Abstract
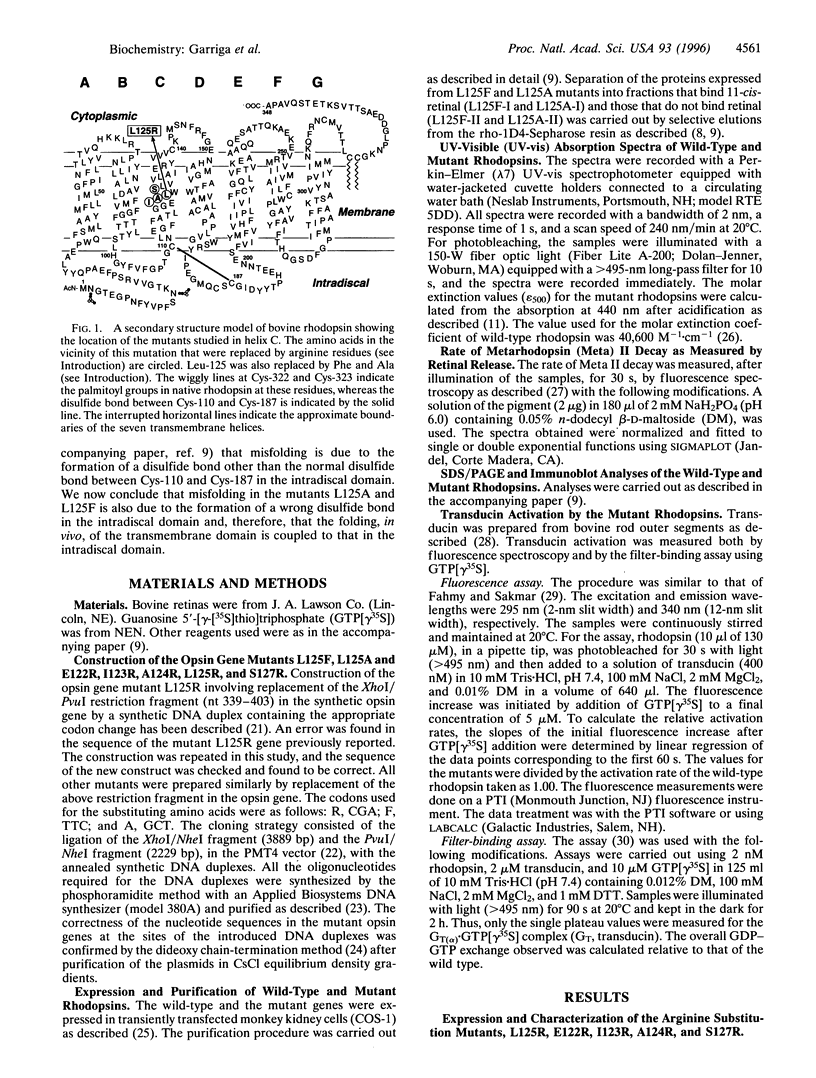
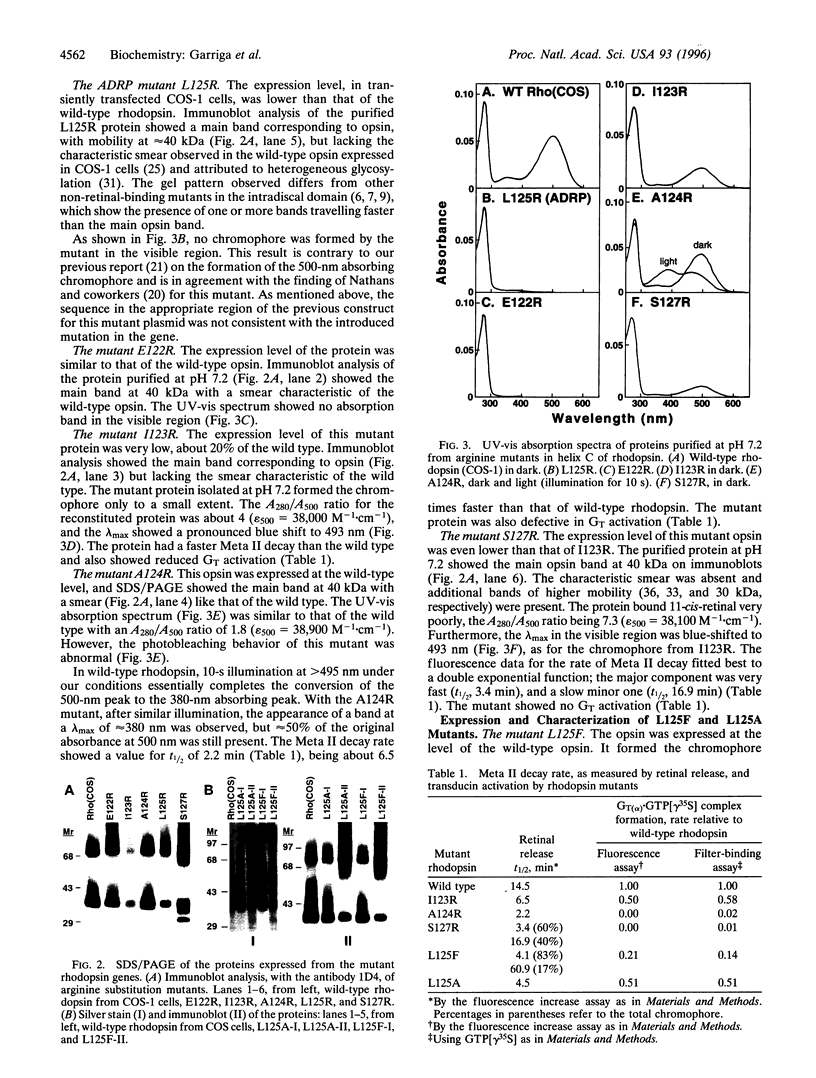
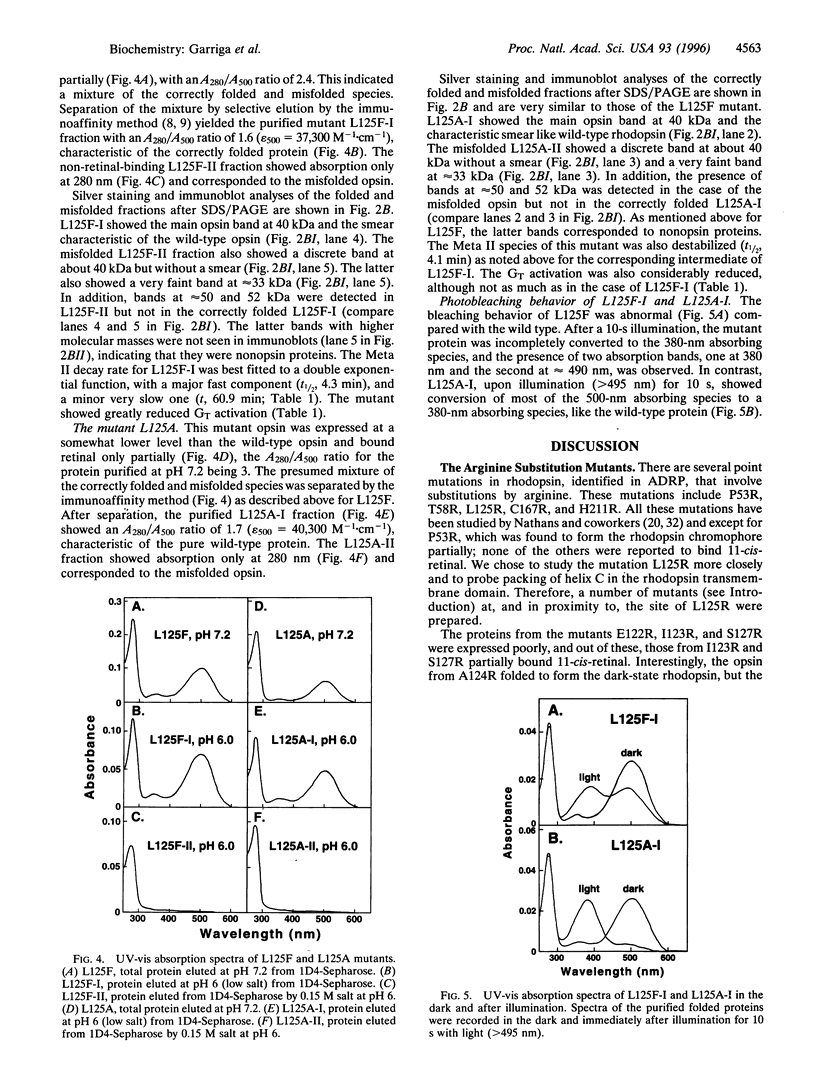
L125R is a mutation in the transmembrane helix C of rhodopsin that is associated with autosomal dominant retinitis pigmentosa. To probe the orientation of the helix and its packing in the transmembrane domain, we have prepared and studied the mutations E122R, I123R, A124R, S127R, L125F, and L125A at, and in proximity to, the above mutation site. Like L125R, the opsin expressed in COS-1 cells from E122R did not bind 11-cis-retinal, whereas those from I123R and S127R formed the rhodopsin chromophore partially. A124R opsin formed the rhodopsin chromophore (lambda max 495 nm) in the dark, but the metarhodopsin II formed on illumination decayed about 6.5 times faster than that of the wild type and was defective in transducin activation. The mutant opsins from L125F and L125A bound 11-cis-retinal only partially, and in both cases, the mixtures of the proteins produced were separated into retinal-binding and non-retinal-binding (misfolded) fractions. The purified mutant rhodopsin from L125F showed lambda max at 500 nm, whereas that from L125A showed lambda max at 503 nm. The mutant rhodopsin L125F showed abnormal bleaching behavior and both mutants on illumination showed destabilized metarhodopsin II species and reduced transducin activation. Because previous results have indicated that misfolding in rhodopsin is due to the formation of a disulfide bond other than the normal disulfide bond between Cys-110 and Cys-187 in the intradiscal domain, we conclude from the misfolding in mutants L125F and L125A that the folding in vivo in the transmembrane domain is coupled to that in the intradiscal domain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anukanth A., Khorana H. G. Structure and function in rhodopsin. Requirements of a specific structure for the intradiscal domain. J Biol Chem. 1994 Aug 5;269(31):19738–19744. [PubMed] [Google Scholar]
- Arnis S., Fahmy K., Hofmann K. P., Sakmar T. P. A conserved carboxylic acid group mediates light-dependent proton uptake and signaling by rhodopsin. J Biol Chem. 1994 Sep 30;269(39):23879–23881. [PubMed] [Google Scholar]
- Berson E. L. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993 Apr;34(5):1659–1676. [PubMed] [Google Scholar]
- Cohen G. B., Oprian D. D., Robinson P. R. Mechanism of activation and inactivation of opsin: role of Glu113 and Lys296. Biochemistry. 1992 Dec 22;31(50):12592–12601. doi: 10.1021/bi00165a008. [DOI] [PubMed] [Google Scholar]
- Davidson F. F., Loewen P. C., Khorana H. G. Structure and function in rhodopsin: replacement by alanine of cysteine residues 110 and 187, components of a conserved disulfide bond in rhodopsin, affects the light-activated metarhodopsin II state. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4029–4033. doi: 10.1073/pnas.91.9.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T., Molday R. S., Khorana H. G. Role of the intradiscal domain in rhodopsin assembly and function. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4991–4995. doi: 10.1073/pnas.87.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Berson E. L. Retinitis pigmentosa and allied diseases. Implications of genetic heterogeneity. Invest Ophthalmol Vis Sci. 1995 Jun;36(7):1197–1200. [PubMed] [Google Scholar]
- Dryja T. P., Hahn L. B., Cowley G. S., McGee T. L., Berson E. L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy K., Sakmar T. P. Regulation of the rhodopsin-transducin interaction by a highly conserved carboxylic acid group. Biochemistry. 1993 Jul 20;32(28):7229–7236. doi: 10.1021/bi00079a020. [DOI] [PubMed] [Google Scholar]
- Fahmy K., Siebert F., Sakmar T. P. A mutant rhodopsin photoproduct with a protonated Schiff base displays an active-state conformation: a Fourier-transform infrared spectroscopy study. Biochemistry. 1994 Nov 22;33(46):13700–13705. doi: 10.1021/bi00250a021. [DOI] [PubMed] [Google Scholar]
- Farrens D. L., Khorana H. G. Structure and function in rhodopsin. Measurement of the rate of metarhodopsin II decay by fluorescence spectroscopy. J Biol Chem. 1995 Mar 10;270(10):5073–5076. doi: 10.1074/jbc.270.10.5073. [DOI] [PubMed] [Google Scholar]
- Ferretti L., Karnik S. S., Khorana H. G., Nassal M., Oprian D. D. Total synthesis of a gene for bovine rhodopsin. Proc Natl Acad Sci U S A. 1986 Feb;83(3):599–603. doi: 10.1073/pnas.83.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke R. R., Sakmar T. P., Oprian D. D., Khorana H. G. A single amino acid substitution in rhodopsin (lysine 248----leucine) prevents activation of transducin. J Biol Chem. 1988 Feb 15;263(5):2119–2122. [PubMed] [Google Scholar]
- Inglehearn C. F., Keen T. J., Bashir R., Jay M., Fitzke F., Bird A. C., Crombie A., Bhattacharya S. A completed screen for mutations of the rhodopsin gene in a panel of patients with autosomal dominant retinitis pigmentosa. Hum Mol Genet. 1992 Apr;1(1):41–45. doi: 10.1093/hmg/1.1.41. [DOI] [PubMed] [Google Scholar]
- Jäger F., Fahmy K., Sakmar T. P., Siebert F. Identification of glutamic acid 113 as the Schiff base proton acceptor in the metarhodopsin II photointermediate of rhodopsin. Biochemistry. 1994 Sep 13;33(36):10878–10882. doi: 10.1021/bi00202a005. [DOI] [PubMed] [Google Scholar]
- Karnik S. S., Sakmar T. P., Chen H. B., Khorana H. G. Cysteine residues 110 and 187 are essential for the formation of correct structure in bovine rhodopsin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8459–8463. doi: 10.1073/pnas.85.22.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal S., Khorana H. G. Structure and function in rhodopsin. 7. Point mutations associated with autosomal dominant retinitis pigmentosa. Biochemistry. 1994 May 24;33(20):6121–6128. doi: 10.1021/bi00186a011. [DOI] [PubMed] [Google Scholar]
- Kaushal S., Ridge K. D., Khorana H. G. Structure and function in rhodopsin: the role of asparagine-linked glycosylation. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4024–4028. doi: 10.1073/pnas.91.9.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Garriga P., Khorana H. G. Structure and function in rhodopsin: correct folding and misfolding in two point mutants in the intradiscal domain of rhodopsin identified in retinitis pigmentosa. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4554–4559. doi: 10.1073/pnas.93.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macke J. P., Davenport C. M., Jacobson S. G., Hennessey J. C., Gonzalez-Fernandez F., Conway B. P., Heckenlively J., Palmer R., Maumenee I. H., Sieving P. Identification of novel rhodopsin mutations responsible for retinitis pigmentosa: implications for the structure and function of rhodopsin. Am J Hum Genet. 1993 Jul;53(1):80–89. [PMC free article] [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Otto H., Heyn M. P., Khorana H. G. The retinylidene Schiff base counterion in bacteriorhodopsin. J Biol Chem. 1991 Oct 5;266(28):18674–18683. [PubMed] [Google Scholar]
- Nakayama T. A., Khorana H. G. Mapping of the amino acids in membrane-embedded helices that interact with the retinal chromophore in bovine rhodopsin. J Biol Chem. 1991 Mar 5;266(7):4269–4275. [PubMed] [Google Scholar]
- Nathans J. Determinants of visual pigment absorbance: identification of the retinylidene Schiff's base counterion in bovine rhodopsin. Biochemistry. 1990 Oct 16;29(41):9746–9752. doi: 10.1021/bi00493a034. [DOI] [PubMed] [Google Scholar]
- Oprian D. D., Molday R. S., Kaufman R. J., Khorana H. G. Expression of a synthetic bovine rhodopsin gene in monkey kidney cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8874–8878. doi: 10.1073/pnas.84.24.8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge K. D., Lu Z., Liu X., Khorana H. G. Structure and function in rhodopsin. Separation and characterization of the correctly folded and misfolded opsins produced on expression of an opsin mutant gene containing only the native intradiscal cysteine codons. Biochemistry. 1995 Mar 14;34(10):3261–3267. doi: 10.1021/bi00010a016. [DOI] [PubMed] [Google Scholar]
- Sakmar T. P., Franke R. R., Khorana H. G. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmar T. P., Franke R. R., Khorana H. G. The role of the retinylidene Schiff base counterion in rhodopsin in determining wavelength absorbance and Schiff base pKa. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3079–3083. doi: 10.1073/pnas.88.8.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Nathans J. Rhodopsin mutations responsible for autosomal dominant retinitis pigmentosa. Clustering of functional classes along the polypeptide chain. J Biol Chem. 1993 Dec 15;268(35):26645–26649. [PubMed] [Google Scholar]
- Sung C. H., Schneider B. G., Agarwal N., Papermaster D. S., Nathans J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8840–8844. doi: 10.1073/pnas.88.19.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALD G., BROWN P. K. The molar extinction of rhodopsin. J Gen Physiol. 1953 Nov 20;37(2):189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M., Johnson G. L. Allosteric behavior in transducin activation mediated by rhodopsin. Initial rate analysis of guanine nucleotide exchange. J Biol Chem. 1987 Mar 15;262(8):3697–3705. [PubMed] [Google Scholar]
- Zhukovsky E. A., Oprian D. D. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989 Nov 17;246(4932):928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]