Abstract
Influenza virus recombinant X-53 produced for use in the 1976 National Immunization Program for swine influenza was found to comprise two types of virions differing in their antigenic, replicative, and plaque-forming characteristics. One type, characteristic of X-53 and designated “L,” was relatively low-yielding in chicken embryos, produced small clear plaques in Madin-Darby dog kidney cells, and was selectively inhibited by heterotypic antibody to the A/sw/Cam/39 strain of swine influenza virus. The other, X-53a or “H,” was high-yielding in chicken embryos, produced large turbid plaques in dog kidney cells, and was not inhibited by concentrations of A/sw/Cam/39 antisera inhibitory to X-53. It was shown that A/NJ/11/76 (HswN1) virus, from which X-53 was derived, and five other swine influenza virus isolates from humans and pigs were dimorphic mixtures of the two types of virus. Segregation of the hemagglutinin genes of L and H variants by further recombination demonstrated that their different properties were pleiotropic phenotypes of mutation in the hemagglutinin gene. Under selective conditions suppressive to the L mutant, mutation of cloned L to H virus was observed. This observation, as well as the apparent ubiquity of the two mutants in nature, suggests that this is another example of viral dimorphism—the stable association of two allelic mutants. Of special significance is the indication that antigenic variants may be selected by selection for properties other than antigenicity, and therefore may represent mutants with pathogenic effects determined by factors other than lesser modulation by host antibody.
Keywords: virulence, recombination, RNA, vaccine
Full text
PDF
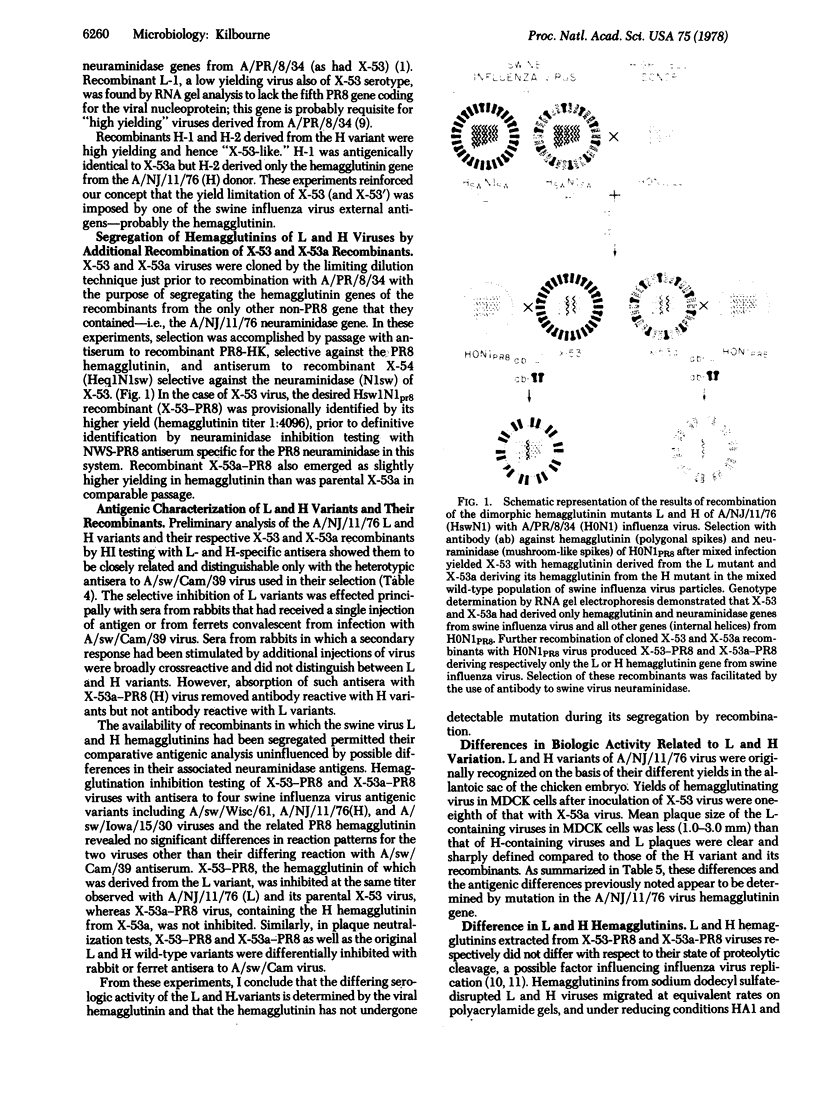


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. D. Genetic analysis of animal viruses. New approaches and new vistas. Br Med Bull. 1967 May;23(2):155–160. doi: 10.1093/oxfordjournals.bmb.a070537. [DOI] [PubMed] [Google Scholar]
- FENNER F., SAMBROOK J. F. THE GENETICS OF ANIMAL VIRUSES. Annu Rev Microbiol. 1964;18:47–94. doi: 10.1146/annurev.mi.18.100164.000403. [DOI] [PubMed] [Google Scholar]
- Gerhard W., Webster R. G. Antigenic drift in influenza A viruses. I. Selection and characterization of antigenic variants of A/PR/8/34 (HON1) influenza virus with monoclonal antibodies. J Exp Med. 1978 Aug 1;148(2):383–392. doi: 10.1084/jem.148.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILBOURNE E. D., MURPHY J. S. Genetic studies of influenza viruses. I. Viral morphology and growth capacity as exchangeable genetic traits. Rapid in ovo adaptation of early passage Asian strain isolates by combination with PR8. J Exp Med. 1960 Mar 1;111:387–406. doi: 10.1084/jem.111.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P., Noble G. R., Dowdle W. R. Swine influenza viruses isolated in 1976 from man and pig contain two coexisting subpopulations with antigenically distinguishable hemagglutinins. Virology. 1977 Oct 1;82(1):111–121. doi: 10.1016/0042-6822(77)90037-x. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D. Future influenza vaccines and the use of genetic recombinants. Bull World Health Organ. 1969;41(3):643–645. [PMC free article] [PubMed] [Google Scholar]
- Kilbourne E. D., Schulman J. L., Schild G. C., Schloer G., Swanson J., Bucher D. Related studies of a recombinant influenza-virus vaccine. I. Derivation and characterization of virus and vaccine. J Infect Dis. 1971 Nov;124(5):449–462. doi: 10.1093/infdis/124.5.449. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- McCormick K. J., Murphy W. H. Genetic heterogeneity of cloned animal virus preparations. Nature. 1969 Jan 18;221(5177):286–286. doi: 10.1038/221286a0. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L., Kilbourne E. D. Genetic composition of a high-yielding influenza A virus recombinant: a vaccine strain against "Swine" influenza. Science. 1976 Oct 15;194(4262):334–335. doi: 10.1126/science.968486. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- WALEN K. H. Demonstration of inapparent heterogeneity in a population of an animal virus by single-burst analyses. Virology. 1963 Jun;20:230–234. doi: 10.1016/0042-6822(63)90110-7. [DOI] [PubMed] [Google Scholar]
- WONG S. C., KILBOURNE E. D. Changing viral susceptibility of a human cell line in continuous cultivation. I. Production of infective virus in a variant of the Chang conjunctival cell following infection with swine or N-WS influenza viruses. J Exp Med. 1961 Jan 1;113:95–110. doi: 10.1084/jem.113.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]




