Abstract
We aimed to investigate the effects of Xylaria nigripes (XN) extracts on the rapid eye movement sleep deprivation (REMSD)-induced memory impairment, and explore related mechanism. Male Sprague Dawley rats were randomly divided into 6 groups: cage control (CC)-NaCl group; tank control (TC)-NaCl group; sleep deprivation (SD)-NaCl group; CC-XN group; TC-XN group and SD-XN group. The rats were administered with intragastric XN and 0.9% of sodium chloride. SD group rats were deprived of REM sleep for 72 h. Morris water maze (MWM) was used to assess the effects of XN on spatial learning and memory. The expression of cAMP-response element binding protein (CREB) and p-CREB were also investigated in all groups. Result showed rats in SD-NaCl group had significantly longer mean escape latencies in finding the platform as compared to the control rats (p<0.05) in MWM test. The SD-NaCl group spent significantly less time in goal quadrant compared with the SD-XN group. REMSD and XN did not alter CREB expression in the hippocampus, while sleep deprivation resulted in reduced phosphorylation of CREB in the hippocampus, which was reversed by XN. XN mitigates spatial memory impairment induced by REMSD in rat. Phosphorylation of CREB in hippocampus might be one of the mechanisms.
Keywords: Xylaria nigripes, rapid eye movement sleep deprivation, learning and memory, cAMP response element-binding protein, phospho-cAMP response element-binding protein
Introduction
Xylaria nigripes (XN), also known as Wu Ling Shen, is a high value medicinal fungus belonging to the family of Xylariaceae. The sclerotia of XN grows several feet underground the fungus combs of the Odontotermes termite species during the spring and summer seasons [1].
Recent studies show that XN have a tranquilizing effect on the central nervous system, it could modulate some physiological functions such as anti-oxidative damage [2-4], anti-inflammatory activity [5] and protecting carbon tetrachloride-induced liver injury in mice [6]. XN is also used to relieving depression and treating trauma [7,8]. In traditional Chinese medicine (TCM), XN is commonly used to regulating wake and sleep. Previous studies have shown that XN could induce sleepiness and maintained sleep time in insomnia patients [9,10].
Sleep loss is a common disorder with a high incidence. Numerous studies showed that sleep plays a key role in learning and memory [11,12]. Rapid eye movement (REM) sleep is an important part in sleep, which is closely related with synaptic plasticity and long-term potentiation [13,14]. So far, no study examined the potential effect of XN on memory impairment induced by sleep loss.
cAMP response element-binding protein (CREB) was firstly described in 1987 as a cAMP-responsive transcription factor regulating the somatostatin gene [15]. Through binding to certain DNA sequences called cAMP response elements (CRE), CREB could regulate the transcription of its downstream genes such as c-fos, neurotrophin brain-derived neurotrophic factor (BDNF) and neuropeptides (such as somatostatin), VGF, and corticotropin-releasing hormone related gene [16]. CREB has a well-documented role in neuronal plasticity and long-term memory formation. The phosphorylated form of CREB (p-CREB) is a critical protein act in hippocampal-dependent learning and synaptic plasticity [17]. Moreover, the decrease of phosphorylated CREB was presently reported to be one of the mechanisms of REMSD-induced cognition impairment. It is also reported that p-CREB levels reduce after sleep deprivation [18].
These reports inspired us whether XN mediate the spatial memory impairment of REM sleep deprivation, and further, whether CREB phosphorylation involved in this process.
In present study, the cultivated XN were used to prepare powders extracts. We studied the role of XN in modulating hippocampus-dependent spatial learning and memory through the MWM method, and examined the change of CREB and p-CREB expression in the rat hippocampus in XN treated group after 72 h of REMSD.
Materials and methods
Preparation of extracts and test solutions
The XN materials were obtained from Zhejiang Jolly Pharmaceutical Co., Ltd (Zhejiang Province, China). Dried XN materials were subjected to water extraction, the extracts were prepared according to the traditional uses of XN materials as procedures described previously [19]. After dried by lyophilisation, the powders of XN were stored at 4°C until use. Test solutions were freshly prepared for each experiment with 0.9% NaCl. The same amount of NaCl (0.9%) served as control.
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Second Military Medical University. Male Sprague Dawley rats (body weight: 220-250 g) were randomly divided into 6 groups: cage control (CC)-NaCl group; tank control (TC)-NaCl group; sleep deprivation (SD)-NaCl group; CC-XN group; TC-XN group and SD-XN group, each group consisted 8 animals. They were housed freely access to food and water under an automatic 12 h light/12 h dark cycle at a temperature of 24°C and a humidity of 50-60%.
Intragastric administration
The rats were administered with intragastric XN (300 mg/kg bodyweight, dissolved in 0.9% NaCl) or control of 3 ml 0.9% NaCl once a day for 6 weeks respectively [20].
Rapid eye movements sleep deprivation
The rats in SD group were deprived of REM sleep for 72 h using the modified multiple platform method [21]. Cage control (CC) group rats were maintained in their home cages. Rats in SD and tank control (TC) group were placed into a water tank (123 × 44 × 44 cm) containing 14 circular platforms (6.5 cm diameter) or 10 circular platforms (14 cm diameter), the water was up to 1 cm beneath the platform. When the SD rats reached the REM sleep phase, they were awakened by touching the water because of muscle atonia. For TC group rats, they were placed on large platforms as the REMSD environment to simulate the sleep deprivation condition while allowing sleep to occur. The water in the tanks was changed daily.
Assessment of spatial learning and memory
The MWM task was performed as described previously [22,23]. The MWM was a black circular tank (167 cm in diameter, 50 cm in deep) filled with water (22-24°C) at a depth of 25 cm. The maze was divided into four quadrants of equal size. A hidden black platform (12 cm in diameter) was placed in the middle of the southwest quadrant, submerged 1.5 cm below the water surface. The rats were trained between 11:00 and 13:00 in four trials for 4 days to find the hidden platform. Each rat was released facing the wall of the maze from different positions around the perimeter of the tank (north, northwest, east and southeast), respectively. In each trial, a rat was allowed to locate the platform up to 120 s and then stayed for 5 s. If the platform was not found in 120 s, the rat was guided onto it and stayed there for 30 s. The escape latency to find the hidden platform was recorded automatically using the Video Tracking System (Ji Liang Technology, Shanghai, China). At the end of REMSD, a probe test was adopted. The platform was removed and each rat started at northeast and had to swim freely for 2 min. Memory retention was measured by quantifying the time spent in the goal target (southwest quadrant). The swimming trail was recorded at the same time.
Western blot
Hippocampal tissues of the rats in each group were dissected and then homogenized in 1% sodium dodecyl sulphate (SDS) containing 1 mM of phenylmethylsulphonyl-fluoride (PMSF) and centrifuged. The supernatants were collected and the total protein concentration was determined using a BCA Protein Assay Kit (Beyotime, Shanghai, China). Whole tissue homogenates (50 μg of total protein) were heated at 100°C for 5 min and resolved on 12% gels using SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. The membranes were blocked with skim milk in Tris buffered saline (TBS) for 2 h at room temperature and incubated with anti-CREB (1:200, Santa Cruz, USA) and anti-GAPDH (1:10000, KangChen, Shanghai, China) antibodies in TBS plus 0.1% Tween-20 (TBST) with 5% bovine serum albumin; anti-p-CREB (1:200, Santa Cruz, USA) and anti-GAPDH (1:10000, KangChen, Shanghai, China) antibodies in TBS supplemented with 0.1% Tween-20 (TBST) and 5% bovine serum albumin overnight at 4°C, respectively. GAPDH was used as a loading control. Membranes were washed in TBS and incubated with horseradish peroxidase-conjugated anti-rabbit antibodies (1:10000, sigma, USA) for 2 h at room temperature. Then the blots were visualized by enhanced chemiluminescence (Bipec Biopharma, Massachusetts, USA) and captured on autoradiography films (Kodak X scientific image film, Xiamen, China). Densitometric scans of the autoradiographs were digitized on a ScanMaker 3860 (Microtek, Shanghai, China) with Scanwizard 5 software (Microtek), and quantified using Quantity One 4.6 (Bio-Rad) image analysis software.
Statistical analysis
Statistical analysis was performed by SPSS 16.0 software. Data were expressed as mean ± S.E.M. The difference of latencies to target were determined by ANOVA with repeated measures (MANOVA) followed by post-hoc test for multi-group comparisons. Two-way classification ANOVA followed by post-hoc test was used to determine whether XN affected memory impairments induced by REMSD. Western blot data were analysed using one-way analysis of variance. P<0.05 was considered as significant.
Results
Spatial learning
Before REMSD, all groups were trained in the MWM for spatial learning assessment. As shown in Figure 1A, all groups showed reductions in latency to find the hidden platform during the training. The differences in latency to find the hidden platform among the 6 groups were significant [F (5, 42) = 21.60, P<0.01]. The rats in SD-NaCl group had significantly longer mean escape latencies in finding the platform during the 4-day training compared with the control rats (p<0.05). The rats treated with XN had slightly shorter mean latencies in finding the platform throughout the 4-day training period compared with the rats in SD-NaCl group. These results indicated that REMSD impaired spatial learning in rats and XN could alleviate the impaired spatial learning.
Figure 1.
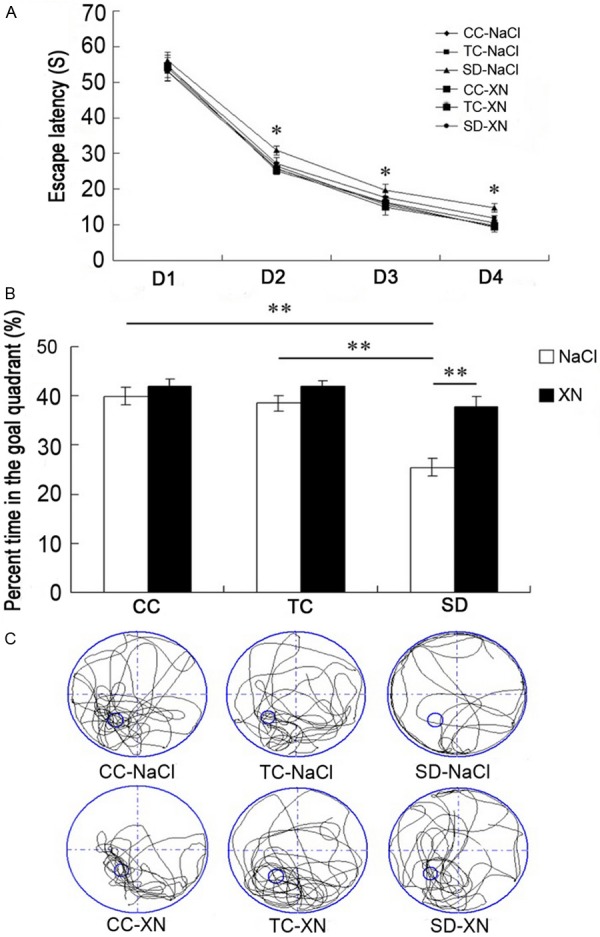
Performance of rats in the Morris water maze during spatial learning and memory. A: Mean latency to find the hidden platform. B: Time (%) rats spent swimming in the target quadrant during the probe test. C: Representative swim trajectories in the probe test. *P<0.05, **P<0.01. CC, cage control; TC, tank control; SD, sleep deprivation.
XN mitigates spatial memory impairment induced by REMSD
After REMSD, a probe trial was performed to evaluate spatial memory. As shown in Figure 1B, the percent times spent in the goal quadrant were different among the 6 groups [F (5, 42) = 110.374, P<0.01]. The CC-NaCl and TC-NaCl groups spent significantly more percent time in the goal quadrant than the SD-NaCl group (39.92 ± 1.75 and 38.50 ± 1.61 vs 25.44 ± 1.80, P<0.01). The rats in SD-XN group spent significantly longer time in the target quadrants compared with the rats in SD-NaCl group (37.70 ± 2.07 vs 25.44 ± 1.80, p<0.01). The different swim trajectories of the groups in the probe test were shown in Figure 1C, the rats swam mainly in the target quadrant in CC and TC groups, and the rats swam equally dispersed in the four quadrants in SD-NaCl group, but the swim trajectory in the SD-XN group was similar to the control groups.
CREB expression in the hippocampus
We used western blot to examine the difference of CREB expression in the hippocampus between the 6 groups. CREB was detected in the whole-tissue homogenate of the hippocampus respectively (Figure 2). Quantitative analysis showed there was no difference in CREB expression among all the groups [F (5, 42) = 0.854, P>0.05], which indicating that REMSD and XN did not alter CREB expression in the whole-tissue homogenate of the hippocampus (p>0.05).
Figure 2.
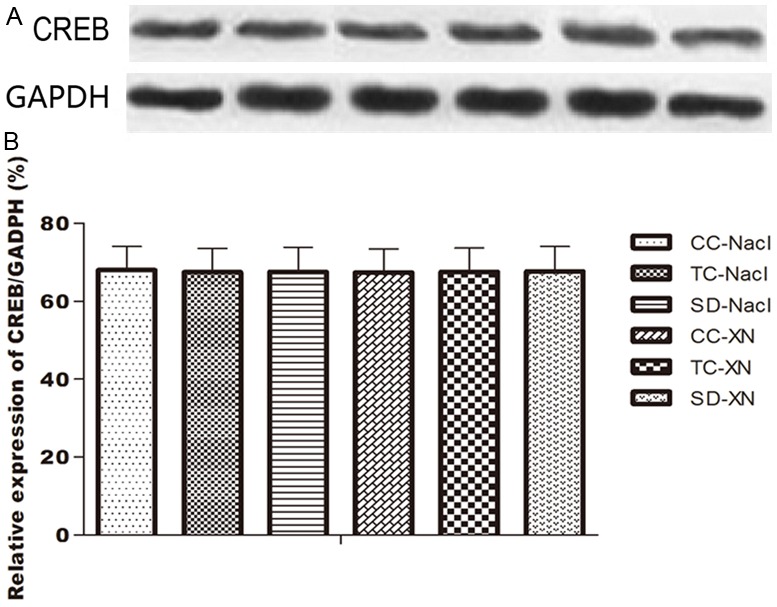
Western blots analysis of CREB expression in the hippocampus. A: Western blot result. B: Histogram result of CREB/GAPDH ratio among the 6 groups. CC, cage control; TC, tank control; SD, sleep deprivation.
Increased p-CREB expression after XN treated
We also used western blot method to examine the difference of p-CREB expression in the hippocampus. Quantitative analysis showed significant difference in p-CREB expression between the 6 groups [F (5, 42) = 214.999, P<0.01] (Figure 3). 72-h after REMSD, the expression of p-CREB in the hippocampus significantly decreased in SD-NaCl group compared with CC-NaCl and TC-NaCl group (0.3044 ± 0.0163 vs 0.6094 ± 0.0239 and 0.5910 ± 0.0283, P<0.01). While the expression of p-CREB increased significantly in the SD-XN group compared with the SD-NaCl group (p-CREB expression/GAPDH: 0.5825 ± 0.0212 vs 0.3044 ± 0.0163, P<0.01).
Figure 3.
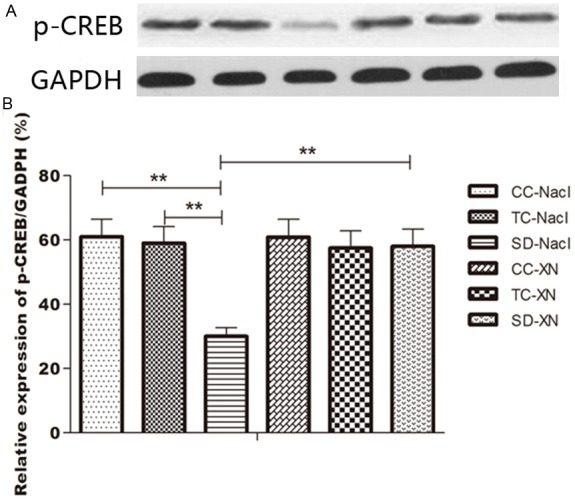
Western blots result of p-CREB in hippocampus. A: Western blot result. B: Histogram result of p-CREB/GAPDH ratio among the 6 groups. *P<0.05, **P<0.01. CC, cage control; TC, tank control; SD, sleep deprivation.
Discussion
In the present study we found that Xylaria nigripes mitigated the spatial memory impairment induced by 72 h REMSD in rats, and XN attenuated the reduction of the p-CREB expression. This is the first time that medicinal fungus XN was shown to possess potent anti-spatial memory impairment activity through inducing CREB phosphorylation in the hippocampus.
Considerable studies have reached a consensus that REM sleep is closely related with cognition, and REM sleep deprivation impairs cognitive function [24,25]. Our data showed that the spatial learning and memory were impaired, which were caused by 72 h REMSD. The results are consistent with our previous studies [26,27]. Xylaria nigripes is a high value medicinal fungus used in TCM and are commonly used to treat insomnia and trauma [3,6,8-10]. Xylaria nigripes contains polysaccharide, rare elements such as Fe, Mn, Zn, Mg, K, Na, P, Ca, B, Co, etc., Vitamin D2, K1, E, and naturally occurring amino acids, such as gamma-aminobutyric acid (GABA) and glutamate decarboxylase [28].
In this study, our data indicated that administration of XN increased the percent time in the goal quadrant, which decreased after REMSD significantly, and also shortened the mean latency which increased after REMSD. The results demonstrated that XN mitigates spatial memory impairment induced by REMSD, and also improves the spatial learning induced by REMSD deficit slightly.
It is well established that CREB, a member of the activating transcription factor (ATF) family, plays an important role in prolonged maintenance of memory and long-term potentiation. The phosphorylated form of CREB (p-CREB) is a critical modulator of hippocampal-dependent learning, memory storage and synaptic plasticity [29]. Report indicated that p-CREB level increased in the hippocampus following learning [30]. For instance, p-CREB increased in the hippocampus following learning of an operant conditioning task [29,31]. Recent findings demonstrate sleep deprivation disrupts hippocampal function by interfering with cAMP signalling [32], p-CREB levels decrease after interrupted sleep deprivation [33].
In our study, the CREB expression did not change after sleep deprivation but CREB phosphorylation was inhibited during the process of REMSD, this indicate that the phosphorylated form of CREB is the critical factors related to learning and memory after sleep deprivation. The decreased expression of p-CREB was activated by XN. Therefore, we speculated that XN might partially reverse the reduction of p-CREB expression in hippocampus induced by REMSD, and mitigate REMSD-induced memory impairment. Thus, we concluded that it might be one of the mechanisms of XN protective function in mitigating memory impairment.
Specific mechanisms of this process is not entirely clear, GABA may play an important role in it. Previous study shows that Xylaria nigripes could promote sleep and decrease autonomic locomotion, and Xylaria nigripes exert its sedative and sleep-promoting effect through activating the receptors of GABA [20]. Furthermore, study has demonstrated that GABA-mediated excitation regulates CREB activation [34], and GABA-B receptor activity upon ethanol exposure could modulate the expression of p-CREB [35,36]. Consistently, other studies showed that CREB phosphorylation could be induced by selective activation of GABA-B receptor via an ERK1/2-dependent pathway, this suggested a role of the ERK/CREB pathway in GABA-B receptor-mediated long-term synaptic plasticity and memory [37]. Accordingly, we speculated that XN may modulate p-CREB signalling to promote learning and memory through activating GABA receptor in rats. This hypothesis requires further studies.
In conclusion, Xylaria Nigripes exerts effective protection against spatial memory impairment induced by rapid eye movement sleep deprivation in rats; Xylaria Nigripes is a potential effective drug to treat spatial memory impairment. Its act mechanism could be elevating phosphorylation of CREB in hippocampus.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (No. 81100991, No. 81070070 and No. 81200061); and grant of the Army Medical Technology “12th Five-Year Plan” Scientific Research Projects of China (Surface Project, No. CWS11J126).
Disclosure of conflict of interest
None.
References
- 1.Rogers JD, Ju YM, Lehmann J. Some Xylaria species on termite nests. Mycologia. 2005;97:914–923. doi: 10.3852/mycologia.97.4.914. [DOI] [PubMed] [Google Scholar]
- 2.Ko HJ, Song A, Lai MN, Ng LT. Antioxidant and antiradical activities of Wu Ling Shen in a cell free system. Am J Chin Med. 2009;37:815–828. doi: 10.1142/S0192415X09007260. [DOI] [PubMed] [Google Scholar]
- 3.Lu XL, Yao XL, Liu Z, Zhang H, Li W, Li Z, Wang GL, Pang J, Lin Y, Xu Z, Chen L, Pei Z, Zeng J. Protective effects of xyloketal B against MPP+-induced neurotoxicity in Caenorhabditis elegans and PC12 cells. Brain Res. 2010;1332:110–119. doi: 10.1016/j.brainres.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 4.Wu G. [A study on DPPH free-radical scavengers from Xylaria nigripes] . Wei Sheng Wu Xue Bao. 2001;41:363–366. [PubMed] [Google Scholar]
- 5.Ko HJ, Song A, Lai MN, Ng LT. Immunomodulatory properties of Xylaria nigripes in peritoneal macrophage cells of Balb/c mice. J Ethnopharmacol. 2011;138:762–768. doi: 10.1016/j.jep.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Song A, Ko HJ, Lai MN, Ng LT. Protective effects of Wu-Ling-Shen (Xylaria nigripes) on carbon tetrachloride-induced hepatotoxicity in mice. Immunopharmacol Immunotoxicol. 2011;33:454–460. doi: 10.3109/08923973.2010.534100. [DOI] [PubMed] [Google Scholar]
- 7.Li DQ, Li XJ, Duan JF, Cai W. Wuling Capsule promotes hippocampal neurogenesis by improving expression of connexin 43 in rats exposed to chronic unpredictable mild stress. Zhong Xi Yi Jie He Xue Bao. 2010;8:662–669. doi: 10.3736/jcim20100710. [DOI] [PubMed] [Google Scholar]
- 8.Shi L, Zhao X, Wang Y, Zhang J, Zhang H, Wu K, Feng B, Wei J. A randomized study on comparing effect and safety of wuling capsule and deanxit in patients with anxiety or depression status. Chin J Neurolo. 2009;42:776–779. [Google Scholar]
- 9.Lu Y, Lu H, Qin D. The clinical effect of wuling capsule on wild depression. Chinese Traditional Patent Medicine. 2010;32:1083–1084. [Google Scholar]
- 10.Song X, He J, Zheng T, Ye R, Yuan Z. Research on treatment effects of Wuling capsule for sub-healthy state insomnia. Chinese Archives of Traditional Chinese Medicine. 2010;28:477–478. [Google Scholar]
- 11.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 12.Zhu B, Dong Y, Xu Z, Gompf HS, Ward SA, Xue Z, Miao C, Zhang Y, Chamberlin NL, Xie Z. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48:348–355. doi: 10.1016/j.nbd.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- 14.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montminy MR, Bilezikjian LM. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987;328:175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- 16.Meyer TE, Waeber G, Lin J, Beckmann W, Habener JF. The promoter of the gene encoding 3’,5’-cyclic adenosine monophosphate (cAMP) response element binding protein contains cAMP response elements: evidence for positive autoregulation of gene transcription. Endocrinology. 1993;132:770–780. doi: 10.1210/endo.132.2.8381074. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Zou X, Watanabe H, van Deursen JM, Shen J. CREB binding protein is required for both short-term and long-term memory formation. J Neurosci. 2010;30:13066–13077. doi: 10.1523/JNEUROSCI.2378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Sleep deprivation prevents stimulation-induced increases of levels of P-CREB and BDNF: protection by caffeine. Mol Cell Neurosci. 2011;46:742–751. doi: 10.1016/j.mcn.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Oyagi A, Ogawa K, Kakino M, Hara H. Protective effects of a gastrointestinal agent containing Korean red ginseng on gastric ulcer models in mice. BMC Complement Altern Med. 2010;10:45. doi: 10.1186/1472-6882-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma ZZ, Zuo PP, Chen WR, Tang LJ, Shen Q, Sun XH, Shen Y. Studies on the sedative and sleeping effects of Wuling mycelia and its pharmacological mechanism. Chin Pharm J. 1999;34:374–377. [Google Scholar]
- 21.Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Hendricks JC, Williams JA, Panckeri K, Kirk D, Tello M, Yin JC, Sehgal A. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat Neurosci. 2001;4:1108–1115. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- 25.Graves LA, Hellman K, Veasey S, Blendy JA, Pack AI, Abel T. Genetic evidence for a role of CREB in sustained cortical arousal. J Neurophysiol. 2003;90:1152–1159. doi: 10.1152/jn.00882.2002. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Huang L, Wu H, Li Y, Zhang L, Yin Y, Xiang Z. Neuropeptide S mitigates spatial memory impairment induced by rapid eye movement sleep deprivation in rats. Neuroreport. 2010;21:623–628. doi: 10.1097/WNR.0b013e328339b5f9. [DOI] [PubMed] [Google Scholar]
- 27.Wang GP, Huang LQ, Wu HJ, Zhang L, You ZD, Zhao ZX. Calcineurin contributes to spatial memory impairment induced by rapid eye movement sleep deprivation. Neuroreport. 2009;20:1172–1176. doi: 10.1097/WNR.0b013e32832f0772. [DOI] [PubMed] [Google Scholar]
- 28.Gong QF, Zhang YM, Tan NH, Chen ZH. [Chemical constitutents in fermental mycelium of Xylaria nigripes] . Zhongguo Zhong Yao Za Zhi. 2008;33:1269–1272. [PubMed] [Google Scholar]
- 29.Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porte Y, Trifilieff P, Wolff M, Micheau J, Buhot MC, Mons N. Extinction of spatial memory alters CREB phosphorylation in hippocampal CA1. Hippocampus. 2010;21:1169–79. doi: 10.1002/hipo.20844. [DOI] [PubMed] [Google Scholar]
- 31.Rapanelli M, Frick LR, Zanutto BS. Differential gene expression in the rat hippocampus during learning of an operant conditioning task. Neuroscience. 2009;163:1031–1038. doi: 10.1016/j.neuroscience.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006;575:807–819. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li XY, Descalzi G, Kim SS, Chen T, Shang YZ, Zhuo M, Houslay MD, Abel T. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HY, Yang BC, Lee ES, Chung JI, Koh PO, Park MS, Kim MO. Modulation by the GABA(B) receptor siRNA of ethanol-mediated PKA-alpha, CaMKII, and p-CREB intracellular signaling in prenatal rat hippocampal neurons. Anat Cell Biol. 2011;44:210–217. doi: 10.5115/acb.2011.44.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naseer MI, Lee HY, Ullah N, Ullah I, Park MS, Kim MO. siRNA-mediated GABA(B) receptor at early fetal rat brain upon acute and chronic ethanol exposure: down regulation of PKA and p-CREB expression. Synapse. 2011;65:109–118. doi: 10.1002/syn.20824. [DOI] [PubMed] [Google Scholar]
- 37.Tu H, Rondard P, Xu C, Bertaso F, Cao F, Zhang X, Pin JP, Liu J. Dominant role of GABAB2 and Gbetagamma for GABAB receptor-mediated-ERK1/2/CREB pathway in cerebellar neurons. Cell Signal. 2007;19:1996–2002. doi: 10.1016/j.cellsig.2007.05.004. [DOI] [PubMed] [Google Scholar]


