Abstract
Objectives
The goal of this study was to systematically review the peripartum cardiomyopathy (PPCM) literature and determine the prevalence of preeclampsia (PE) in women with PPCM. Secondary analyses included evaluation of the prevalence of hypertensive disorders, multiple gestations, and multiparity.
Background
PPCM is a significant cause of maternal and infant morbidity and mortality worldwide, yet its etiology remains unknown. PE is often cited as a risk factor for the development of PPCM, and recent research suggests that PE and PPCM share mechanisms that contribute to their pathobiology. No comprehensive evaluation of the relationship between PE and PPCM exists.
Methods
A systematic predetermined search strategy was performed in multiple databases to identify studies describing ≥3 women with PPCM. Prevalence rates of PE, hypertension, multiple gestations, and multiparity were pooled.
Results
Data from 22 studies (979 patients) were included in this analysis. The pooled prevalence of 22% (95% confidence interval [CI] 16 to 28) was more than quadruple the 5% average worldwide background rate of PE in pregnancy (p < 0.001). There were no geographic or racial differences detected in the prevalence of PE in women with PPCM. The rates of hypertension during pregnancy (37% [95% CI 29 to 45) and multiple gestations (9% [95% CI 7 to 11]) were also elevated.
Conclusions
The prevalence of PE, hypertensive disorders, and multiple gestations in women with PPCM is markedly higher than that in the general population. These findings support the concept of a shared pathogenesis between PE and PPCM and highlight the need for awareness of the overlap between these 2 diseases.
Keywords: antiangiogenic, peripartum cardiomyopathy, preeclampsia
Peripartum cardiomyopathy (PPCM) affects women around the time of childbirth and is marked by unexplained systolic dysfunction and the development of symptomatic heart failure (1,2). PPCM affects 1 in 3,000 pregnancies, although it is more common in women of African descent (3,4) and can occur in as many as 1% of all pregnancies in certain “hot spots” such as Haiti and Nigeria (5,6). The outcome is devastating for both mothers and infants. PPCM accounts for 5% of heart transplants in US women, an option not available in most of the world (7). As many as 25% of women with PPCM in developing countries die within 5 years (8), with an associated infant mortality rate as high as 50% to 75% (9,10). In the United States, the mortality rate for women with PPCM approximates 6% for white non-Hispanic women and is 4-fold higher in women of African descent (11).
The etiology of PPCM remains enigmatic. Numerous hypotheses have been proposed, including viral myocarditis, autoimmunity, and fetal microchimerism. Hull and Hidden (12) first described a connection between postpartum heart failure and hypertensive heart disease in 1938 when they noted that >85% of cases of “‘toxic’ postpartal” heart disease were associated with hypertension, which was double the frequency seen in their control group. Demakis and Rahimtoola (13), in their classic 1971 description of PPCM, reported that “toxemia,” an older term for preeclampsia (PE), was detected in 22% of affected women. Since the publication of these seminal studies, PE has often been cited as an independent risk factor for the development of PPCM (14–16), but not all clinical studies support this conclusion (17).
Recent research suggests that PPCM is a vascular disease, triggered by late–gestational secretion of potent antiangiogenic agents from the placenta and pituitary (18,19). In this context, the frequently noted association of PPCM with PE is of special interest because PE is similarly caused by excess secretion of such antivascular factors from the placenta (20). Multiple gestations also have higher circulating levels of these antiangiogenic factors and are a risk factor for both PPCM and PE (21,22). In light of these observations, the objective of the current paper was to review the PPCM literature to determine the frequency of association between PPCM and PE. In secondary analyses, we reviewed the observed frequency of any hypertensive disorder, as well as multiple gestations in pregnancies affected by PPCM.
Methods
Literature search
The methods and inclusion criteria were specified in advance and documented in a protocol (Online Appendix). We followed the guidelines from MOOSE (Meta-analyses of Observational Studies in Epidemiology) (23) and the Cochrane Handbook for Systematic Reviews (24). Studies were identified by searching electronic databases, scanning reference lists, and consultation with experts in the field. This search strategy was applied to MEDLINE (1966 to present), EMBASE (1966 to present), and Web of Science (1945 to present). Cochrane and DARE (Database of Abstracts of Reviews of Effectiveness) databases were reviewed. We used the following search strategy to search MEDLINE and adapted it for the other databases: “Cardiomyopathies” [MeSH] OR “Cardiomyopathies”[tiab] OR “Cardiomyopathy”[tiab]) AND (“Pregnancy”[MeSH] OR “Pregnancies”[tiab] OR “Gestation” [tiab] OR “pregnancy”[tiab]. The analysis was restricted to articles and abstracts published in English. No publication date restrictions were imposed. The last search was run on January 7, 2013.
Study selection
Independent screening of articles and abstracts was performed by 2 authors (Drs. Bello and Rendon) by using predefined data fields, including study quality indicators and the following criteria: 1) the publication described ≥3 women with PPCM; 2) the publication reported numbers of cases and sample size or the prevalence of PE and/or hypertensive disorders; and 3) PPCM was defined according to the 1992 National Heart, Lung, and Blood Institute Workshop criteria of left ventricular ejection fraction (LVEF) <45%, fractional shortening <30%, or both (25,26). Recent definitions of PPCM have loosened both the requirements that LVEF be <45% and that presentation occur within 1 month before or 5 months after delivery. Nevertheless, we chose to adhere strictly to the National Institutes of Health criteria promoted by the 1992 National Heart, Lung, and Blood Institute workshop because these criteria remain widely accepted and because the criteria are more clearly defined. We also extracted data on patient characteristics, study design, and location. For those articles on which the 2 data extractors disagreed (n = 3), the third author (Dr. Arany) independently reviewed the articles and made a final decision on the inclusion or exclusion of the study.
For the purpose of the current review, cases described as PE or eclampsia were grouped as PE, and hypertension during pregnancy was defined as either chronic hypertension, gestational hypertension, PE, eclampsia, or hypertension not otherwise specified. When there was overlap of patients between publications, the distinct patients were included once and analyzed as a single sample (rather than as 2 studies) (15,27).
Statistical analysis
For each included study, the prevalence of PE and its 95% confidence interval (CI) was calculated. If a study included an explicit prevalence rate of 0%, we increased the prevalence of PE to 5% to calculate SEs and CIs. Meta-analysis was performed by using a variable effects model to determine the pooled prevalence of PE in women with PPCM. The analysis was also stratified according to country of origin. Heterogeneity was assessed by using the Cochran's Q test and I2 statistics. For the secondary analyses of hypertensive disorders and twins, the heterogeneity was high, and we chose to display the results in narrative format rather than as a meta-analysis. Spearman's correlation was performed to evaluate the relationship between African descent and PE in women with PPCM. Statistical analyses were performed by using Stata/SE version 12.0 (StataCorp, College Station, Texas).
Results
A total of 267 full-text articles and abstracts of primary data were identified as potentially relevant publications, and 22 (5,11,14–17,27–42) were included in the primary analysis (Fig. 1). Table 1 describes the characteristics of the studies and patients included in the analysis. Overall, 979 patients were included, approximately one-half of studies were from the United States, and most were published within the last decade. Sample size varied from 6 to 110, and the majority of women were multiparous. The fraction of women of African descent varied widely, even within the United States.
Figure 1. Process of Study Selection.
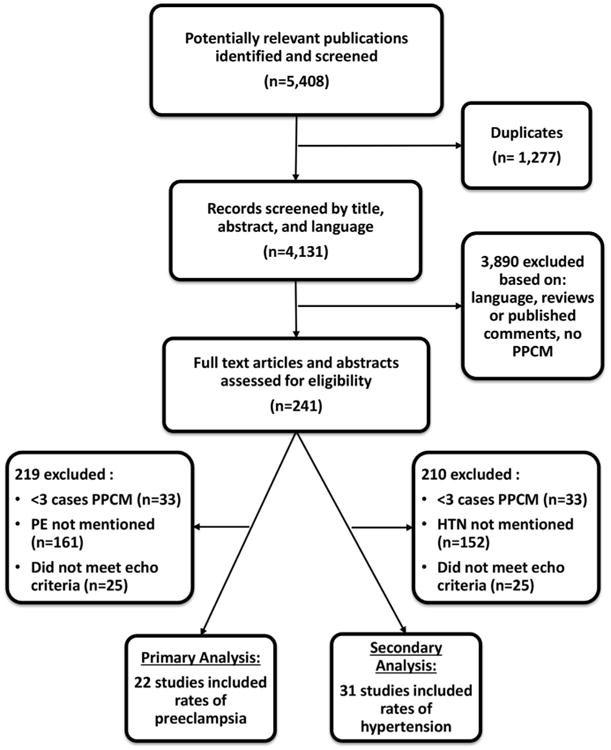
HTN = hypertension; PE = preeclampsia; PPCM = peripartum cardiomyopathy.
Table 1. Characteristics of Studies Included in the Meta-Analysis.
| Year | Country | No. of Patients | Mean Age (yrs) | African Descent (%) | |
|---|---|---|---|---|---|
| Amos et al. (13) | 2006 | United States | 55 | 29 | 51 |
| Chapa et al. (32) | 2005 | United States | 32 | 27 | 78 |
| Chee et al. (45) | 2013 | Malaysia | 8 | 31 | – |
| Elkayam et al. (34) | 2001 | United States | 44 | 29 | 36 |
| Elkayam et al. (33) | 2005 | United States | 100 | 31 | 19 |
| Felker et al. (17) | 2000 | United States | 42 | 29 | 46 |
| Fett et al. (5) | 2005 | Haiti | 98 | 32 | 100 |
| Gunderson et al. (16) | 2011 | United States | 110 | – | 29 |
| Harper et al. (11) | 2012 | United States | 79 | - | 59 |
| Hasan et al. (35) | 2010 | Pakistan | 32 | 32 | 0 |
| Horgan et al. (46) | 2013 | Ireland | 12 | 35 | 0 |
| Horne et al. (36) | 2011 | United States | 71 | 30 | 7 |
| Mandal et al. (40) | 2011 | India | 36 | - | - |
| Modi et al. (41) | 2005 | United States | 30 | 23 | 80 |
| Pillarisetti et al. (43) | 2011 | United States | 81 | 30 | 60 |
| Samonte et al. (42) | 2012 | Philippines | 9 | 29 | – |
| Sebillotte et al. (37) | 2010 | Martinique | 13 | 30 | 100 |
| Shah et al. (44) | 2012 | Pakistan | 61 | 31 | 30 |
| Suri et al. (38) | 2012 | India | 28 | 28 | 0 |
| Vettori et al. (39) | 2011 | Brazil | 6 | 27 | 50 |
| Witlin et al. (15,27) | 1997 | United States | 32 | 32 | 75 |
The overall prevalence of PE varied from a low of 0% (n = 17) to a high of 78% (n = 38) in the individual studies (Table 2). The summary estimate of the prevalence of PE was 22% (95% CI 16 to 28) (Fig. 2), which is >4 times higher than the 3% to 5% average worldwide prevalence of PE (p < 0.001) (43–46). There was substantial heterogeneity among the included studies (Q test, p < 0.001; I 79%). To investigate potential causes of heterogeneity, a subgroup meta-analysis looking at the rate of PE by country was performed. The studies from the United States had an estimated prevalence of 24% (95% CI 17 to 32), higher than the rest of the world (17% [95% CI 8 to 26); these studies had markedly reduced heterogeneity (Fig. 3).
Table 2. Rates of Hypertensive Disorders, Multiple Gestations, and Multiparity During Pregnancy in Women With Peripartum Cardiomyopathy.
| Year | Country | No. | Mean Age (yrs) | PE (%) | Any HTN During Pregnancy (%) | Twins or Multiples (%) | Multiparous (%) | |
|---|---|---|---|---|---|---|---|---|
| Amos et al. (13) | 2006 | United States | 55 | 29 | 45 | 56 | – | 63 |
| Chapa et al. (31) | 2005 | United States | 32 | 27 | 16 | 16* | 13 | 50 |
| Chee et al. (44) | 2013 | Malaysia | 8 | 31.2 | 13 | 25 | 38 | – |
| Elkayam et al. (33) | 2005 | United States | 100 | 30.7 | 33 | 43 | 13 | – |
| Elkayam et al. (32) | 2001 | United States | 44 | 29 | 23 | 32 | - | 100 |
| Felker et al. (16) | 2000 | United States | 42 | 29 | 0 | 43 | 12 | 53 |
| Fett et al. (5) | 2005 | Haiti | 98 | 32.2 | 4 | 19 | - | 76 |
| Gentry et al. (55) | 2010 | United States | 28 | 26 | – | 29 | - | 58 |
| Gunderson et al. (15) | 2011 | United States | 110 | – | 12 | 50 | 8 | 61 |
| Habli et al. (56) | 2008 | United States | 70 | 29 | – | 23 | - | – |
| Harper et al. (10) | 2012 | United States | 79 | – | 28 | 63 | 9 | – |
| Hasan et al. (34) | 2010 | Pakistan | 32 | 31.8 | 19 | 50 | 6 | 97 |
| Horgan et al, (45) | 2013 | Ireland | 12 | 34.7 | 75 | 75 | 17 | 75 |
| Horne et al. (35) | 2011 | United States | 71 | 30 | 25 | 68 | – | – |
| Huang et al. (46) | 2012 | China | 52 | 25.2 | – | 12 | – | – |
| Lampert et al. (50) | 1993 | United States | 15 | 30 | – | 53 | – | – |
| Mandal et al. (39) | 2011 | India | 36 | – | 11 | 11* | – | 61 |
| Modi et al. (40) | 2009 | United States | 44 | 25.2 | – | 45 | 17 | – |
| Pillarisetti et al. (42) | 2011 | United States | 81 | 30 | 33 | 33 | – | 60 |
| Safirstein et al. (47) | 2012 | United States | 55 | 31.6 | - | 42 | 15 | 51 |
| Saltzberg et al. (48) | 2012 | United States | 107 | 31.2 | - | 14 | 15 | 66 |
| Samonte et al. (41) | 2012 | Philippines | 9 | 29 | 78 | 78* | 11 | 78 |
| Sebillotte et al. (36) | 2010 | Martinique | 13 | 30 | 8 | 31 | 15 | 54 |
| Shah et al. (43) | 2012 | Pakistan | 61 | 30.9 | 20 | 51 | 8 | 95 |
| Shani et al. (49) | 2008 | Israel | 25 | 34 | - | 48 | 2 | 36 |
| Sliwa et al. (51) | 2003 | South Africa | 100 | 31.6 | - | 2 | 5 | 8 |
| Suri et al. (37) | 2012 | India | 28 | 28.3 | 25 | 21 | 0 | – |
| Vettori et al. (38) | 2011 | Brazil | 6 | 26.5 | 50 | 83 | 17 | 83 |
| Witlin et al. (14,26) | 1997 | United States | 32 | 31.8 | 25 | 69 | – | 89 |
| Yang et al. (52) | 2002 | China | 11 | 28.7 | - | 18 | – | 27 |
| Overall (95% CI) | 37 (29–45) | 9 (7–11) | 67 (60–74) |
No mention of chronic or gestational hypertension; preeclampsia rate was therefore used. HTN = hypertension; PE = preeclampsia.
Figure 2. Prevalence Estimates for PE in Women With PPCM.
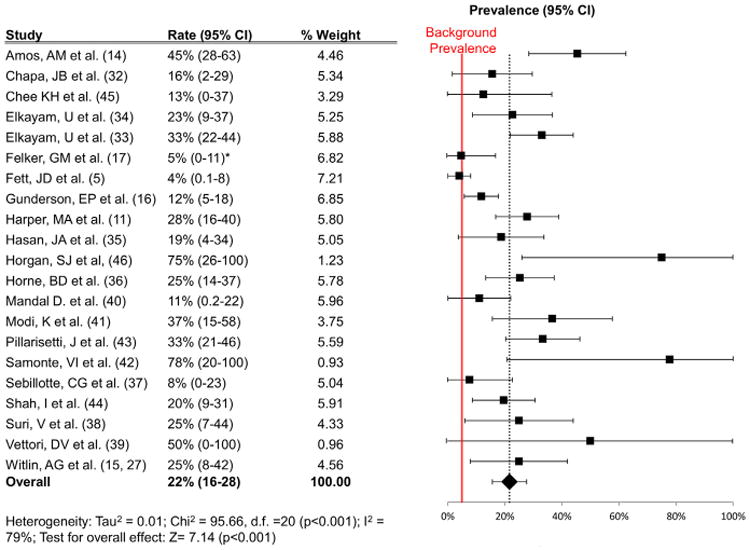
Results of the meta-analysis of the primary outcome (mean prevalence and 95% confidence interval [CI] of PE in women with PPCM). The red line represents the background worldwide prevalence rate of 5%. *Reported rate 0%; 5% used for statistical purposes. Abbreviations as in Figure 1.
Figure 3. Prevalence Estimates for PE in Women With PPCM According to Country.
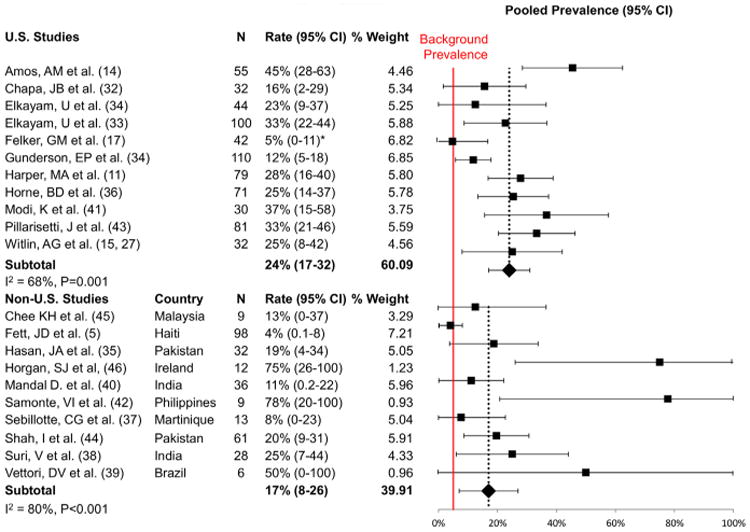
Data are given as United States versus non–United States. Results of the meta-analysis stratified according to study country (mean prevalence and 95% CI of PE in women with PPCM). The red line represents the background worldwide prevalence rate of 5%. *Reported rate 0%; 5% used for statistical purposes. Abbreviations as in Figures 1 and 2.
A secondary analysis, including an additional 10 studies (47–56) and excluding 1 study from the primary analysis because of patient overlap (37), was performed to assess the prevalence of any hypertensive disorder (preeclamptic or not), twin/multiple gestations, and multiparity during pregnancies affected by PPCM (Table 2). There was high heterogeneity observed among these studies, which precluded the performance of a formal meta-analysis. The rates of both hypertensive disorders and twins were elevated above background rates in women with PPCM. The rate of any hypertensive disorder ranged from 2% to 83%, with a weighted average prevalence of 37%, well above the estimated prevalence of 22% to 27% (57). The prevalence of twins or multiple gestation, when reported (N = 16), ranged from 0% to 38% with an average of 9%, again higher than the average estimated prevalence of 3% (58). Sixty-seven percent of women with PPCM were multiparous.
PPCM is more common in women of African descent (4,11,59), raising the possibility that the relationship between PE and PPCM may differ in this group. Figure 4 displays the rates of PE as a function of prevalence of African heritage in each of the studies included in the primary analysis. There was no correlation found between rates of PE and African heritage in women with PPCM at the study level (correlation coefficient −0.35, p = 0.4).
Figure 4. Rates of PE as a Function of Prevalence of African Heritage.
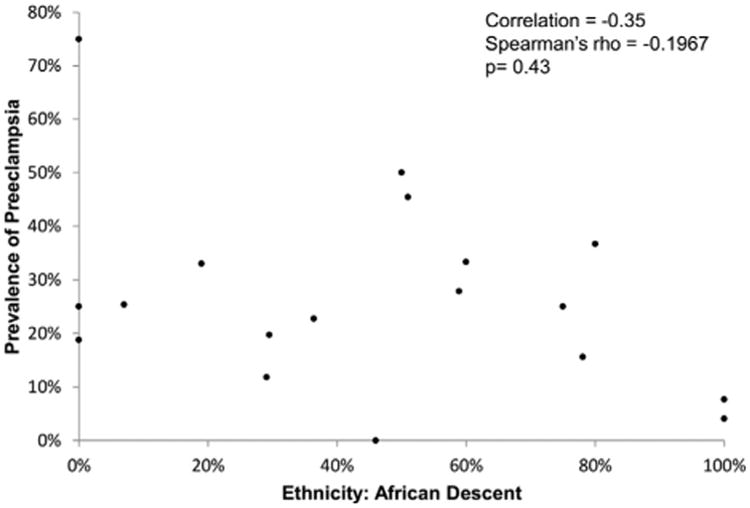
Rate of PE as a function of prevalence of African heritage. Abbreviation as in Figure 1.
Discussion
Our analysis found that patients with PPCM have a prevalence of PE >4 times the average global rate expected in the general population. The included studies come from countries around the globe and are representative of the general population of PPCM. This rate varied by study but was elevated in all but 2 studies. The first study, by Fett et al. (5), investigated women in Haiti, where the incidence of PPCM is extraordinarily high, suggesting that the cause of PPCM in that country may differ from elsewhere. In addition, some women with PE were excluded from the study (J. Fett, personal communication). The second study (17) reported a prevalence of 0%, but this may be an underestimate of the true prevalence of PE in this cohort because an earlier study of a subset of the same cohort (60) reported a prevalence of 11%. These observations underscore an inherent limitation of meta-analyses, which rely on reported studies. Nevertheless, the observations suggest that some of the observed elevated heterogeneity likely relates to the exclusion of some cases of PE from the studies, and that our calculated pooled prevalence of PE in PPCM is a conservative value that likely underestimates the true prevalence.
Historically, many PPCM studies purposefully excluded women with PE or eclampsia to avoid misclassification of PE-associated pulmonary edema as PPCM (e.g., the study by Hasan et al. [31]). It is important to appreciate that PE-associated pulmonary edema is a distinct clinical entity that occurs in the presence of high blood pressure and increased cardiac afterload, but unlike PPCM, it occurs despite a normal ejection fraction. We recommend, however, that women with PE not be excluded from future studies of PPCM, in light of their strong association.
The strong epidemiological relationship observed between PE and PPCM suggests that PE may be part of a pathway that leads to impairments in cardiac function. Consistent with this notion, several studies have recently shown that women with PE have evidence of diastolic dysfunction, as well as impaired systolic strain despite preservation of global systolic function as assessed by ejection fraction (61–63). Gestational hypertension without PE does not cause similar cardiac dysfunction, indicating that aspects of PE other than elevation of blood pressure contribute to cardiac dysfunction (63,64). Moreover, cardiac dysfunction associated with PE persists for at least a year, despite normalization of blood pressure (64).
It is important to note that PPCM is unlikely to be simply a manifestation of severe PE. LVEF in women with PE, even if complicated by acute pulmonary edema, is usually either unchanged or minimally reduced. In contrast, LVEF is usually severely depressed in women with PPCM. The results of our meta-analysis indicate that there is an elevated prevalence of PE in women with PPCM, but it remains true that up to 80% of women with PPCM have no history of PE and, conversely, >90% of women with PE do not develop PPCM. PE thus likely causes cardiac dysfunction directly, but it is neither necessary nor sufficient to cause PPCM. Other factors, yet unknown, must coexist to trigger fullblown cardiac failure.
The strong association between PE and PPCM, and the evidence that PE causes cardiac dysfunction independently of PPCM, suggests that the 2 diseases may share mechanisms of pathophysiology. PE is widely accepted to be a vascular disease (20). The late-gestational placenta of all pregnancies secretes potent antiangiogenic factors into the maternal circulation that are greatly up-regulated in women with PE (65). The most prominent such factor, a soluble version of the vascular endothelial growth factor receptor 1 (sVEGFR1), binds and neutralizes circulating vascular endothelial growth factor. At elevated concentrations, sVEGFR1 disrupts homeostasis in many vascular beds, including the glomerulus, which leads to the hypertension and proteinuria that are characteristic of PE (66,67). Recent evidence from 2 mouse models suggests that PPCM is similarly a disease of the vasculature (18,19). In 1 model, which is deficient in peroxisome proliferator-activated receptor gamma coactivator 1-alpha, sVEGFR1 was sufficient to trigger the development of dilated cardiomyopathy even in the absence of pregnancy. This pathophysiological overlap between PE and PPCM may thus explain their strong epidemiological connection.
The notion that placental sVEGFR1 triggers PPCM may also explain 2 perplexing aspects of PPCM: first, why the disease affects women only during pregnancy; and second, why the disease affects women in late pregnancy, rather than during the second trimester when hemodynamic stresses are most pronounced (68). In both cases, the timing matches the secretion from the placenta of sVEGFR1 and other antiangiogenic factors. We also found a consistently high association between PPCM and twin pregnancies. Twin gestations have larger placentas that secrete more sVEGFR1 into the maternal circulation (22), and twin gestations are also an established risk factor for PE. Again, the pathophysiological overlap between PE and PPCM may explain these observations. Multiparity could similarly cause a stepwise cumulative insult to cardiac function. These clinical and experimental observations suggest a 2-hit hypothesis as a cause of PPCM. The first hit is the late-gestational circulation of cardiotoxic sVEGFR1 and other antiangiogenic factors, and the second hit is a yet-unidentified inability of some women's hearts to withstand this antiangiogenic insult. PE and twin pregnancies accentuate the first hit, thus increasing the likelihood of triggering frank PPCM.
PE and hypertensive disorders of pregnancy are also increasingly recognized as risk factors for future cardiovascular disease. A history of PE confers a >2-fold increased risk of future cardiovascular disease (67), a magnitude of effect that is similar to conventional risk factors, including diabetes and dyslipidemia. American Heart Association/American College of Cardiology guidelines now recommend screening for a history of PE as a part of routine cardiovascular risk evaluation in all women (70). The experimental data discussed here suggest that PE may in part have direct vasculo-toxic effects that predispose to future vascular disease, but it remains unclear whether the increased cardiovascular risk is a direct result of the pathobiology of PE or due to shared risk factors.
PPCM is more common and carries a worse prognosis in women of African heritage (4,10,59). This observation has raised the possibility that PPCM in this population may differ from other racial backgrounds, including in its relationship to PE. We did not find any correlation between African ancestry and prevalence of PE in cohorts of women with PPCM. The strength of this conclusion is somewhat limited by the fact that data were not available at the individual patient level, although there were high rates of women of African descent in many of the included studies. Unfortunately, only 2 studies from the Caribbean, predominantly of African descent, reported ejection fraction data and met inclusion criteria (5,32). Several large, high-quality studies of PPCM from Africa excluded women with PE and were thus also not included in the current analysis.
Study limitations
Ejection fraction in PPCM frequently improves to near-normal, although sometimes only over years (1–3). Few studies have evaluated factors that predict which women will recover systolic function; low ejection fraction on presentation is thus far the only validated prognostic factor. Whether the presence of PE with PPCM portends a worse or better prognosis is not known and warrants further study. Data on outcomes were not included in many of the studies evaluated here, and thus the correlation between outcomes and incidence of PE could not be evaluated.
Conclusions
The current study demonstrates that PE, hypertensive disorders of pregnancy, and multiple gestations are strong risk factors for the development of PPCM throughout the world. The results of the study also support the novel concept that PE and PPCM share a common antivascular pathobiology. PE and PPCM both contribute to significant cardiovascular morbidity and mortality in women. Our analysis highlights the need for better understanding of the mechanistic overlap between PE and PPCM, and a need to include patients with both PE and PPCM in the same clinical studies. This analysis also highlights the need for increased awareness of, and participation in, registries such as the PeriPartum CardioMyopathy Registry of the EURObservational Research Programme by the European Society of Cardiology. In light of potentially unique aspects of PPCM in the United States (Fig. 2), a similar registry in the United States would also be highly informative, as will the results of the ongoing prospective observational IPAC (Investigations in Pregnancy Associated Cardiomyopathy) trial (ClinicalTrials.gov NCT01085955).
Supplementary Material
Acknowledgments
The authors thank Mr. P. Scott Lapinski, of the Countway Library at Harvard Medical School, for his assistance in designing and running the searches for this review.
Dr. Bello is supported by the National Heart, Lung, and Blood Institute (T32 5T32HL007374-33). Dr. Arany is supported by the National Heart, Lung, and Blood Institute and the Ellison Foundation (New York, New York). Dr. Rendon has reported that she has no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- CI
confidence interval
- LVEF
left ventricular ejection fraction
- PE
preeclampsia
- PPCM
peripartum cardiomyopathy
- sVEGFR1
soluble vascular endothelial growth factor receptor-1
Footnotes
Appendix: For supplementary data on the study protocol, please see the online version of this article.
References
- 1.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2012;58:659–70. doi: 10.1016/j.jacc.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368:687–93. doi: 10.1016/S0140-6736(06)69253-2. [DOI] [PubMed] [Google Scholar]
- 3.Deneux-Tharaux C, Berg C, Bouvier-Colle MH, et al. Underreporting of pregnancy-related mortality in the United States and Europe. Obstet Gynecol. 2005;106:684–92. doi: 10.1097/01.AOG.0000174580.24281.e6. [DOI] [PubMed] [Google Scholar]
- 4.Brar SS, Khan SS, Sandhu GK, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100:302–4. doi: 10.1016/j.amjcard.2007.02.092. [DOI] [PubMed] [Google Scholar]
- 5.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80:1602–6. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 6.Isezuo SA, Abubakar SA. Epidemiologic profile of peripartum cardiomyopathy in a tertiary care hospital. Ethn Dis. 2007;17:228–33. [PubMed] [Google Scholar]
- 7. [Accessed June 4, 2013];US Department of Health and Human Services. Available at: http://optn.transplant.hrsa.gov/latest/Data/rptData.asp.
- 8.Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, Sareli P. Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol. 2000;35:701–5. doi: 10.1016/s0735-1097(99)00624-5. [DOI] [PubMed] [Google Scholar]
- 9.Clark SJ, Kahn K, Houle B, et al. Young children's probability of dying before and after their mother's death: a rural South African population-based surveillance study. PLoS Med. 2013;10:e1001409. doi: 10.1371/journal.pmed.1001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fett JD, Murphy JG. Infant survival in Haiti after maternal death from peripartum cardiomyopathy. Int J Gynaecol Obstet. 2006;94:135–6. doi: 10.1016/j.ijgo.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: population-based birth prevalence and 7-year mortality. Obstet Gynecol. 2012;120:1013–9. doi: 10.1097/aog.0b013e31826e46a1. [DOI] [PubMed] [Google Scholar]
- 12.Hull E, Hidden E. Postpartal heart failure. Southern Med J. 1938;31:265–70. [Google Scholar]
- 13.Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44:964–8. doi: 10.1161/01.cir.44.5.964. [DOI] [PubMed] [Google Scholar]
- 14.Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152:509–13. doi: 10.1016/j.ahj.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Witlin AG, Mabie WC, Sibai BM. Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol. 1997;176(1 Pt 1):182–8. doi: 10.1016/s0002-9378(97)80033-6. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson EP, Croen LA, Chiang V, Yoshida CK, Walton D, Go AS. Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol. 2011;118:583–91. doi: 10.1097/AOG.0b013e318229e6de. [DOI] [PubMed] [Google Scholar]
- 17.Felker GM, Jaeger CJ, Klodas E, et al. Myocarditis and long-term survival in peripartum cardiomyopathy. Am Heart J. 2000;140:785–91. doi: 10.1067/mhj.2000.110091. [DOI] [PubMed] [Google Scholar]
- 18.Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–8. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 20.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–92. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 21.Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451–8. doi: 10.1161/HYPERTENSIONAHA.112.195065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bdolah Y, Lam C, Rajakumar A, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198:14. doi: 10.1016/j.ajog.2007.10.783. [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration. 2009 Available at: http://www.cochrane-handbook.org.
- 25.Manolio TA, Baughman KL, Rodeheffer R, et al. Prevalence and etiology of idiopathic dilated cardiomyopathy (summary of a National Heart, Lung, and Blood Institute workshop. Am J Cardiol. 1992;69:1458–66. doi: 10.1016/0002-9149(92)90901-a. [DOI] [PubMed] [Google Scholar]
- 26.Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999;94:311–6. doi: 10.1016/s0029-7844(99)00293-8. [DOI] [PubMed] [Google Scholar]
- 27.Witlin AG, Mabie WC, Sibai BM. Peripartum cardiomyopathy: a longitudinal echocardiographic study. Am J Obstet Gynecol. 1997;177:1129–32. doi: 10.1016/s0002-9378(97)70028-0. [DOI] [PubMed] [Google Scholar]
- 28.Chapa JB, Heiberger HB, Weinert L, Decara J, Lang RM, Hibbard JU. Prognostic value of echocardiography in peripartum cardiomyopathy. Obstet Gynecol. 2005;105:1303–8. doi: 10.1097/01.AOG.0000161382.30233.ba. [DOI] [PubMed] [Google Scholar]
- 29.Elkayam U, Akhter MW, Singh H, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050–5. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 30.Elkayam U, Tummala PP, Rao K, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344:1567–71. doi: 10.1056/NEJM200105243442101. [DOI] [PubMed] [Google Scholar]
- 31.Hasan JA, Qureshi A, Ramejo BB, Kamran A. Peripartum cardiomyopathy characteristics and outcome in a tertiary care hospital. J Pak Med Assoc. 2010;60:377–80. [PubMed] [Google Scholar]
- 32.Horne BD, Rasmusson KD, Alharethi R, et al. Genome-wide significance and replication of the chromosome 12p11.22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ Cardiovasc Genet. 2011;4:359–66. doi: 10.1161/CIRCGENETICS.110.959205. [DOI] [PubMed] [Google Scholar]
- 33.Sebillotte CG, Deligny C, Hanf M, et al. Is African descent an independent risk factor of peripartum cardiomyopathy? Int J Cardiol. 2010;145:93–4. doi: 10.1016/j.ijcard.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 34.Suri V, Aggarwal N, Kalpdev A, Chopra S, Sikka P, Vijayvergia R. Pregnancy with dilated and peripartum cardiomyopathy: maternal and fetal outcome. Arch Gynecol Obstet. 2013;287:195–9. doi: 10.1007/s00404-012-2543-8. [DOI] [PubMed] [Google Scholar]
- 35.Vettori DV, Rohde LE, Clausell N. Asymptomatic left ventricular dysfunction in puerperal women: an echocardiographic-based study. Int J Cardiol. 2011;149:353–7. doi: 10.1016/j.ijcard.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Mandal D, Mandal S, Mukherjee D, et al. Pregnancy and subsequent pregnancy outcomes in peripartum cardiomyopathy. J Obstet Gynaecol Res. 2011;37:222–7. doi: 10.1111/j.1447-0756.2010.01378.x. [DOI] [PubMed] [Google Scholar]
- 37.Modi K, Jariatul K, Illum S, Ghali JK, Reddy PC. Contemporary profile of peripartum cardiomyopathy. J Cardiac Fail. 2005;11:S179. Abstract. [Google Scholar]
- 38.Samonte VI, Ngalob QG, Mata DGB, Punzalan FER, Reyes EP. Clinical and echocardiographic profile and outcomes of peripartum cardiomyopathy: the Philippine General Hospital Experience. J Cardiac Fail. 2012;18:S67–S8. doi: 10.1136/heartasia-2013-010356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pillarisetti J, Reddy V, Kondur A, et al. Post partum cardiomyopathy: predictors of recovery and current state of implantable cardioverter defibrillator use. Heart Rhythm. 2011;8:S438. doi: 10.1016/j.jacc.2014.04.014. Abstract. [DOI] [PubMed] [Google Scholar]
- 40.Shah I, Shahzeb, Hafizullah M, Shah S, Faheem MR. Peripartum cardiomyopathy: risk factors, hospital course and prognosis; experiences at Lady Reading Hospital Peshawar. Pak Heart J. 2012;45:108–15. [Google Scholar]
- 41.Chee KH, Azman W. Prevalence and outcome of peripartum cardiomyopathy in Malaysia. Int J Clin Pract. 2009;63:722–5. doi: 10.1111/j.1742-1241.2007.01449.x. [DOI] [PubMed] [Google Scholar]
- 42.Horgan SJ, Margey R, Brennan DJ, O'Herlihy C, Mahon NG. Natural history, management, and outcomes of peripartum cardiomyopathy: an Irish single-center cohort study. J Matern Fetal Neonatal Med. 2013;26:161–5. doi: 10.3109/14767058.2012.726299. [DOI] [PubMed] [Google Scholar]
- 43.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–69. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993-1997 and 2001-2005. Obstet Gynecol. 2009;113:1075–81. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- 45.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533–8. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 46.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 47.Huang G, Zhang L, Long-Le M, Le-Xin W. Clinical characteristics and risk factors for peripartum cardiomyopathy. Afr Health Sci. 2012;12:26–31. [PMC free article] [PubMed] [Google Scholar]
- 48.Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, Staniloae C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the Internet. Int J Cardiol. 2012;154:27–31. doi: 10.1016/j.ijcard.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 49.Saltzberg MT, Szymkiewicz S, Bianco NR. Characteristics and outcomes of peripartum versus nonperipartum cardiomyopathy in women using a wearable cardiac defibrillator. J Card Fail. 2012;18:21–7. doi: 10.1016/j.cardfail.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Shani H, Kuperstein R, Frenkel Y, Arad M, Sivan E, Simchen M. Peripartum cardiomyopathy- risk factors and prognosis. Am J Obstet Gynecol. 2008;199:S149. Abstract. [Google Scholar]
- 51.Lampert MB, Hibbard J, Weinert L, Briller J, Lindheimer M, Lang RM. Peripartum heart failure associated with prolonged tocolytic therapy. Am J Obstet Gynecol. 1993;168:493–5. doi: 10.1016/0002-9378(93)90479-3. [DOI] [PubMed] [Google Scholar]
- 52.Sliwa K, Zhanje F, Candy G, et al. Peripartum cardiomyopathy: clinical profile in 100 prospective patients studied at a single centre. Eur Heart J. 2003;24:613. Abstract. [Google Scholar]
- 53.Yang J, Liu J, Bian X. Peripartum cardiomyopathydreport of 16 cases. Chin Med Sci J. 2002;17:117–20. [PubMed] [Google Scholar]
- 54.Modi KA, Illum S, Jariatul K, Caldito G, Reddy PC. Poor outcome of indigent patients with peripartum cardiomyopathy in the United States. Am J Obstet Gynecol. 2009;201:171, e1–5. doi: 10.1016/j.ajog.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 55.Gentry MB, Dias JK, Luis A, Patel R, Thornton J, Reed GL. African-American women have a higher risk for developing peripartum cardiomyopathy. J Am Coll Cardiol. 2010;55:654–9. doi: 10.1016/j.jacc.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Habli M, O'Brien T, Nowack E, Khoury S, Barton JR, Sibai B. Peripartum cardiomyopathy: prognostic factors for long-term maternal outcome. Am J Obstet Gynecol. 2008;199:22. doi: 10.1016/j.ajog.2008.06.087. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization international collaborative study of hypertensive disorders of pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am J Obstet Gynecol. 1988;158:80–3. [PubMed] [Google Scholar]
- 58.Boyle B, McConkey R, Garne E, et al. Trends in the prevalence, risk and pregnancy outcome of multiple births with congenital anomaly: a registry-based study in 14 European countries 1984-2007. BJOG. 2013;6:1471–0528. doi: 10.1111/1471-0528.12146. [DOI] [PubMed] [Google Scholar]
- 59.Goland S, Modi K, Hatamizadeh P, Elkayam U. Differences in clinical profile of African-American women with peripartum cardiomyopathy in the United States. J Card Fail. 2013;19:214–8. doi: 10.1016/j.cardfail.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Felker G, Hu W, Hare J, Hruban R, Baughman K, Kasper E. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore) 1999;78:270–83. doi: 10.1097/00005792-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Melchiorre K, Thilaganathan B. Maternal cardiac function in preeclampsia. Curr Opin Obstet Gynecol. 2011;23:440–7. doi: 10.1097/GCO.0b013e32834cb7a4. [DOI] [PubMed] [Google Scholar]
- 62.Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy. 2012;31:454–71. doi: 10.3109/10641955.2012.697951. [DOI] [PubMed] [Google Scholar]
- 63.Shahul S, Rhee J, Hacker MR, et al. Subclinical left ventricular dysfunction in preeclamptic women with preserved left ventricular ejection fraction: a 2D speckle-tracking imaging study. Circ Cardiovasc Imaging. 2012;5:734–9. doi: 10.1161/CIRCIMAGING.112.973818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709–15. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 65.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 66.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–91. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 68.Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL. A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol. 1994;170:849–56. doi: 10.1016/s0002-9378(94)70297-7. [DOI] [PubMed] [Google Scholar]
- 69.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:1. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–62. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


