and the MPS IH comparison group is confounded by the smaller number of children in the older subgroup. Nonetheless, comparisons of variable means suggests that the disease shows slower progression in the children diagnosed later.
SBRS Orality ratings
All but one of the parents of the MPS IIIA children endorsed their child having at least one of the 6 oral behaviors at least “sometimes” (about 25% of the time). The median number of oral behaviors across all these children was 5; the median frequencies for each behavior ranged from at least “occasionally” (5-10% of the time) to at least “half the time.” The one child reported to show no such behaviors was 11 yrs old and had been diagnosed after age 6.
Auditory Brainstem Response in children with MPS IIIA
In ABR analysis, the latency of Waveform I (at the cochlear nerve) indicates peripheral hearing while the interpeak interval between Waveform I and Waveform V (medullary/pontine brainstem) reflects central auditory pathway function. ABR data were available for 26 children with MPS IIIA whose response to the startle sound was recorded; information about the post-startle response to the trigger toy was missing for two of these children. As Table 4 shows, there were no significant differences in the available Waveform I latency measures for those children who startled vs. those who did not or between those who held/returned to the trigger toy vs. those who dropped/left it. Similarly, there were no significant contrasts in Waveform (V – I) interpeak interval for these behaviorally defined subgroup comparisons.
Table 4.
Mean latency (SD) of ABR Waveform I (Wf I) and the interpeak interval between Waveform V - Waveform I [(Wf (V - I)]. In healthy children over a range of ages, the latencies of Wf 1 and interpeak interval Wf (V – I) are about 1.5 msec and 4 msec, respectively (Gillberg, Rosenhall, & Johansson 1983; Gorga, Kaminski, Beauchaine, Jesteadt, & Neely, 1989; Purdy, Kelly, & Davies, 2002.)
| Response to noise bursts | Post-burst response to trigger toy | |||
|---|---|---|---|---|
| No startle | Startle | Leave toy | Hold/return to toy | |
| N | 18 | 8 | 6 | 18 |
| Wf I (msec) | 1.64 (0.23) | 1.68 (0.28) | 1.67 (0.23) | 1.67 (0.26) |
| Wf (V – I, msec) | 4.46 (0.36) | 4.52 (0.23) | 4.42 (0.36) | 4.49 (0.34) |
The ABRs were taken under anesthesia and after PE tubes had been adjusted. However, ABRs are reportedly unchanged by these anesthetics (Savoia et al. 1988, Morlet et al. 1997) and the startle stimulus was intense enough to reach the inner ear through bone conduction, unaffected by PE tube adjustments. These results therefore strongly suggest that individual differences in hearing were not responsible for differences in reflex or behavioral response to the startle noise.
MRI results
For the children in the NH study, there was no association between first visit amygdala or hippocampus volumes and Risk Room behaviors. There were significant volume reductions between first and second visits. The rate of these reductions was significantly larger for amygdala (10% per 6 months) than for hippocampus (3%), t(23) = 4.92, p < .001. For children diagnosed before age 6, greater volume reductions in the amygdala were associated with shorter latencies to first contact a mask (p <.05) according to a Cox proportional hazards model, score test (1, N = 17) = 5.03, p = .025. There was no such association for changes in hippocampal volume score test (1, N = 17) = .16, p = .69.
DISCUSSION
Our results document many of the anecdotally reported behaviors of children with MPS IIIA. These children differed significantly from those with MPS IH on almost every measure. They left their mothers sooner, wandered more, were more likely to approach frightening objects, were less likely to respond with whole body startle to a loud noise, and much more likely to contact the object associated with the startle noise afterwards. They also interacted less with mother when she returned from a brief departure, and they were less compliant with a clean-up command. The wandering, lack of fear, and impaired social interaction all fit characteristics of KBs. The abnormal oral tendencies of these children were quantified in the SBRS parent report questionnaire. Inappropriate sexual behavior is also part of K-Bs; masturbatory activity has been reported in child clinical cases (Jha et al., 2005; Pradhan et al.,1998) and was documented among the older children by our questionnaires.
Three quarters of children with MPS IH startled to a loud noise, but fewer than a third of the MPS IIIA group did so. Although MPS IIIA affects hearing, the lack of difference in the ABRs of children with MPS IIIA who startled compared with those who did not suggests that differences in hearing do not explain differences in startle within this group. Note that many of the MPS IH children have hearing loss and wear hearing aids. Nearly 75% of children with MPS IIIA did not drop the toy they were touching when the sound was triggered or returned to that toy soon after. Only one of six children with MPS IH did so. The lack of fear conditioning in children with MPS IIIA may not be due to the absence of startle; 5 of 8 children with MPS IIIA who startled also returned to the toy while only 1 of 6 MPS IH group who startled did so. This deficit in fear learning may contribute to the heedlessness of danger these children routinely show.
The more pronounced findings in children diagnosed with MPS IIIA before age 6 are consistent with the predictable course of the classic form of this disease. In children diagnosed later, disease progression is slower and less predictable; the small N of this subgroup precludes further conclusions.
The relatively small number of children in the MPS IH comparison group and the lack of ABR data for this group are limitations to this study. Differences found between the MPS IIIA and IH groups suggest, but do not prove, that the behavioral profile delineated here is specific to MPS IIIA. However, our extensive experience with children in various stages of other MPS diseases has yielded no parent reports or clinical observations resembling the MPS IIIA profile. The lower cognitive ability of children with MPS IIIA raises the question of how much this contributes to their behavioral profile. This is difficult to answer because increasing loss of amygdala function, which arguably gives rise to the KB-S profile, is accompanied by general brain atrophy, which engenders declining cognitive ability. However, children with severe MR do not routinely present with the profile of KB-S reported here.
In children diagnosed early with MPS IIIA brain volumes declined over 6 months in both structures measured. Notably, the decline was significantly greater in the amygdala than in the hippocampus, suggesting neuroanatomical specificity. The significant difference between volume declines in amygdala vs. hippocampus is particularly noteworthy, given that they are neighboring limbic structures in the temporal lobe The rate of amygdala decline was associated with shorter latency to touch a mask, suggesting that amygdala dysfunction may be responsible for some of the reduction in fearfulness seen in children with MPS IIIA.
Urbach-Wiethe disease, an autosomal recessive disorder affecting the amygdala, presents with some aspects of K-Bs (Hurlemann et al., 2007; Tranel et al., 2006) While there are case reports of K-Bs in children involving various etiologies (e.g., Jha et al., 2005; Pradhan et al., 1998), no childhood equivalent of Urbach-Wiethe has been identified. The pattern of behavioral abnormalities and associated accentuated rate of amygdala atrophy found in children with MPS IIIA suggests that it may be the first pediatric disease whose behavioral phenotype is identified as a K-Bs variant. Clinically, this identification sets the stage for theoretically grounded and empirically focused approaches to monitoring disease progression and assessing the effects of therapeutic intervention.
Acknowledgements
We thank Kendra Bjoraker for her observations and interpretations of the behaviors of children with MPS IIIA. Kevin Donley and Neha Rajagopal assisted at early stages of this project. Kent Osman and Joel Westacott contributed to the instrumentation of the startle apparatus. Testing was carried out in the University of Minnesota’s Center for Neurobehavioral Development and we thank the staff of the CNBD for their considerable assistance. We also thank the MPS Society for their help in disseminating information about the study and recruiting families. Finally, we appreciate the participation of the children and their parents without whom this study would not have been possible. This study was supported by NIH grant U54NS065768 and a grant from Shire HGT. The MRI, cognitive, and ABR data were contributed by ShireHGT for the children in the study “A 12-month Longitudinal, Prospective, Observational, Natural History Study of Patients with Sanfilippo Syndrome Type A (MPS IIIA)”.
Footnotes
This score is unrelated to the standard A-D classification of attachment reactions in the Strange Situation.
References
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews. 2002;1:21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Nestrasil I, Rudser K, Shapiro EG. Reliability of manual and automated tracing of hippocampal volumes in MPS patients and normal controls: A report of the neuroimaging core of the lysosomal disease network. Molecular Genetics and Metabolism. 2010;99(2):S9. [Google Scholar]
- Angrilli A, Mauri A, Palomba D, Flor H, Birbaumer N, Sartori G, Di Paola F. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain: A journal of neurology. 1996;119:1991–2000. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- Aygun D, Guven H, Altintop L, Doganay Z. Postcontusional Klüver-Bucy syndrome. The American Journal of Emergency Medicine. 2003;21:246–247. doi: 10.1016/s0735-6757(03)00017-2. [DOI] [PubMed] [Google Scholar]
- Baehner F, Schmiedeskamp C, Krummenauer F, Miebach E, Bajbou M, Whybra C, Kohlschutter A, Kampmann C, Beck M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. Journal of Inherited Metabolic Disease. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Buss KA, Goldsmith HH. Manual and normative data for the Laboratory Temperament Assessment Battery—Toddler Version. University of Wisconsin—Madison, Department of Psychology; 2000. (Tech. Rep.) [Google Scholar]
- Cleary MA, Wraith JE. Management of mucopolysaccharidosis type III. Archives of Disease in Childhood. 1993;69:403–406. doi: 10.1136/adc.69.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville GA, Bax MA. Early presentation in the mucopolysaccharide disorders. Child Care Health and Development. 1996;22:31–36. [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Rosenhall U, Johansson E. Auditory brainstem responses in childhood psychosis. Journal of Autism and Developmental Disorders. 1983;13:181–195. doi: 10.1007/BF01531818. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Kaminski JR, Beauchaine KL, Jesteadt W, Neely ST. Auditory brainstem responses from children three months to three years of age. Journal of Speech and Hearing Research. 1989;32:281–288. doi: 10.1044/jshr.3202.281. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Dougherty LR, Maloney B, Durbin CE, Olino TM, Nurnberger JI, Lahiri DK, et al. Temperamental fearfulness in childhood and the serotonin transporter promoter region polymorphism: a multimethod association study. Psychiatric Genetics. 2006;17:135–142. doi: 10.1097/YPG.0b013e3280147847. [DOI] [PubMed] [Google Scholar]
- Héron B, Mikaeloff Y, Froissart R, Caridade G, Maire I, Caillaud C, Levade T, et al. Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. American journal of medical genetics Part A. 2011;155A:58–68. doi: 10.1002/ajmg.a.33779. [DOI] [PubMed] [Google Scholar]
- Hopwood J. Sanfilippo Syndrome: Clinical genetic diagnosis and therapies. In: Barranger J, editor. Lysosomal storage disorders. Springer; New York: 2007. pp. 415–432. [Google Scholar]
- Hurlemann R, Wagner M, Hawellek B, Reich H, Pieperhoff P, Amunts K, et al. Amygdala control of emotion-induced forgetting and remembering: evidence from Urbach-Wiethe disease. Neuropsychologia. 2007;45:877–884. doi: 10.1016/j.neuropsychologia.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Janszky J, Fogarasi A, Magalova V, Tuxhorn I, Ebner A. Hyperorality in epileptic seizures: periictal incomplete Klüver-Bucy syndrome. Epilepsia. 2005;46:1235–1240. doi: 10.1111/j.1528-1167.2005.69404.x. [DOI] [PubMed] [Google Scholar]
- Jha S, Kumar R, Kumar M, Kumar R. Cerebral birth anoxia, seizures and Klüver — Bucy syndrome: some observations. Journal of Pediatric Neurology. 2005;3:227–232. [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andrease NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–64. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. The Journal of the American Medical Association. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Morlet D, Bertrand O, Salord F, Boulieu R, Pernier J, Fischer C. Dynamics of MLAEP changes in midazolam-induced sedation. Electroencephalography and Clinical Neurophysiology. 1997;104:437–446. doi: 10.1016/s0168-5597(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Neylan T. Temporal lobe and behavior: Klüver and Bucy’s classic. Journal of Neuropsychiatry. 1997;9:606–620. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Poorthuis BJHM, Wevers RA, Kleijer WJ, Groener JEM, de Jong JGN, van Weely S, et al. The frequency of lysosomal storage diseases in The Netherlands. Human Genetics. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- Potegal M, Shapiro E. Behavioral phenotypes in MPS III. Molecular Genetics and Metabolism. 2009;96(2):S36. [Google Scholar]
- Pradhan S, Singh MN, Pandey N. Klüver Bucy syndrome in young children. Clinical Neurology and Neurosurgery. 1998;100:254–258. doi: 10.1016/s0303-8467(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Purdy SC, Kelly AS, Davies MG. Auditory Brainstem Response, Middle Latency Response, and Late Cortical Evoked Potentials in Children with Learning Disabilities. Journal of the American Academy of Audiology. 2002;13:367–382. [PubMed] [Google Scholar]
- Roberts MW. Clinic observations of structured parent-child interaction designed to evaluate externalizing disorders. Psychological Assessment. 2001;13:46–58. [PubMed] [Google Scholar]
- Robertson SP, Klug GL, Rogers JG. Cerebrospinal fluid shunts in the management of behavioural problems in Sanfilippo syndrome (MPS III) European Journal of Pediatrics. 1998;157:653–655. doi: 10.1007/s004310050904. [DOI] [PubMed] [Google Scholar]
- Savoia G, Esposito C, Belfiore F, Amantea B, Cuocolo R. Propofol infusion and auditory evoked potentials. Anaesthesia. 1988;43(Suppl):46–49. doi: 10.1111/j.1365-2044.1988.tb09069.x. [DOI] [PubMed] [Google Scholar]
- Stone JL, Calderon-Arnulphi M, Watson KS, Patel K, Mander NS, Suss N, et al. Brainstem auditory evoked potentials—A review and modified studies in healthy subjects. Journal of Clinical Neurophysiology. 2009;26:167–175. doi: 10.1097/WNP.0b013e3181a76a6e. [DOI] [PubMed] [Google Scholar]
- Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cognitive Neuropsychiatry. 2006;11:219–232. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. Journal of Neuroscience. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


