Abstract
Diffuse large B-cell lymphoma (DLBCL) presented as a primary dural lesion is an extremely rare entity, which may be misdiagnosed as meningioma. Patients with symptomatic meningioma are usually treated with tumor resection. The five previously described cases of intracranial dural DLBCL were treated with surgery followed by chemotherapy and/or radiotherapy, with a favorable outcome, but with potential sequels. We reported the first case of DLBCL, presented as a primary dural lesion, successfully treated with chemotherapy only in a 52-year-old woman presented in October 2011 with rapidly progressive headaches, nausea and vomiting. Magnetic resonance imaging of the brain and head computed tomography showed a lesion en plaque in the right parieto-occipital region, tracking the dura matter, and osteolytic lesions. The patient underwent an open tumor biopsy, and the diagnosis of DLBCL was established. The patient received dexamethasone, cisplatin and cytarabine (DHAP) followed by methotrexate plus cytarabine, and obtained a durable complete response. Thus, intracranial dural DLBCL must be considered in differential diagnosis of meningeal lesions, particularly when a rapid progression of symptoms and osteolytic lesions are present, because an early diagnosis and rapid initiation of treatment, even though with chemotherapy, is associated with favorable outcome.
Keywords: Primary dural lymphoma, diffuse large B-cell lymphoma, chemotherapy
Introduction
Primary dural lymphoma (PDL) is an extremely rare form of extranodal non-Hodgkin lymphoma (NHL), defined as lymphomatous involvement of the dura matter, in the absence of systemic disease [1].
PDL is distinct from primary central nervous system lymphoma (PCNSL) in terms of tumor biology, clinical presentation and prognosis. Unlike PCNSL, PDL originates outside the central nervous system of immunologically stable individuals, and therefore is not protected by the blood-brain barrier until central nervous system invasion and seeding occurs [2]. On histological examination, the vast majority of intra-cranial PDL corresponds to low-grade lymphomas, and carries a more favorable prognosis in comparison to PCNSL, which is an aggressive, diffuse large B-cell lymphoma (DLBCL) in over 95% of cases [3,4]. On neuro-imaging, PDL mimics and is undistinguishable from other dural-based neoplasms, particularly meningiomas [5,6], which are the most common intra-cranial tumor. The differential diagnosis of dural-based lesions found on neuro-imaging is important because asymptomatic patients often receive a presumptive diagnosis of meningioma, and are followed radiographically without biopsy or surgical resection as part of initial workup, while that PDL of high grade seems to need treatment with tumor resection, radio and/or chemotherapy [7].
We report herein the diagnostic challenges and treatment outcome in a patient with PDL that mimicked a meningioma on neuro-imaging, with the biopsy surprisingly showing histological features of a DLBCL, rather than a low grade lymphoma. Tumor complete remission with chemotherapy makes this case unique in its type.
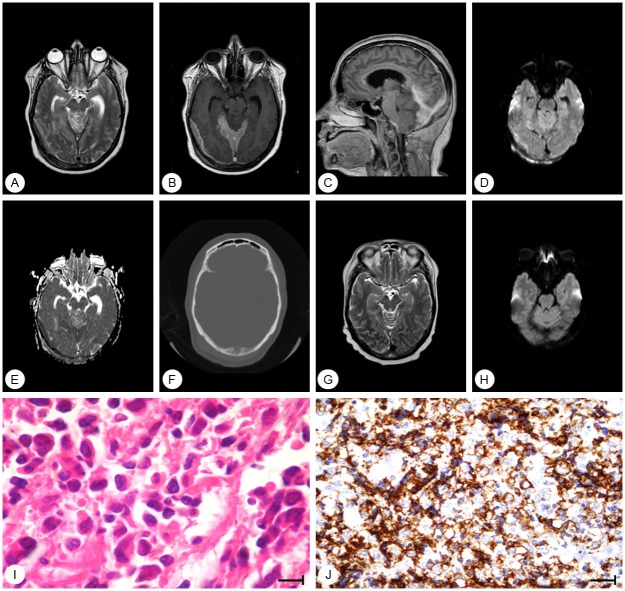
Clinical history
A 52-year-old Caucasian woman with no significant past medical history presented in October 2011 with right hemifacial paresthesia for the past four weeks. The initial physical and neurological examination was unremarkable. The patient received a diagnosis of atypical trigeminal neuralgia, which improved with amitriptyline. A month later, the patient was admitted to an Urgent Care facility with rapidly progressive headaches, nausea and vomiting. The neurologic examination disclosed the presence of a right lower limb hyperreflexia. A magnetic resonance imaging (MRI) of the brain revealed dural/patchy meningeal thickening in the right posterior fossa and occipital region, hypointense on T2 sequences, with adjacent brain edema (Figure 1A). T1 post-gadolinium images showed an intense enhancement on the dural lesion (Figure 1B, 1C). Diffusion-weighted image showed a hyperintense area in the dural parieto-occipital lesion (Figure 1D) with low intensity on the apparent diffusion coefficient (ADC) map (Figure 1E), confirming restricted diffusion. Computed tomography (CT) of the head showed similar findings, and disclosed signs of bone lysis (Figure 1F). Cerebrospinal fluid examination was unremarkable. No additional lesions were seen in a CT of the chest, abdomen and pelvis, and the bone marrow biopsy showed no evidence of malignancy. Serum immunology studies for HIV and EBV were negative. The differential diagnosis included a meningioma en plaque versus, less likely, intracranial dural metastasis, a low-grade PDL and a hemangiopericytoma.
Figure 1.
(A) T2 weighted magnetic resonance imaging (MRI) shows that the extraaxial (patchy meningeal) lesions are hypointense. There is also hyperintensity in the superior cerebellar vermis (vasogenic edema); T1-weighted (B) axial and (C) sagittal images, after contrast show dural (patchy meningeal) enhancement in the right parieto-occipital region, tracking the dura. (D) Diffusion-weighted image (DWI) and (E) apparent diffusion coefficient (ADC) map demonstrate a hyperintense area in the dural parietooccipital lesion, with low ADC. The superior cerebellar vermis shows high intensity in DWI and high intensity in the ADC map secondary to “T2 shine through” and not restricted diffusion. (F) Axial head computed tomography (bone window) demonstrates infiltration and lysis of the occipital bone. After treatment, (G) T2 and (H) DWI on MRI show no signal of the tumor. (I) Histological and (J) immunohistochemical analyses of the tumor at diagnosis show neoplastic cells with large and pleomorphic nuclei (HE, 1000x), and neoplastic cells with expression of CD20 on membrane (800x).
The patient underwent a ventricular peritoneal shunt due to hydrocephalus and open biopsy of the tumor. Histological examination showed diffusely infiltrative large cells with scant cytoplasm, evident nucleoli, and frequent mitotic figures (Figure 1I). Immunohistochemical profiling revealed expression of CD20 (Figure 1J) and CD10, and high counts of proliferation marker Ki-67 (40%). The diagnosis of a DLBCL of the dura was established (IE, Ann Arbor staging system).
The patient was treated with two cycles of DHAP (dexamethasone, 40 mg orally on days 1 through 4, cisplatin 100 mg/m² intravenously (IV) by continuous infusion over 24 hours, followed by cytarabine in two pulses each at a dose of 2000 mg/m² given [8], followed by four cycles of methotrexate (3.5 g/m²) on day 1 plus two pulses 12 hours apart of cytarabine (2000 mg/m²) every two weeks [9] between November 2011 and June 2012. A repeat MRI of the brain done one month later showed a complete response (Figure 1G, 1H). As of September 2013, the patient remains disease-free, at 22 months following her initial diagnosis.
Discussion
We report an extremely rare case of DLBCL presenting as PDL, which on MRI mimicked particularly a meningioma, and that responded favorably to chemotherapy.
Intra-cranial PDL is a rare form of NHL that usually presents with focal neurologic symptoms reflecting tumor location, including seizures, focal sensory, headache and, occasionally, intra-cranial hypertension [7,10-13]. In the vast majority of the patients, the lymphoma is of a low-grade [3,4]. A diffuse large B-cell histology such as seen in our patient is extremely rare in PDL, and only five other patients have been reported in the literature (Table 1). Including our patient, 5/6 cases occurred in women [10-13] and one in a man [7], with a median age at diagnosis of 51 years (range: 42-61 years).
Table 1.
Reported cases of primary intracranial dural diffuse large B-cell lymphoma
| Case | Age (years) | Sex | Location | Therapy | Survival Status (months) |
|---|---|---|---|---|---|
| Amaker et al., 2000 | 49 | F | Left frontal | S + C + R | Alive (14) |
| Galarza et al., 2006 | 61 | M | Vertex | S + C + R | Alive (23) |
| Yamada et al., 2006 | 59 | F | Frontal bilateral | S + C | Alive (30) |
| Sacho et al., 2010 | 46 | F | Right parietal | S + C | Dead1 |
| Said et al., 2011 | 42 | F | Left vertex | S + C | Alive (34) |
| Current case | 52 | F | Parieto-occipital | C | Alive (22) |
F: female; M: male; S: surgery; C: chemotherapy; R: radiation therapy.
Patient died from progression of intra-cranial mass.
The pathogenesis of PDL is poorly understood. The dura is devoid of any lymphoid tissue, but it has been hypothesized that a benign meningeal chronic inflammatory condition could precede the malignant transformation of lymphoid cells, as suggested for other extranodal lymphomas [3]. Our patient had a negative HIV and EBV testing, and no history of immunosuppression, which is in line with other patients reported in the literature [3]. Radiographically, PDL presents as dural-based lesion with a variable signal intensity on T1 and long-TR sequences, with an usually homogeneous, intense pattern of contrast enhancement on T1 post-gadolinium sequences. The diffusion may be restricted, reflecting compact cellularity. Such findings are undistinguishable from other dural-based lesions such as dural-based metastasis, meningiomas and hemangiopericytomas [3,5,6,14]. Dural metastasis is common in women with breast cancer [15]. However, dural-based lesions are rarely a presenting or isolated finding of systemic cancer, and therefore this diagnosis was unlikely in our patient. As hemangiopericytomas are extremely rare tumors, the main diagnosis for our case was meningioma. In a series of 15 patients with PDL, a diagnosis of meningioma was mistakenly proposed in 14, based on the radiographic features. Our patient presented with radiographic features of a meningioma en plaque, defined by a carpet or sheet-like lesion that infiltrates the dura, and provokes bone hyperostosis and sometimes osteolytic lesions [14,16]. In addition to displaying restricted diffusion MRI and vasogenic edema, which would be unusual in meningiomas, our patient showed signs of occipital bone lysis, rather than the typical hyperostosis that accompanies meningiomas en plaque [3,16]. These unusual findings, coupled with the fact that symptoms were rapidly progressing, promptly lead to a surgical biopsy, favored over a wait-and-see approach. This eventually allowed for rapidly and accurately establishing the diagnosis of DLBCL and initiating the treatment.
There has been no standard treatment in intracranial dural DLBCL. Therapy for extra-nodal high-grade NHL of less than 10 cm can usually be successfully managed with Adriamycin-containing regimens followed by radiation therapy in the involved field. Other approaches include other aggressive chemotherapy courses with or without subsequent radiation therapy, and radiation therapy alone in selected cases [17]. In all the five previously reported cases, patients were treated with tumor resection followed by CHOP with or without rituximab or methotrexate-based regimes [7,10-13]; additional whole-brain radiation therapy was used in two patients [7,11] (Table 1). We chose systemic chemotherapy for the tumor eradication in our case for three reasons: 1) the patients was immunologically stable; 2) the tumor was highly proliferative; and 3) free access of chemotherapeutic agents to the dural tumor without passing through the blood-brain barrier was considered to be an advantage. The DHAP regimen was initially selected because of the possibility of transformation from a low-grade lymphoma, the most common form of PDL [3]. After a multidisciplinary discussion, a decision was made to switch treatment to a methotrexate and cytarabine regimen, with the intent of preventing or treating a potentially undiagnosed central nervous system invasion. Radiotherapy was not offered as part of the treatment to avoid neurotoxicity, considering that this patient could potentially achieve long-term survival, which is associated with a cumulative life-time risk of cognitive deterioration [18].
The prognosis of intracranial dural DLBCL seems to be favorable. Four out of five previously reported cases were alive at the time of the last evaluation [7,10,11,13]; only one immunosuppressed patient died from rapid progression of intra-cranial mass effect, which highlights the need to rapidly establish the diagnosis and initiate treatment [12]. Overall patients responded well to treatment, irrespective of the regimen chosen.
In conclusion, intracranial dural DLBCL is an extremely uncommon NHL that must be considered in the differential diagnosis of meningeal lesions. Clues for the diagnosis include a rapid progression of symptoms, restricted diffusion on MRI, and the presence of bone lytic lesions on CT, which are usually not seen in meningiomas. Recognizing this entity is important because an early diagnosis and rapid initiation of treatment, even though with chemotherapy only, is associated with a high response rates and favorable outcome.
Disclosure of conflict of interest
The authors declare no competing interests.
References
- 1.Woodman R, Shin K, Pineo G. Primary non-Hodgkin Lymphoma of the brain: a review. Medicine. 1985;64:425–30. doi: 10.1097/00005792-198511000-00006. [DOI] [PubMed] [Google Scholar]
- 2.De Angelis LM. Brain tumors. N Engl J Med. 2001;344:114–23. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto FM, Abrey LE. Primary dural lymphomas: a review. Neurosurg Focus. 2006;21:E5–9. doi: 10.3171/foc.2006.21.5.6. [DOI] [PubMed] [Google Scholar]
- 4.Mneimneh WS, Ashraf MA, Li L, El-Kadi O, Qian J, Nazeer T, Hayner-Buchan A. Primary dural lymphoma: a novel concept of heterogeneous disease. Pathol Int. 2013;63:68–72. doi: 10.1111/pin.12025. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs A, Kracht LW, Grossman A, Ruger MA, Thomas AV, Thiel A, Herholz K. Imaging in neurooncology. NeuroRx. 2005;2:333–47. doi: 10.1602/neurorx.2.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sotoudeh H, Yazdi HR. A review on dural tail sign. World J Radiol. 2010;2:188–92. doi: 10.4329/wjr.v2.i5.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galarza M, Gazzeri R, Elfeky HA, Johnson RR. Primary diffuse large B-cell lymphoma of the dura mater and cranial vault: case report and literature review. Neurosurg Focus. 2006;21:E10–3. doi: 10.3171/foc.2006.21.5.11. [DOI] [PubMed] [Google Scholar]
- 8.Velasquez WS, Cabanillas F, Salvador P, McLaughlin P, Fridrik M, Tucker S, Jagannath S, Hagemeister FB, Redman JR, Swan F, Barlogie B. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP) Blood. 1988;71:117–22. [PubMed] [Google Scholar]
- 9.Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G, Ilariucci F, Rossi G, Soffietti R, Stelitano C, Vallisa D, Zaja F, Zoppegno L, Aondio GM, Avvisati G, Balzarotti M, Brandes AA, Fajardo J, Gomez H, Guarini A, Pinotti G, Rigacci L, Uhlmann C, Picozzi P, Vezzulli P, Ponzoni M, Zucca E, Caligaris-Cappio F, Cavalli F International Extranodal Lymphoma Study Group (IELSG) High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374:1512–20. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- 10.Amaker BH, Ghatak NR, Jebraili SA, Ferreira-Gonzalez A, Kornstein MJ. Primary T-cell-rich B-cell lymphoma masquerading as a meningioma. Arch Pathol Lab Med. 2000;124:1700–3. doi: 10.5858/2000-124-1700-PTCRBC. [DOI] [PubMed] [Google Scholar]
- 11.Yamada SM, Ikawa N, Toyonaga S, Nakabayashi H, Chang Park K, Shimizu K. Primary malignant B-cell-type dural lymphoma: case report. Surg Neurol. 2006;66:539–43. doi: 10.1016/j.surneu.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Sacho RH, Kogels M, du Plessis D, Jowitt S, Josan VA. Primary diffuse large B cell central nervous system lymphoma presenting as an acute space-occupying subdural mass. J Neurosurg. 2010;113:384–7. doi: 10.3171/2010.2.JNS091554. [DOI] [PubMed] [Google Scholar]
- 13.Said R, Rizk S, Dai Q. Clinical challenges of primary diffuse large B-cell lymphoma of the dura: Case report and literature review. ISRN Hematol. 2011;2011:945212. doi: 10.5402/2011/945212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu PH, Giannini C, Judkins AR, Schwalb JM, Burack R, O’Neill BP, Yachnis AT, Burger PC, Scheithauer BW, Perry A. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J. Clin. Oncol. 2005;23:5718–27. doi: 10.1200/JCO.2005.17.624. [DOI] [PubMed] [Google Scholar]
- 15.Nayak L, Abrey LE, Iwamoto FM. Intracranial dural metastases. Cancer. 2009;115:1947–53. doi: 10.1002/cncr.24203. [DOI] [PubMed] [Google Scholar]
- 16.Baek JU, Cho YD, Yoo JC. A no steolytic meningioma en plaque of the sphenoid ridge. J Korean Neurosurg Soc. 2008;43:34–6. doi: 10.3340/jkns.2008.43.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrey LE, Yahalom J, De Angelis LM. Treatment for primary CNS lymphoma: the next step. J. Clin. Oncol. 2000;18:3144–50. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 18.Omuro AM, Martin-Duverneuil N, Delattre JY. Complications of radiotherapy to the central nervous system. Handb Clin Neurol. 2012;105:887–901. doi: 10.1016/B978-0-444-53502-3.00030-6. [DOI] [PubMed] [Google Scholar]