Abstract
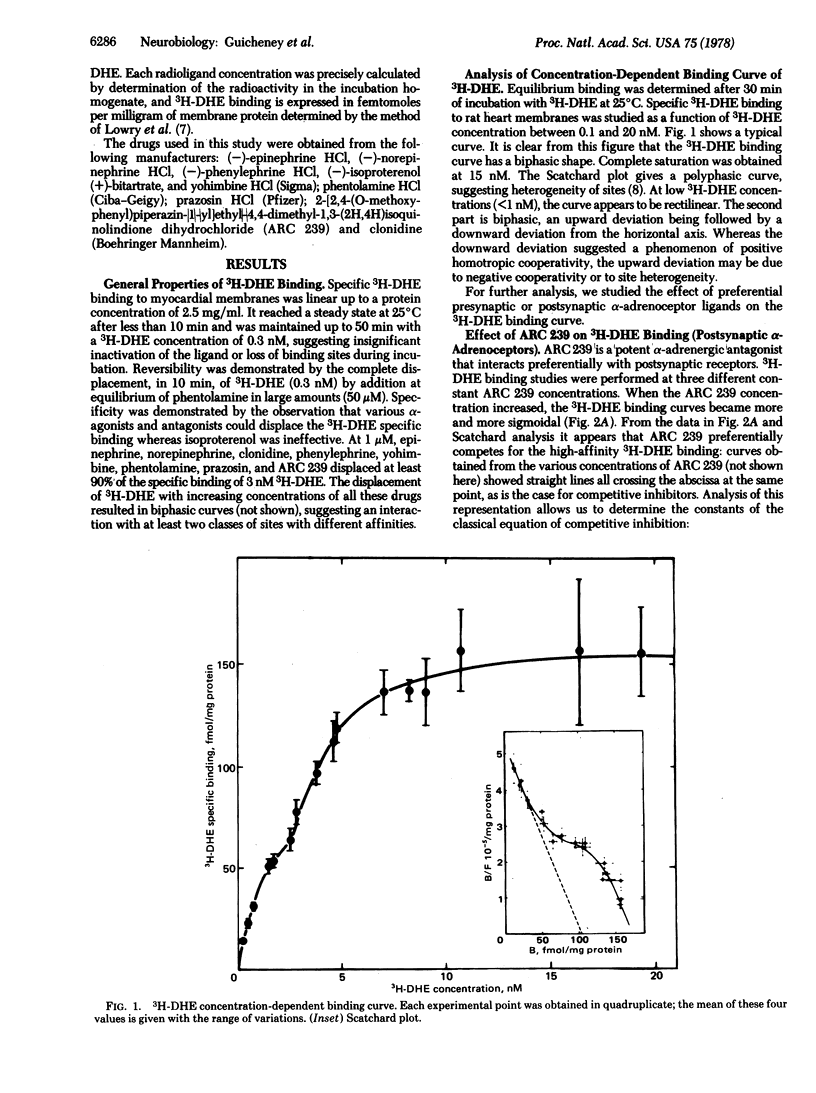
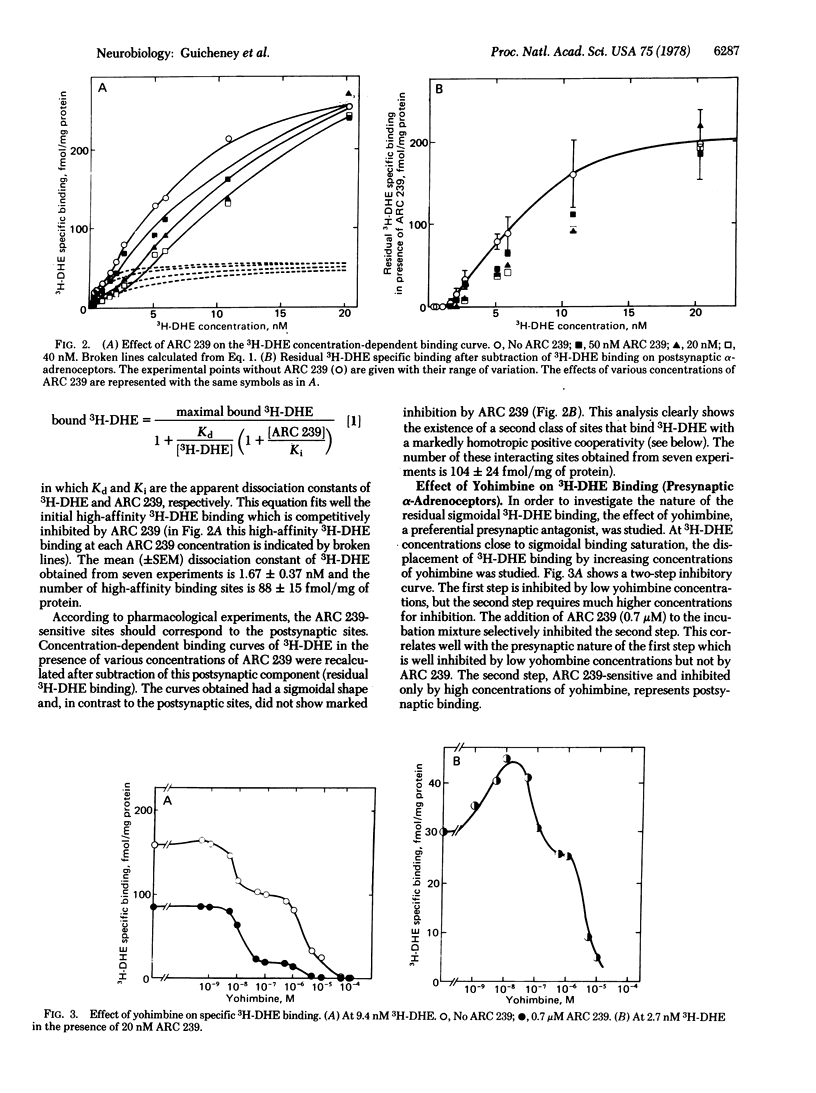
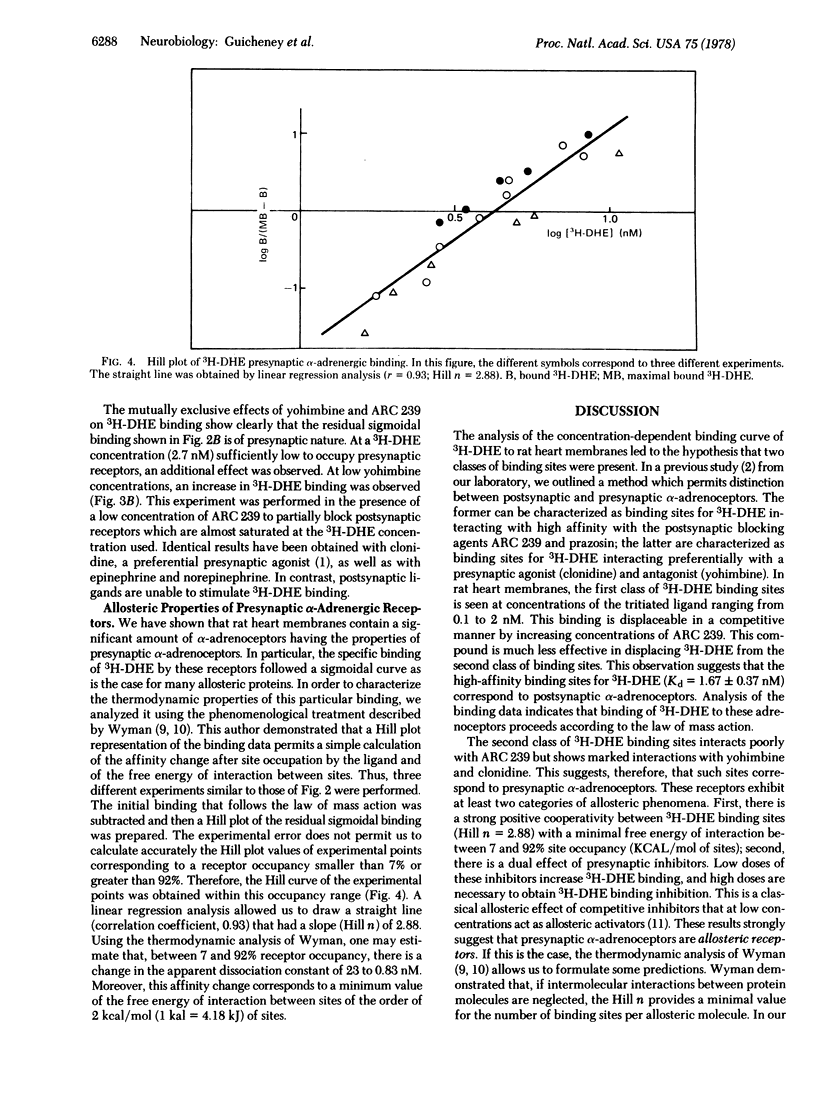
In crude rat cardiac membrane preparations, [3H]dihydroergocryptine (3H-DHE) appears to bind to two classes of sites with limited capacity, differing in their specificities and their affinities. The first class of binding sites interacts preferentially with the postsynaptic alpha-adrenoceptor blocker ARC239, as can be expected for postsynaptic alpha-adrenoceptors. The binding of 3H-DHE to these receptors follows the law of mass action, with a high affinity for 3H-DHE (Kd 25 degrees C = 1.67 +/- 0.37 nM). Postsynaptic saturating levels of 3H-DHE are necessary to occupy the second class of binding sites. These sites exhibit a preferential affinity for presynaptic ligands such as clonidine and yohimbine, as would be expected for presynaptic alpha-adrenergic receptors. This presynaptic binding shows a markedly positive homotropic cooperativity (Hill n = 2.88) with initial and final apparent Kds of 23 and 0.83 nM, respectively. Free energy of interaction between sites is of the order of 2 kcal (8.36 kJ)/mol of sites. These characteristics provide a rational molecular basis for the functional role of presynaptic alpha-adrenoceptors that mediate the inhibition of norepinephrine release from nerve endings.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhandwala M. F., Coats J. T., Buckley J. P. Effects of several catecholamines on sympathetic transmission to the myocardium: role of presynaptic alpha-adrenoceptors. Eur J Pharmacol. 1977 Apr 7;42(3):257–265. doi: 10.1016/0014-2999(77)90292-8. [DOI] [PubMed] [Google Scholar]
- Miach P. J., Dausse J., Meyer P. Direct biochemical demonstration of two types of alpha-adrenoreceptor in rat brain. Nature. 1978 Aug 3;274(5670):492–494. doi: 10.1038/274492a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Tittler M., Weinreich P., Seeman P. New detection of brain dopamine receptors with (3H)dihydroergocryptine. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3750–3753. doi: 10.1073/pnas.74.9.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Mullikin D., Lefkowitz R. J. Identification of alpha-adrenergic receptors in uterine smooth muscle membranes by [3H]dihydroergocryptine binding. J Biol Chem. 1976 Nov 25;251(22):6915–6923. [PubMed] [Google Scholar]



